Effects of dietary consumption of cranberry powder on metabolic parameters in growing rats fed high fructose diets
Ramesh C.
Khanal
ab,
Theodore J.
Rogers
a,
Samuel E.
Wilkes
a,
Luke R.
Howard
b and
Ronald L.
Prior
*ac
aUSDA, Arkansas Children's Nutrition Center, 15 Children's Way, Little Rock, AR 72202, USA. E-mail: rprior1@cablelynx.com; Tel: +1-501-743-8949
bDepartment of Food Science, University of Arkansas, Fayetteville, AR 72704, USA
cUSDA, ARS, Little Rock, AR 72202, USA
First published on 22nd September 2010
Abstract
The effect of dietary consumption of a cranberry powder (CP) containing increased amounts of procyanidins and other phytochemicals on metabolic parameters associated with metabolic syndrome was investigated in growing rats fed a high fructose diet. Dietary treatments were control (starch based), high fructose (HF), and HF containing either 3.3, 6.6, or 33 g CP/kg diet. Fasting plasma glucose and triglycerides tended to be higher with HF feeding and were reduced by feeding CP. The area under curve following an oral glucose tolerance test was 35–50% higher in animals fed HF diet vs. control and was decreased to control levels by the low or medium but not high CP diet. Feeding CP tended to lower fasting plasma insulin. Homeostatic models of insulin resistance (HOMA-IR) and β-cell function (HOMA-BCF) were lowest in animals fed low or medium CP diets (p < 0.05). Rats fed the control starch diet had slightly higher food intake, final body weight, and abdominal fat compared to animals fed other diets. Kidney weight was higher in HF group and feeding CP decreased kidney weight to normal levels. In the fed state, plasma triglyceride was increased with HF diet, whereas insulin was lower in animals fed HF diet. Overall, inclusion of CP in the diet was effective in modulating some aspects of metabolic parameters associated with metabolic syndrome and the medium level of CP in the diet produced a better response than the lower and higher CP levels.
Abbreviations
| HF | High fructose |
| LC | Low Cranberry powder |
| MC | Medium Cranberry powder |
| HC | High Cranberry powder |
| HOMA-IR | Homeostatic model of insulin resistance |
| HOMA-BCF | Homeostatic model of β-cell function |
| QUICKI | quantitative insulin-sensitivity check index |
| OGTT | Oral glucose tolerance test |
Introduction
Resistance to insulin is a state in which a given concentration of insulin produces subnormal glucose response. Such resistance is characteristic of type II diabetes and of metabolic syndrome. Therefore, some patients with type II diabetes require larger doses of insulin to achieve and control hyperglycemia. In a recent study, cinnamon extract appeared to have moderate effects in reducing fasting plasma glucose concentrations in diabetic patients with poor glycemic control.1 The effectiveness of cinnamon supplementation in patients with type II diabetes has received considerable attention after this study was published in 2003, and a reasonable amount of literature is developing relating to the effects of cinnamon on insulin sensitivity and glycemic control in diabetes. The insulinomimetic activity of cinnamon has been shown to be related to its content of A-type procyanidins.2,3 Phenolic phytochemicals are implicated to have potential for prevention and management of many chronic oxidation-linked diseases such as diabetes and cardiovascular disease.4 A-type procyanidins have been found in cranberry extracts and are suggested to be the active component inhibiting the adherence of uropathogenic Escherichia coli to the uroepithelial-cell surfaces.5–7 A-type procyanidins have been found in only a limited number of other foods, such as peanuts and cinnamon.8,9 Other foods are also identified to have A-type linkages,9 but at lower concentrations than in cinnamon or cranberry.Cranberry is a rich source of phenolic phytochemicals that have been shown to have high antioxidant activity10,11 and its procyanidin profile contains A-type linkages in the monomer, dimer, trimer and higher oligomers.9 While the bioavailability of trimers and higher oligomeric procyanidins is questionable, procyanidin monomers and dimers have been shown to be absorbed from the small intestine.12 Cranberry powder (CP) used in the current study is a proprietary product prepared by Decas Botanical Synergies (Carver, MA) and contains increased levels of procyanidins compared to whole cranberry.
The objective of this study was to determine if phytochemicals in cranberry were effective in normalizing selected metabolic parameters associated with metabolic syndrome in high fructose fed growing male Sprague-Dawley rats. Along with the current one, a second manuscript comparing the effects of freeze dried whole fruit powders of black berry, blueberry, and cranberry on selected metabolic parameters associated with high fructose feeding has also been submitted.
