Antioxidative and antibacterial effects of seeds and fruit rind of nutraceutical plants belonging to the Fabaceae family
Sumitra
Chanda
*,
Sandeep
Dudhatra
and
Mital
Kaneria
Phytochemical, Pharmacological and Microbiological Laboratory, Department of Biosciences, Saurashtra University, Rajkot, 360 005, Gujarat, India. E-mail: svchanda@gmail.com
First published on 25th October 2010
Abstract
In the present study, the seeds and fruit rind of six plants of the Fabaceae family were selected to evaluate their potential as antioxidant and antibacterial agents. The dried powders were individually extracted with various organic solvents by the cold percolation method, were evaluated for antibacterial activity and methanol extracts used for antioxidant activities. Total phenol, protein and sugar contents were also measured. Antioxidant activities were measured by DPPH free radical scavenging activity, superoxide anion radical scavenging activity and reducing capacity assessment. Antibacterial activity was measured by the agar well diffusion method against four Gram positive and four Gram negative bacteria. The methanol extract of the fruit rind of C. indica showed the maximum DPPH free radical scavenging activity, superoxide anion radical scavenging activity, a high reducing capacity assessment and also had the highest total phenol content. There was a direct correlation between the phenol content and the antioxidant activity. The antibacterial activity of all the extracts was more pronounced on Gram positive bacteria than on Gram negative bacteria. Thus, the fruit rind of C. indica showed the best antioxidant and antibacterial activities.
Introduction
Reactive oxygen species (ROS) are a class of highly reactive molecules derived from oxygen, and generated by metabolic processes in human beings and by external factors such as pollution, radiation or some dietary habits. ROS are detrimental to cells, as they destroy macromolecules such as DNA, protein and lipids when overproduced during conditions such as excessive exercise, hypoxia and/or in antioxidant system failure. ROS have been implicated in more than 100 diseases from malaria to hemorrhagic shock and AIDS.2Synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG) and citric acid are commonly used as antioxidants in foods to prevent or retard lipid oxidation. But, according to the toxicologists and nutritionists, synthetic antioxidants can show carcinogenic effects in living organisms,7 enlarge liver size and increase microsomal enzyme activity.3,12 Thus, evaluation of the antioxidative activity of naturally-occurring substances has become a focus of interest in recent years.14 The use of plants and herbs as antioxidants in processed foods is becoming of increasing importance in the food industry as an alternative to synthetic antioxidants.20
The frequency of life threatening infections caused by pathogenic microorganisms has increased worldwide, and is becoming an important cause of morbidity and mortality in immunocompromised patients in developing countries.1 The steadily increasing bacterial resistance to existing drugs is a serious problem, and therefore there is a dire need to search for new classes of antibacterial substances, especially from natural sources. Unlike synthetic drugs, antimicrobials of plant origin are not associated with side effects and have a great therapeutic potential to heal many infectious diseases.13,29
Any part of a plant can be therapeutically used to treat infectious diseases. There are numerous reports on the antimicrobial activity of crude plant extracts of different parts of plants, for example Punica granatum leaf,25Nelumbo nucifera rhizome,36Trapa natans L. fruit rind,30Satureja cuneifolia,28Diospyros ebenum Roxb. leaf,8Pistachia vera green hull33 and Ficus carica latex,5etc.
The Fabaceae family is the second biggest family among the dicotyledons, and out of 12,000 species, 951 species are grown in India. The aim of this work was to analyze the antioxidant and antibacterial activities of seeds and fruit rind extracts of six nutraceutical plant species belonging to the Fabaceae family.
Materials and methods
Plant material
Fresh pods of six different plants belonging to the Fabaceae family were collected in 2008 from the local market of Rajkot, Gujarat. The ethnobotanical information4 of the studied plants is given in Table 1. The pods were thoroughly washed with tap water, the seeds and fruit rind separated, shade dried, crushed in a homogenizer to a fine powder and stored in air tight bottles.| No. | Botanical Name | Vernacular Name | Therapeutic uses |
|---|---|---|---|
| 1 | Cajanus indica Spr. | Tuver | Bechic, diuretic, astringent, strongly antidysenteric, laxative, vulnerant, restoring lost taste, leprosy, cough, mouth ulcers, tumors, bronchitis, heart diseases, piles, biliousness, diarrhoea, weakness and also used in increasing efficiency of liver |
| 2 | Pisum sativum L. | Vatana | Contraceptive, fungistatic and spermicidal |
| 3 | Vicia faba L. | Vaal | Hemolytic anemia, Parkinson's disease, malaria and help in controlling hypertension |
| 4 | Vigna mungo L. | Udad | Nervine disorders, debility, paralysis, facial paralysis, osteo-arthritis, liver disorders and constipation for relief from such ailments |
| 5 | Vigna radiate (L.) Wilczek | Mag | Dyspepsia, pyrexia, diarrhoea, skin diseases, leprosy, inflammations, seminal weakness, burning sensation, colic, flatulence, haemorrhages, cough, haemoptysis, agalactia, emaciation, consumption, facial paralysis, hemiplegia, vitiated condition of vata and pitta, fever and general debility |
| 6 | Vigna unguiculata (Linn.) Walp. | Chori | Astringent, appetiser, laxative, anthelmintic, anaphrodisiac, diuretic, galactogogue, liver tonic, anorexia, constipation, helmnathiasis, strangury, agalactia, jaundice and general debility |
Crude extraction
The dry powder of seeds and fruit rind (10 g) was first de-fatted with hexane (100 ml), and then individually extracted with ethyl acetate, methanol and water by the cold percolation method.30 The extraction flasks were kept on a rotary shaker for 24 h at 120 rpm. Thereafter, it was filtered through 8 layers of muslin cloth and centrifuged at 5000 rpm for 15 min. The supernatant was concentrated to dryness under reduced pressure. The extraction procedure was repeated at least three times, and the dry extract was pooled, weighed and stored at 4 °C in air tight bottles.Determination of protein and sugar content
The protein content of all the samples was measured by Lowry's method.19 The reducing, non-reducing and total sugar content of all the samples was estimated using Miller's reagent.24Determination of total phenol content
The total phenol content in ethyl acetate, methanol and water extracts of the different plants was determined by the Folin–Ciocalteu reagent method.22 0.5 ml of sample (1 mg ml−1) and 0.1 ml (0.5 N) of Folin–Ciocalteu reagent were mixed and the mixture incubated at room temperature for 15 min. Then, 2.5 ml of saturated sodium carbonate was added and the resulting mixture was again incubated at room temperature for 30 min. The absorbance of the mixture was measured at 760 nm using a spectrophotometer. The total phenol values are expressed in terms of gallic acid equivalents per gram of extracted compound. The assay was carried out in triplicate, with mean values being presented.Antioxidant testing assays
Antibacterial assay
Results and discussion
Extractive yield