Results
All animals were fed isocaloric and isonitrogenous diets with all the other major nutrients held constant (Table 1). Control and HF diets had no known source of polyphenols. The CP contained 1.51 mg/g total anthocyanins, 56.2 mg/g procyanidins, 85 μg/g epicatechin, and 137 μg/g catechin. Based upon information provided by the supplier, the CP contained 94.2 mg total phenolics (gallic acid equivalents)/g, 214 mg glucose/g and 70.6 mg fructose/g. The HPLC chromatograms of procyanidins and anthocyanins are given in Fig. 1. The relative distribution of procyanidins is presented in Table 2, which was based upon area under the curve obtained from the HPLC chromatogram; dimers, trimers, tetramers, and polymers were the major procyanidins present in CP contributing approximately 88% of the reported procyanidins, while the monomer was present only in small amounts at slightly more than 3.5% of the total. Among the anthocyanins, peonidin 3-galactoside was present in highest amounts, followed by peonidin 3-arabinoside, cyanidin 3-arabinoside, and cyanidin 3-galactoside, respectively (Table 2). Details related to the intake and urinary excretion pattern of 19 phenolic acids are presented elsewhere.13 Average daily intakes of these different polyphenols by rats in the CP fed groups is presented in Table 3. Since the diets were purified, it was assumed that rats in control or HF group did not receive any of the polyphenols in appreciable amounts that would otherwise confound the results.| Ingredients | Control | HF | HF + LC | HF + MC | HF + HC |
|---|---|---|---|---|---|
| a HF = High fructose, HF + LC = High fructose + low (3.3 g/kg diet) cranberry powder, HF + MC = HF + medium (6.6 g/kg diet) cranberry powder, HF + HC = High fructose + high (33 g/kg diet) cranberry powder. | |||||
| A. Ingredient composition | |||||
| Casein, 80 mesh | 200 | 200 | 200 | 200 | 200 |
| L-Lysine | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Corn starch | 397.5 | 0 | 0 | 0 | 0 |
| Maltodextrin | 132 | 99.5 | 96.2 | 92.9 | 67.5 |
| Sucrose | 100 | 0 | 0 | 0 | 0 |
| Fructose | 0 | 530 | 530 | 530 | 530 |
| Cellulose | 50 | 50 | 50 | 50 | 49 |
| Soybean oil | 70 | 70 | 70 | 70 | 70 |
| t-BHQ | 0.014 | 0.014 | 0.014 | 0.014 | 0.014 |
| Vitamin mixture | 10 | 10 | 10 | 10 | 10 |
| Mineral mixture | 35 | 35 | 35 | 35 | 35 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Cranberry powder | 0 | 0 | 3.3 | 6.6 | 33.0 |
| Total | 1000.014 | 1000.014 | 1000.014 | 1000.014 | 1000.014 |
| B. Nutrient composition | |||||
| Crude protein, % | 20.3 | 20.3 | 20.3 | 20.3 | 20.5 |
| Fat, % | 7.0 | 7.0 | 7.0 | 7.0 | 7.1 |
| Crude fiber, % | 5.0 | 5.0 | 5.0 | 5.0 | 5.1 |
| Energy, kcal/kg | 3998 | 4000 | 3997 | 3995 | 3978 |
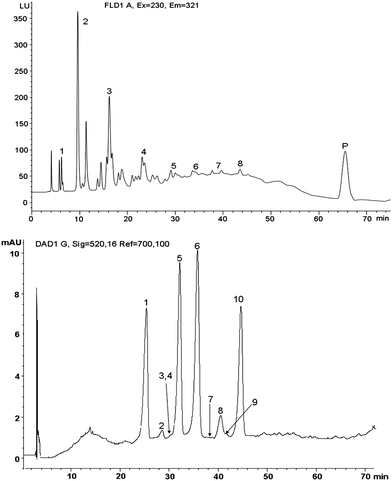 | ||
| Fig. 1 Top panel - Fluorescence trace of HPLC chromatogram of procyanidins extracted from cranberry powder (CP) included in the rat diet. Numbers directly above or by the peaks indicate the degree of polymerization, including the polymer (P). Bottom panel – Diode array traces of HPLC chromatogram of anthocyanins obtained from CP. Peak 1 = Cyanidin 3-galactoside, 2 = cyanidin 3-glucoside, 3 = pelargonidin 3-galactoside, 4 = petunidin 3-galactoside, 5 = cyanidin 3-arabinoside, 6 = peonidin 3-galactoside, 7 = pelargonidin 3-arabinoside, 8 = peonidin 3-glucoside, 9 = malvidin 3-galactoside, 10 = peonidin 3-arabinoside. | ||
| Procyanidins, DPa | Distribution, % | |
|---|---|---|
| Monomer | 1 | 3.65 |
| Dimer | 2 | 24.47 |
| Trimer | 3 | 30.05 |
| Oligomers | 4 | 15.06 |
| 5 | 3.84 | |
| 6 | 2.33 | |
| 7 | 1.48 | |
| 8 | 0.86 | |
| Polymer | >10 | 18.24 |
| Anthocyaninsb | (mg/g dry weight) | |
|---|---|---|
| a DP = Degree of polymerization. b Two other anthocyanins, peonidin 3,5-digalactoside and delphinidin 3-arabinoside were also detected in small amounts. | ||
| Cyanidin 3-galactoside | 0.1492 | |
| Cyanidin 3-glucoside | 0.0124 | |
| Pelargonidin 3-galactoside | 0.0062 | |
| Petunidin 3-galactoside | 0.0093 | |
| Cyanidin 3-arabinoside | 0.1846 | |
| Peonidin 3-galactoside | 0.6359 | |
| Pelargonidin 3-arabinoside | 0.0011 | |
| Peonidin 3-glucoside | 0.1002 | |
| Malvidin 3-galactoside | 0.0090 | |
| Peonidin 3-arabinoside | 0.3980 | |
| Total | 1.5081 | |
| Treatments | PCNa, mg/d | Total phenolics, mg/d | Total ACYb, μg/d | Catechin, μg/d | Epicatechin, μg/d |
|---|---|---|---|---|---|