The successful determination of biologically-active compounds from plant material is largely dependent on the type of solvent used in the extraction procedure. Several parameters such as extraction temperature, solvent type and solvent concentration can influence the yield during the extraction process.17 Traditional healers primarily use water, but plant extracts from organic solvents showed better antimicrobial properties. Therefore, a number of organic solvents, such as chloroform, methanol, ethanol, acetone, dichloromethane, etc., have been used to evaluate the antimicrobial property of different plants.31,35 In the present work, plant extraction was performed in aqueous as well as organic solvents.The extractive yield differed in different solvents, as shown in Table 2. The extractive yield was greater in water (aqueous) than in organic solvents in both the seeds and fruit rind of all the six plants. The maximum yield was obtained in the aqueous and methanol extracts of the fruit rind of P. sativum. It appears that in the screened plants, polar compounds were greater in concentration than those of non-polar compounds. There are many reports in the literature where the extractive yield varies with different solvents.10,15
| No. | Plant name | Part | % Yield (g g−1 dry powder of the plant) | Total protein content (mg g−1) | Sugar content (mg g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | Ethyl acetate | Methanol | Aqueous | Reducing sugar | Non-reducing sugar | Total sugar | ||||
| 1 | C. indica | Seed | 0.77 | 0.24 | 3.23 | 4.44 | 62.40 ± 0.02 | 1.61 ± 0.12 | 22.62 ± 0.06 | 24.23 ± 0.09 |
| Fruit rind | 1.4 | 0.49 | 4.98 | 5.56 | 15.51 ± 0.04 | 34.5 ± 0.03 | 64.40 ± 0.14 | 98.90 ± 0.13 | ||
| 2 | P. sativum | Seed | 1.48 | 0.40 | 5.39 | 11.03 | 70.20 ± 0.06 | 0.92 ± 0.01 | 17.14 ± 0.16 | 18.06 ± 0.09 |
| Fruit rind | 0.47 | 0.46 | 15.71 | 18.67 | 13.18 ± 0.03 | 3.56 ± 0.01 | 1.06 ± 0.01 | 4.62 ± 0.02 | ||
| 3 | V. faba | Seed | 0.58 | 0.21 | 3.68 | 9.17 | 86.31 ± 0.08 | 0.81 ± 0.01 | 12.57 ± 0.03 | 13.38 ± 0.06 |
| Fruit rind | 0.77 | 0.52 | 5.84 | 13.61 | 6.89 ± 0.02 | 7.95 ± 0.03 | 6.52 ± 0.02 | 14.47 ± 0.08 | ||
| 4 | V. mungo | Seed | 0.60 | 0.30 | 2.66 | 5.51 | 77.21 ± 0.12 | 0.99 ± 0.01 | 14.81 ± 0.07 | 15.80 ± 0.06 |
| Fruit rind | 0.50 | 0.43 | 2.37 | 8.51 | 10.95 ± 0.01 | 1.53 ± 0.02 | 0.70 ± 0.02 | 2.23 ± 0.02 | ||
| 5 | V. radiata | Seed | 0.49 | 0.20 | 3.79 | 6.48 | 67.50 ± 0.11 | 2.08 ± 0.02 | 35.56 ± 0.16 | 37.64 ± 0.15 |
| Fruit rind | 0.51 | 0.38 | 2.22 | 7.69 | 16.69 ± 0.06 | 3.15 ± 0.03 | 4.95 ± 0.03 | 8.10 ± 0.05 | ||
| 6 | V. unguiculata | Seed | 0.72 | 0.28 | 5.05 | 9.38 | 46.50 ± 0.09 | 1.41 ± 0.01 | 11.97 ± 0.08 | 13.38 ± 0.06 |
| Fruit rind | 0.66 | 0.46 | 3.08 | 8.24 | 14.25 ± 0.02 | 3.04 ± 0.02 | 1.14 ± 0.05 | 4.18 ± 0.02 | ||
Total protein and sugar content
Some chemical substances in plants like proteins, carbohydrates, vitamins and fiber also contribute to their antioxidant capacity. The protein content of seeds was more than that of fruit rind in all six plants (Table 2). The reducing sugars were less compared to non-reducing sugars in both the seeds and fruit rind of all six of these plants (Table 2). Amongst the twelve parts, the highest protein content was observed in the seed of V. faba and the highest sugar content was in the fruit rind of C. indica.Total phenol content