| a PCN = procyanidins. b ACY = anthocyanin. c HF = High fructose, HF + LC = High fructose + low (3.3 g/kg diet) cranberry powder, HF + MC = HF + medium (6.6 g/kg diet) cranberry powder, HF + HC = High fructose + high (33 g/kg diet) cranberry powder. | |||||
| HF + LC | 3.98 | 6.68 | 106.97 | 9.72 | 6.03 |
| HF + MC | 7.75 | 13.0 | 207.92 | 18.9 | 11.7 |
| HF + HC | 39.81 | 66.8 | 1069.72 | 97.2 | 60.3 |
Animals were randomized to treatments at the beginning of the study such that initial weights were not different (P > 0.95) between treatments (Table 4). Initial body weight was used as a blocking factor in the experimental design, which was incorporated during statistical analysis, to minimize its effect on the eventual results. Rats fed the control starch diet consumed the highest amount of food throughout the experiment (Fig. 2). When analyzed with a repeated measures design, all treatments had a significant effect (P < 0.05) on food consumption and cumulative weight gain vs. control (Fig. 2). As a result, animals on control diet were also the heaviest. However, when final and fasted body weight (at the time of sacrifice) and total food intake was analyzed using a randomized complete block design (Table 4), only a tendency (P = 0.06 and 0.07) for the same was observed. Increased body weight, however, did not necessarily result in the higher organ weights (Table 4), except abdominal fat, which was highest in animals fed the control diet. Feeding the high fructose diet decreased abdominal fat accretion, but dietary CP did not significantly (P > 0.1) alter this response. When organ weights were expressed as percent of body weight, high fructose fed animals had the largest kidney weight relative to their body weight compared to animals on control diet, but was not significantly different from other animals on any of the CP diets. A similar trend was observed with liver weights. Cardiac hypertrophy was not observed in this study as indicated by non-significant changes in heart weight as a % of body weight.
| Items | Control | HF | HF + LC | HF + MC | HF + HC | SEM | P |
|---|---|---|---|---|---|---|---|
| a HF = High fructose, HF + LC = High fructose + low (3.3 g/kg diet) cranberry powder, HF + MC = HF + medium (6.6 g/kg diet) cranberry powder, HF + HC = High fructose + high (33 g/kg diet) cranberry powder. *§ Means without a common superscript are significantly different at the P value indicated in the last column. | |||||||
| A. Body weight, g | |||||||
| Initial | 175.1 | 175.8 | 174.8 | 175.4 | 175.2 | 2.8 | 0.95 |
| Final | 518.2 | 462.2 | 480.9 | 474.1 | 475.5 | 15.8 | 0.07 |
| At sac | 493.6 | 434.0 | 464.0 | 455.9 | 459.8 | 14.4 | 0.06 |
| B. Tissue weight, g | |||||||
| Heart | 1.79 | 1.62 | 1.63 | 1.58 | 1.62 | 0.07 | >0.10 |
| Kidney | 3.26 | 3.23 | 3.30 | 3.32 | 3.12 | 0.15 | >0.10 |
| Liver | 12.2 | 11.9 | 12.8 | 13.5 | 12.1 | 0.75 | >0.10 |
| Abdominal fat | 24.6* | 16.2§ | 19.1*§ | 17.6§ | 16.6§ | 2.2 | 0.003 |
| C. Tissue weight, % BW | |||||||
| Heart | 0.362 | 0.377 | 0.351 | 0.348 | 0.352 | 0.012 | >0.10 |
| Liver | 2.48 | 2.75 | 2.73 | 2.96 | 2.62 | 0.11 | 0.06 |
| Kidney | 0.662§ | 0.748* | 0.711*§ | 0.726*§ | 0.680*§ | 0.021 | 0.04 |
| Fat | 4.94* | 3.75§ | 4.03*§ | 3.80§ | 3.56§ | 0.24 | 0.002 |
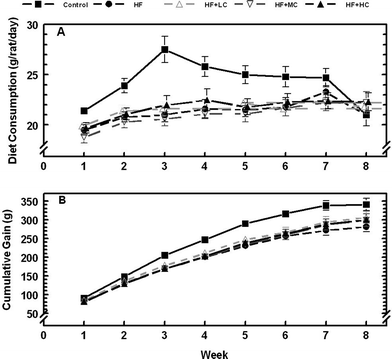 | ||
| Fig. 2 Weekly dietary intake (top panel) and cumulative body weight (bottom panel) of rats fed a control low fructose diet, a high fructose diet (HF) and high fructose diets with different levels of cranberry powder (Low, LC; Medium, MC; High, HC). | ||
Both fasting glucose and triglyceride levels were highest in high fructose fed animals compared with the animals on control or CP diets (Table 5). Addition of CP to the diet tended to have positive effects (P = 0.10) in lowering the blood glucose and triglyceride levels. Changes in serum glucose level after the oral glucose load was consistently and significantly (P < 0.05) higher with high fructose and high fructose + high CP diets compared to animals in other diets after 60 min of oral glucose load (Fig. 3). The total area under curve after the OGTT, was highest in animals fed the high fructose diet. Addition of CP in the diet, primarily at low and medium levels, lowered the OGTT area under the curve down to control levels (Table 5 and Fig. 3). Serum insulin was low in animals fed diets that contained CP and was highest in animals fed high fructose diet (P = 0.08). No effect of the diet was observed on fasting cholesterol.