Phenolics are antioxidants with redox properties that allow them to act as reducing agents, hydrogen donors and singlet oxygen quenchers. Phenolic compounds are commonly found in both edible and non-edible plants,18 like fruits and vegetables, their products, leguminous plants, herbs and spices.9 However, there are very few reports on fruit rind, especially plants belonging to the Fabaceae family. In all six plants, the fruit rind had a considerably greater total phenol content than the seeds (Table 3). Among the four solvents, the methanol extracts had considerably more total phenol content following aqueous extraction. Among the twelve parts, the methanol extract of the fruit rind of C. indica had the maximum total phenol content.| Plant name | Part | Total phenol content (mg g−1) | IC50 value (μg ml−1) | |||
|---|---|---|---|---|---|---|
| Ethyl acetate | Methanol | Aqueous | DPPH | SO | ||
| C. indica | Seed | 4.18 ± 0.21 | 6.22 ± 0.09 | 15.62 ± 0.08 | >1000 | >1000 |
| Fruit rind | 13.62 ± 0.15 | 158.98 ± 2.14 | 43.13 ± 1.43 | 39 | 420 | |
| P. sativum | Seed | 6.84 ± 0.08 | 8.45 ± 0.12 | 11.12 ± 0.08 | >1000 | >1000 |
| Fruit rind | 7.76 ± 0.05 | 11.62 ± 0.14 | 11.26 ± 0.07 | >1000 | >1000 | |
| V. faba | Seed | 4.57 ± 0.03 | 4.35 ± 0.06 | 10.9 ± 0.05 | >1000 | >1000 |
| Fruit rind | 11.35 ± 0.08 | 14.36 ± 0.07 | 42.96 ± 0.14 | >1000 | >1000 | |
| V. mungo | Seed | 9.68 ± 0.03 | 22.7 ± 0.09 | 6.08 ± 0.08 | >1000 | >1000 |
| Fruit rind | 16.5 ± 0.15 | 43.95 ± 0.23 | 51.99 ± 0.14 | 560 | >1000 | |
| V. radiata | Seed | 7.95 ± 0.05 | 16.29 ± 0.09 | 20.59 ± 0.12 | >1000 | >1000 |
| Fruit rind | 13.78 ± 0.12 | 55.77 ± 0.24 | 74.86 ± 0.32 | 220 | >1000 | |
| V. unguiculata | Seed | 7.56 ± 0.08 | 8.33 ± 0.16 | 13.78 ± 0.11 | >1000 | >1000 |
| Fruit rind | 8.67 ± 0.11 | 32.88 ± 0.27 | 47.16 ± 0.17 | 480 | >1000 | |
Antioxidant testing assays
Antioxidants are known to exhibit their biochemical effects through numerous mechanisms, including the prevention of chain initiation, reductive capacity and radical scavenging mechanisms. Several methods have been used to measure the antioxidant activity of biological materials. However, the most commonly used ones are those involving chromogen compounds of radical nature, which stimulate the reductive oxygen species. These methods are popular due to their ease, speed and sensitivity.DPPH free radical scavenging activity
The IC50 values of the DPPH free radical scavenging activity of methanol extracts of seeds and fruit rinds of the six plants is shown in Table 3. The methanolic extracts of the fruit rinds showed varied levels of DPPH free radical scavenging activity, while the methanolic extracts of the seeds showed IC50 values greater than 1000 μg ml−1 (Table 3). The fruit rinds of P. sativum and V. faba showed the higher IC50 values (>1000 μg ml−1), while in the other four extracts, the value of the IC50 ranged between 39–560 μg ml−1. The lowest IC50 value was shown by C. indica fruit rind.Superoxide anion radical scavenging activity
The IC50 values of superoxide anion radical scavenging activity are shown in Table 3. Out of all the methanolic extracts of the seeds and fruit rinds, only the fruit rind of C. indica showed a superoxide anion radical scavenging activity (IC50 = 420 μg ml−1), while other methanolic extracts of seeds and fruit rinds showed IC50 values of more than 1000 μg ml−1 (Table 3). Here, gallic acid was used as a standard (IC50 = 185 μg ml−1).Reducing capacity assessment