| Items | Control | HF | HF + LC | HF + MC | HF + HC | SEM | P |
|---|---|---|---|---|---|---|---|
| a HF = High fructose, HF + LC = High fructose + low (3.3 g/kg diet) cranberry powder, HF + MC = HF + medium (6.6 g/kg diet) cranberry powder, HF + HC = High fructose + high (33 g/kg diet) cranberry powder. b Oral glucose tolerance test area under curve expressed as mg/dL × minutes−1. c Homeostasis model assessment – Insulin resistance (HOMA-IR) = [Fasting glucose (mg/dL) × fasting insulin (mU/L)/405. d Homeostasis model assessment – Beta cell function (HOMA-BCF) = [20 × serum insulin (mU/L)]/[plasma glucose (mmol/L)- 3.5]. e Quantitative insulin sensitivity check index (QUICKI) = 1/log HOMA-IR. *§ Means without a common superscript are significantly different at the P value indicated in the last column. | |||||||
| Glucose, mg/dL | 182.9 | 213.1 | 196.1 | 185.3 | 191.0 | 11.8 | 0.10 |
| Cholesterol, mg/dL | 83.5 | 87.3 | 81.3 | 74.4 | 79.0 | 8.2 | 0.86 |
| Triglycerides, mg/dL | 82.4 | 125.6 | 82.2 | 81.7 | 90.1 | 12.2 | 0.10 |
| OGTT | 10457 | 14031 | 9472 | 10466 | 13788 | 1307 | 0.15 |
| Insulin, mU/L | 49.4 | 54.2 | 31.0 | 24.8 | 36.3 | 6.7 | 0.08 |
| HOMA-IR | 22.4*§ | 28.5* | 15.2§ | 11.3§ | 17.2*§ | 3.1 | 0.04 |
| HOMA-BCF | 148.2* | 130.2*§ | 84.1§ | 73.1§ | 102.2*§ | 17.9 | 0.05 |
| QUICKI | 0.74 | 0.69 | 0.85 | 0.95 | 0.81 | 0.09 | 0.24 |
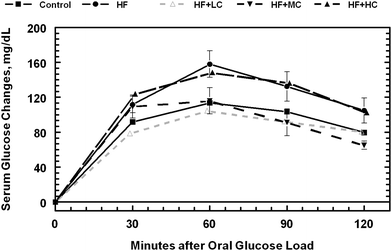 | ||
| Fig. 3 Oral glucose tolerance test responses in rats fed a control low fructose diet, a high fructose diet and high fructose diets with different levels of cranberry powder (Low, LC; Medium, MC; High, HC). See Table 3 for AUC. | ||
When the three models of homeostatic assessment scores were calculated in animals fasted overnight, both HOMA-IR and HOMA-BCF were altered by diet, but the QUICKI model of insulin sensitivity was not statistically significant (P > 0.1) among treatments. While insulin resistance was lowest in animals fed medium level of CP in the diet, it was highest in animals fed high fructose diet. Insulin resistance was reduced by the addition of CP in the diet at all levels (P < 0.04). Similarly, β-cell function was lowest in animals fed medium level of CP in the diet (P 0.05) and highest in animals fed control or high fructose diets. Numerically, QUICKI was lowest in rats fed a high fructose diet and highest in animals fed medium level of CP in the diet.
Weekly postprandial blood glucose, cholesterol, and triglycerides were also tested in animals from wk 2 through 7, whereas insulin was tested in animals from wk 5–7. While there were no significant effects of diet (P > 0.1) on blood glucose concentration (Fig. 4A), there was a significant treatment × week interaction, a situation in which blood glucose level varied from week to week depending on the dietary treatment. Serum insulin (Fig. 4B), on the other hand, was consistently and significantly higher (P < 0.05) in animals fed the control diet compared to animals on high fructose diet; animals on the three CP diets were in between. The concentration of serum triglycerides was consistently higher (P < 0.05) in animals fed high fructose diet (Fig. 5A) even though they had the least amount of abdominal fat as mentioned previously. Inclusion of CP in the diet of rats reduced plasma triglyceride concentrations. Plasma cholesterol concentration increased as the experiment progressed with the increasing age of the animals (Fig. 5B). Although there was a significant treatment × week interaction (P < 0.05) on plasma cholesterol concentrations, diet had no significant effect (P > 0.1) on its weekly values.
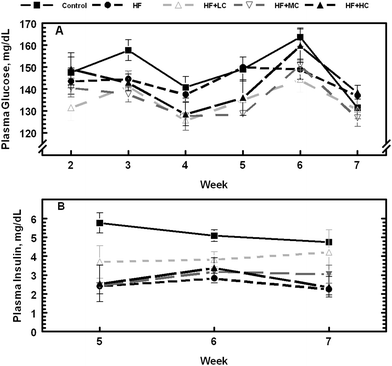 | ||
| Fig. 4 Changes in postprandial serum glucose and insulin levels in rats fed a control low fructose diet, a high fructose diet, and high fructose diets with different levels of cranberry powder (Low, LC; Medium, MC; High, HC). | ||
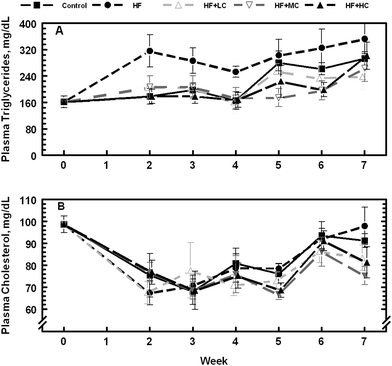 | ||
| Fig. 5 Changes in postprandial serum cholesterol and triglyceride levels in rats fed a control low fructose diet, a high fructose diet, and high fructose diets with different levels of cranberry powder (Low, LC; Medium, MC; High, HC). | ||
Discussion
Polyphenols are one of the major natural dietary compounds believed to be responsible for promotion of health and protection against many chronic diseases, including metabolic syndrome. Originally known as Syndrome X,14 metabolic syndrome refers to the clustering of cardiometabolic risk factors, including abdominal obesity, hyperglycemia, dyslipidemia, elevated blood pressure, increased body mass index/waist circumference, and a decreased high density lipoprotein cholesterol, that are thought to be linked to insulin resistance.15,16 Although it was believed initially to be associated with increased risk of cardiovascular disease, metabolic syndrome has a stronger association with type 2 diabetes than that previously demonstrated for coronary heart disease.17 In the current study, we investigated whether different levels of CP, a concentrated cranberry product, as a source of polyphenols in the diet were effective in modulating some of the metabolic parameters associated with metabolic syndrome.Dietary CP had positive effects in preventing some of the metabolic profiles associated with metabolic syndrome. Response on two of the parameters, fasting blood glucose and triglycerides were borderline and showed a tendency (P = 0.1) to be affected by CP in the diet. Similarly, OGTT responses were slightly improved and similar to animals on control diet when low or medium, but not when the high level of CP was added to the high fructose diet. However, the dietary effect was more pronounced in HOMA scores with insulin resistance being the least in animals when CP was included in the diet. A lower insulin resistance combined with a tendency for reduced blood glucose suggested that animals fed CP were able to utilize the available glucose even at a reduced concentration of circulating insulin, which was reflected in the reduced HOMA-BCF scores when CP was included in the diet. Whereas a higher concentration of fasting insulin was needed to clear out the available glucose in control and HF animals. Lower concentrations of post-prandial glucose and insulin were in line with some previous reports when high fructose diets were fed.18–20 This may be because fructose produces a smaller postprandial rise in plasma glucose than other common carbohydrates21,22 and reduces circulating insulin.20 In the purified diet used in this study, substituting fructose for the starch in the diet removed 75% of the dietary sources of glucose. Maltodextrin, which is a short chain of molecularly linked dextrose (glucose) molecules (fewer than 20) manufactured by regulating the hydrolysis of starch, was included in the diet to aid in the pelleting process, which limited the possibility of removing all glucose sources from the high fructose diets. Fructose metabolism is not regulated by insulin and it has a lower glycemic index than foods rich in starch.23,24 However, fructose increases post-prandial serum triglyceride levels19,20 as was observed in this study. Only 0.1% of fructose was converted to fatty acids at 240 min and lower insulin excursion after fructose resulted in less activation of adipose tissue lipoprotein lipase and impaired triglyceride clearance.19 It may be one of the reasons why there was lower amount of abdominal fat accretion in HF group than those in control group. Moreover, it has been shown previously that increased abdominal fat may not always be associated with metabolic syndrome.25 While sustained elevation of plasma triglycerides with high fructose feeding suggested its possible contribution to atherogenesis and cardiovascular disease, its reduction by the concentrated cranberry product may be helpful in minimizing the effect.
There is a concern that dietary fructose may stimulate energy intake and promote weight gain and obesity.20 As a result, a definitive link through clinical trials may need to be established between the two.21 We actually observed a tendency for lower body weight in HF fed animals compared to animals fed a control diet with equivalent amounts of starch. This was accompanied by a reduced food intake in animals fed HF diet compared to the control diet. We have observed similar results in other experiments with slightly higher or lower level of fructose in the diet (unpublished data). Given the fact that diets were purified and had all the major nutrients at iso-level in the current study (Table 1), results suggested that high fructose feeding may possibly augment some of the factors associated with metabolic syndrome without stimulating energy intake and/or promoting weight gain and obesity. This may partially explain the higher incidence of diabetes and metabolic syndrome among South Asians at lower body mass index and waist circumference.25
The response to high fructose feeding in growing rats in producing elevated fasting plasma triglycerides, enhanced abdominal fat accretion, insulin resistance, and cardiac hypertrophy were not as marked as predicted from the literature using this model.26,27 High fructose feeding in rats is commonly used to induce a strong response on metabolic parameters associated with the metabolic syndrome, albeit more commonly with adult than with growing rats. However, the authors' experience with feeding high fructose diet in developing a strong response of metabolic syndrome in growing rats is rather mixed. We have conducted several experiments in this regard, all with purified diet and nutrient concentrations held constant (unpublished data). One of the observations that we have consistently made is a slightly reduced food intake, lower body weight at termination of the experiment, and the complete lack of abdominal obesity even after having elevated levels of postprandial plasma triglycerides. A number of previous studies have used a natural “chow” based diet and added fructose to it without properly balancing all the nutrients in the process. As a result, animals may become marginally deficient or deficient in some other nutrients resulting in confounding of the effects on metabolic parameters and symptomatic responses, including those associated with metabolic syndrome. To avoid this, a purified diet was used in the current study with all the other major nutrients held constant across treatment. It is possible that when other nutrients are equally balanced and starch is the only item that is completely (or close to) replaced with fructose, a few of the typical signs of metabolic syndrome may not develop at all, while others, such as postprandial plasma triglycerides or insulin resistance may appear. It was observed that balancing all the nutrients across the treatments in purified diets does not generate appreciable oxidative stress in high fructose fed growing animals over those on control diets (unpublished results) that would otherwise be highly conducive for clear signs of metabolic syndrome. A previous study with Sprague-Dawley rats also showed no difference in fasting plasma glucose, cholesterol, triglycerides, and insulin concentration as well as glucose tolerance curves and weight gain between animals fed control (AIN based diet with 53% corn starch), a high fructose (53%) or high fat (25% soybean oil) diet for 3 months.28 The Wistar may be a better rat model in that it is more sensitive to fructose in the diet.
Although it is not possible to determine which of the polyphenols present in CP were responsible for any or all of the observed effects, previous data have suggested that procyanidins may be more responsible than anthocyanins in modulating factors associated with metabolic syndrome.29 Similarly, the effect of total or individual phenolic acids on metabolic syndrome is not clear. Whether and how much of the increased excretion of 4-hydroxycinnamic acid and 3-hydroxyphenylacetic acid and decreased excretion of hippuric acid and 4-hydroxyphenylacetic acid13 have contributed to the observed results is not known. This is the first study detailing the effects of different levels of a commercially available concentrated cranberry product on certain parameters associated with metabolic syndrome in growing animals. The doses provided were calculated based upon previous work using procyanidins on insulin sensitivity and metabolic syndrome.29–31 The high dose of CP was based upon previous data,30 which we predicted would be on the high end of a practical dose. Depending upon the method of extrapolation from the rat to the human, the high dose of CP in the rat (33 g/kg diet) would equate to approximately 54 g of CP for a 70 kg human based upon metabolic weight extrapolation between the rat and human which would provide 3 g of procyanidins. Extrapolating based upon caloric intake, a 70 kg human would consume ∼21 g of the CP or 1.2 g procyanidins per day. The two lower doses were estimated to be practical levels for the rat and when translated to a human would also be reasonable providing ∼200–600 mg procyanidins per day. Overall, inclusion of CP had positive effects in some of the parameters investigated. The high (33 g/kg diet) dose did not provide any additional benefit and may not been as strong based upon the HOMA-IR estimate. Consumption of high level of procyanidins may affect other physiological processes, such as binding or interacting with protein or lipids, which procyanidins (also called tannins) from grape, tea, or other sources are associated with.32–34 It should be noted, however, that medium level included in the current study is, by no means, the best or optimal dose since there is a wide gap between medium and high dose used in the current study. Further experiments are needed to confirm the findings as well as determine the optimum level of inclusion in the diet.
In summary, the cranberry powder was effective in improving some, but not all, of the metabolic parameters associated with metabolic syndrome investigated in the current study. Of the three levels of CP included in the diet, the medium level at 6.6 g/kg diet was the most effective in improving factors associated with metabolic syndrome in the high fructose fed growing rats used in the current study. This highlights the importance of performing dose response studies and that more is not always better. Given the mixed polyphenol content of the cranberry product used, it may be unreasonable to pinpoint the effects observed to one or more specific polyphenols or their constituent compounds.
Experimental
Chemicals
All chemicals used in the study were HPLC grade or higher and were obtained either from Fisher Scientific (Hampton, NH, USA), SigmaAldrich (St. Louis, MO, USA), or SynerMed (Monterey Park, CA, USA).Animals and diet
The protocol was approved by Animal Care and Use Committee of the University of Arkansas for Medical Sciences, Little Rock, AR. Metabolic parameters associated with metabolic syndrome in growing rats were investigated by feeding an American Institute of Nutrition (AIN) based purified diet containing 53% by weight of fructose (0% kcal from starch). Isocaloric and isonitrogenous purified diets were formulated according to Table 1. Purified diets were prepared by Research Diets Inc. (New Brunswick, NJ, USA). Male Sprague-Dawley rats (Charles River Laboratories Intl. Inc., Wilmington, MA; 44 d old, 175.0 ± 8.4 g) were used in a randomized complete block design with initial body weight used as the blocking factor. Animals within block were assigned at random to one of the five treatments, 1) Control (starch based diet); 2) Fructose-rich diet (AIN based diet containing 53% by weight of fructose (HF), 0 kcal from starch); 3) Fructose-rich diet with low, 3.3 g CP per kg diet (HF + LC); 4) Fructose-rich diet with medium, 6.6 g CP per kg diet (HF + MC); or 5) Fructose-rich diet with high, 33 g CP per kg diet (HF + HC). Animals were housed two per cage and provided ad libitum access to food and water. Food consumption and body weight changes were monitored weekly. Cages were changed weekly.Analysis of polyphenols
Total procyanidins in CP were determined by the aldehyde condensation of 4-dimethylaminocinnamaldehyde (DMAC) method as described previously.35 The CP was also analyzed for monomeric, oligomeric and polymeric procyanidins using a high performance liquid chromatograph (HPLC) on a diol column, details of which have been described previously.36 This was done to determine the distribution of individual procyanidins based on the proportionate area under the curve in the HPLC chromatogram. Procedures for determining total phenolics have been described recently,13 whereas anthocyanins were determined using diode array detector in an Agilent 1200 series HPLC (Agilent Technologies, Santa Clara, CA, USA) equipped with a binary pump. Details about the HPLC conditions, column description, mobile phase, gradient, etc. have been described previously.37Sample collection and analysis
Weekly post-prandial blood samples were collected from the tail vein using a Microvette capillary tube (Sarstedt AG & Co., Nümbrecht Germany), while fasting trunk blood was collected at the time of sacrifice. Animals were sacrificed on d 57 and 58 of the experiment after euthanizing in a CO2 chamber and decapitating the head. Organ (kidney, heart, liver, total fat) weights were recorded after the sacrifice. Animals were randomized before blood collection and sacrificed such that treatments were evenly distributed across the time frame or when sacrificed in successive days. Plasma was separated from the blood by centrifugation at 3480 × g for 15 min at 4 °C. Plasma samples were analyzed using commercially available kits for glucose, cholesterol, and triglycerides (Synermed Intl. Inc., Westfield, IN, USA) in a 96-well plate format using a dual pump FluoStar Galaxy (Durham, NC, USA) microplate reader. Plasma insulin was determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Linco Research Inc., St. Charles, MO, USA) in a Benchmark Plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA). All samples were stored at −80 °C prior to analyses. Samples were randomized during each analysis such that treatments were evenly distributed across the plates to minimize the variation caused by running multiple plates and generating multiple standard curves. However, it was not possible to include it in the statistical model given the smaller sample size.Oral glucose tolerance test (OGTT)
At the end of week 6, an OGTT was performed in six rats/treatment over a period of three consecutive days. Rats were food deprived for 12 h before the administration of an oral glucose load of 2 g/kg body weight from a 200 g/L glucose solution. Blood samples (150 μL) were collected via tail vein at time 0 (before administration), 30, 60, 90, and 120 min and analyzed for plasma glucose. Animals were randomized before OGTT such that same number of animals per treatment was included in each day.Insulin sensitivity indices
The Homeostasis model assessment (HOMA) is a computer-generated model consisting of a number of non-linear empirical equations solved numerically to predict glucose and insulin concentrations in the fasting state for any combination of pancreatic β-cell function (HOMA-BCF) and insulin sensitivity (HOMA-IR). The relative-value of the homeostasis model was calculated as an index of insulin resistance (HOMA-IR)17 using the formula: HOMA-IR = Fasting glucose (mmol/L) × fasting insulin (μIU/mL)/22.5. Beta cell function was assessed by the beta cell homeostasis assessment (HOMA-BCF) score: HOMA-BCF = [20 × serum insulin (mU/L)]/[plasma glucose (mmol/L)- 3.5]. Insulin values were expressed in International Units (1 IU = 0.04167 mg).29,38 Similarly, quantitative insulin-sensitivity check index (QUICKI) was calculated from log-transformed HOMA-IR using a formula, QUICKI = 1/log HOMA-IR. Insulin values were expressed as SI units (1 μIU/mL = 6.945 pmol/L).Statistical analysis
Statistical analyses of fasting glucose, cholesterol, triglycerides, insulin, HOMA-IR, HOMA-BCF, QUICKI, final body weight, organ weights, and organ weights as percent of body weight were carried out in SigmaPlot (Systat Software Inc., San Jose, CA, USA). Treatment, block, and their interactions were included in the model as the fixed factors in a randomized complete block design. Statistical analysis of weekly food intake, weight gain, post-prandial glucose, cholesterol, triglycerides, and insulin were carried out in SAS (SAS, Cary, NC, USA) using PROC MIXED. Treatment, week, and their interactions were included in the model with week as the repeated measures on rats. Changes in serum glucose concentration after the oral glucose load was analyzed in PROC MIXED of SAS using treatment, time and their interaction as the fixed factors with time as the repeated measure on the rats. Compound symmetry was used as covariance structure.Acknowledgements
Financial support was provided in part by Decas Botanicals Inc., the Arkansas Biosciences Institute and the U.S. Department of Agriculture, Agriculture Research Service. Mention of a trade name, proprietary product or specific equipment does not constitute a guarantee by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products that may be suitable.References
- B. Mang, M. Wolters, B. Schmitt, K. Kelb, R. Lichtinghagen, D. O. Stichtenoth and A. B. Hahn, Eur. J. Clin. Invest., 2006, 36, 340–344 CrossRef CAS.
- C. L. Broadhurst, M. M. Polansky and R. A. Anderson, J. Agric. Food Chem., 2000, 48, 849–852 CrossRef CAS.
- R. A. Anderson, C. L. Broadhurst, M. M. Polansky, W. F. Schmidt, A. Khan, V. P. Flanagan, N. W. Schoene and D. J. Graves, J. Agric. Food Chem., 2004, 52, 65–70 CrossRef CAS.
- B. Halliwell, Annu. Rev. Nutr., 1996, 16, 33–50 CrossRef CAS.
- L. Y. Foo, Y. Lu, A. B. Howell and N. Vorsa, J. Nat. Prod., 2000, 63, 1225–1228 CrossRef CAS.
- A. B. Howell, Mol. Nutr. Food Res., 2007, 51, 732–737 CrossRef CAS.
- A. B. Howell, J. D. Reed, C. D. Krueger, R. Winterbottom, D. G. Cunningham and M. Leahy, Phytochemistry, 2005, 66, 2281–2291 CrossRef CAS.
- L. Gu, M. A. Kelm, J. F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz, S. Gebhardt and R. L. Prior, J. Nutr., 2004, 134, 613–617 CAS.
- Gu, M. A. Kelm, J. F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz and R. L. Prior, J. Agric. Food Chem., 2003, 51, 7513–7521 CrossRef CAS.
- J. A. Vinson, X. H. Su, L. Zubik and P. Bose, J. Agric. Food Chem., 2001, 49, 5315–5321 CrossRef CAS.
- X. Wu, G. Beecher, J. Holden, D. Haytowitz, S. E. Gebhardt and R. L. Prior, J. Agric. Food Chem., 2004, 52, 4026–4037 CrossRef CAS.
- T. Shoji, S. Masumoto, N. Moriichi, H. Akiyama, T. Kanda, Y. Ohtake and Y. Goda, J. Agric. Food Chem., 2006, 54, 884–892 CrossRef CAS.
- R. L. Prior, T. J. Rogers, S. E. Wilkes, R. C. Khanal, X. Wu and L. R. Howard, J. Agric. Food Chem., 2010, 58, 3940–3949 CrossRef CAS.
- G. M. Reaven, Diabetes, 1988, 37, 1595–1607 CrossRef CAS.
- G. T. Chew, S. K. Gan and G. F. Watts, Med. J. Australia, 2008, 185, 445–449 Search PubMed.
- S. Cowey and R. W. Hardy, Am. J. Physiol., 2006, 169, 1505–1522 CAS.
- E. S. Ford, C. Li and N. Sattar, Diabetes Care, 2008, 31, 1898–1904 CrossRef.
- E. J. Schaefer, J. A. Gleason and M. L. Dansinger, J. Nutr., 2009, 139, 1257S–1262S CrossRef CAS.
- M. F. Chong, B. A. Fielding and K. N Frayn, Amer, J. Clin. Nutr., 2007, 85, 1511–1520 Search PubMed.
- K. L. Teff, S. S. Elliott, M. Tschöp, T. J. Kieffer, D. Rader, M. Heiman, R. R. Townsend, N. L. Keim, D. D'Alessio and P. J. Havel, J. Clin. Endocrinol. Metab., 2004, 89, 2963–2972 CrossRef CAS.
- J. P. Bantle, J. Nutr., 2009, 139, 1263S–1268S CrossRef CAS.
- J. P. Bantle, D. C. Laine, G. W. Castle, J. W. Thomas, B. J. Hoogwerf and F. C. Goetz, N. Engl. J. Med., 1983, 309, 7–12 CrossRef CAS.
- R. R. Henry, P. A. Crapo and A. W. Thorburn, Annu. Rev. Nutr., 1991, 11, 21–39 CrossRef CAS.
- D. J. Jenkins, T. M. Wolever, R. H. Taylor, H. Barker, H. Fielden, J. M. Baldwin, A. C. Bowling, H. C. Newman, A. L. Jenkins and D. V. Goff, Am. J. Clin. Nutr., 1981, 34, 362–366 CAS.
- E. A. Enas, V. Mohan, M. Deepa, S. Farooq, S. Pazhoor and H. Chennikara, J. Cardiometab. Syndrome., 2007, 2, 267–275 Search PubMed.
- S. Delbosc, E. Paizanis, R. Magous, C. Araiz, T. Dimo, J. P. Cristol, G. Cros and J. Azay, Atherosclerosis, 2005, 179, 43–49 CrossRef CAS.
- M. J. Pagliassotti, P. A. Prach, T. A. Koppenhafer and D. A. Pan, Am. J. Physiol., 1996, 271, R1319–R1326 CAS.
- A. H. Stark, B. Timar and Z. Madar, Eur. J. Nutr., 2000, 39, 229–234 CrossRef CAS.
- N. A. Al-Awwadi, C. Araiz, A. Bornet, S. Delbosc, J. P. Cristol, N. Linck, J. Azay, P. L. Teissedre and G. Cros, J. Agric. Food Chem., 2005, 53, 151–157 CrossRef CAS.
- M. Pinent, M. Blay, M. C. Bladé, M. J. Salvadó, L. Arola and A. Ardévol, Endocrinology, 2004, 145, 4985–4990 CrossRef CAS.
- S. H. Kim, S. H. Hyun and S. Y. Choung, J. Ethnopharmacol., 2006, 104, 119–123 CrossRef.
- R. A. Frazier, E. R. Deaville, R. J. Green, E. Stringano, I. Willoughby, J. Plant and I. Mueller-Harvey, J. Pharm. Biomed. Anal., 2010, 51, 490–495 CrossRef CAS.
- P. Sarni-Manchado, V. Cheynier and M. Moutounet, J. Agric. Food Chem., 1999, 47, 42–47 CrossRef CAS.
- Y. S. Tarahovsky, Plant Signal. Behav., 2008, 3, 609–611 Search PubMed.
- R. L. Prior, E. Fan, H. Ji, A. Howell, C. Nio, M. J. Payne and J. Reed, J. Sci. Food Agric., 2010, 90, 1473–1478 CrossRef CAS.
- R. C. Khanal, L. R. Howard, C. Brownmiller and R. L. Prior, J. Food Sci., 2009, 74, H52–H58 CrossRef CAS.
- X. Wu, L. Gu, R. L. Prior and S. McKay, J. Agric. Food Chem., 2004, 52, 7846–7856 CrossRef CAS.
- D. R. Matthews, J. P. Hosker, A. S. Rudenski and B. A. Naylor, Diabetologia, 1985, 28, 412–419 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