The reducing capacity assessments of all the methanolic extracts of the seeds and fruit rinds are shown in Fig. 1. The fruit rind of C. indica showed a high reducing capacity assessment compared to all the other extracts, while seeds of V. unguiculata showed a moderate reducing capacity assessment. In the seeds, the reducing capacity assessment of the plants was in the order: C. indica > V. radiata > V. faba > V. mungo > P. sativum > V. unguiculata. In the fruit rinds, the reducing capacity assessment of the plants was in the order: C. indica > V. radiata > V. mungo > V. unguiculata > P. sativum > V. faba.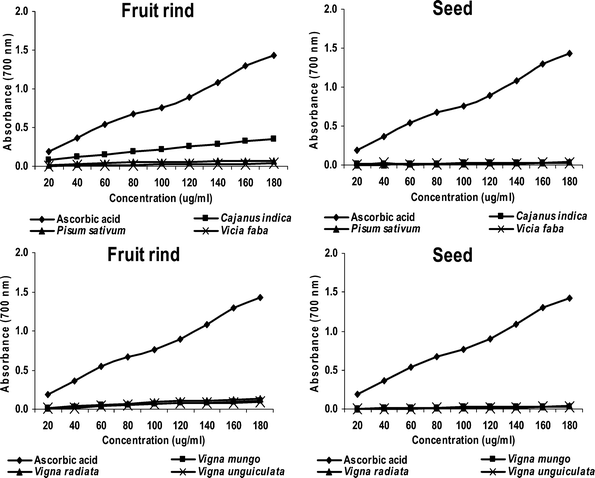 | ||
| Fig. 1 Reducing capacity assessments of methanol extracts of the seed and fruit rind of the screened plants. | ||
Phenolic compounds are considered to be the most important antioxidative plant components. They also have an ability to scavenge free radicals and active oxygen species, such as singlet oxygen, free radicals and hydroxyl radicals.11 A highly positive correlation between total phenols and the antioxidant activities of many plants has been reported.21,8,15 Natural antioxidants strengthen the endogenous defence mechanism and restore the optimal balance by neutralizing reactive oxygen species. Therefore, screening plants for total phenol content may give a clue as to their antioxidant properties. Thus, the search for crude drugs of plant origin with antioxidant properties has become a central focus of study in recent years. In this study, the methanolic extracts of the fruit rind of C. indica showed a high antioxidant activity as compared to the others that might be due to its chemical composition, which is specifically rich in phenolic compounds.
Antibacterial activity
The antibacterial activity of the various solvent extracts exhibited different levels of antibacterial activity against the selected bacterial strains (Table 4 and Table 5). All the extracts showed activity against B. cereus. The hexane and ethyl acetate extracts of the seeds and fruit rinds of all six plants, and the methanolic and aqueous extracts of the fruit rinds of all six plants, except P. sativum, showed activity against K. aerogenes. Ethyl acetate and methanol extracts of the fruit rind of C. indica showed activity against B. subtilis, B. megaterium, C. rubrum and P. pictoruim. Hexane and ethyl acetate extracts of the seeds of P. sativum showed activity against B. subtilis. The ethyl acetate extract of the fruit rind of V. faba showed activity against B. subtilis, B. megaterium, C. rubrum and P. pictoruim. The antibacterial activity of the fruit rind of C. indica against B. cereus was comparable with standard antibiotics like Amikacin, Chloroamphenical and Azithromycin (Table 6).| Plant name | Extracts | Zone of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | ||||||||
| BC | BS | BM | CR | PA | PS | PP | KA | ||
| a HE = hexane; EA = ethyl acetate; ME = methanol; AQ = aqueous; BC = Bacillus cereus; BS = Bacillus subtilis; BM = Bacillus megaterium; CR = Corynebacterium rubrum; PA = Pseudomonas aeruginosa; PS = Pseudomonas stutzeri; PP = Pseudomonas pictoruim; KA = Klebsiella aerogenes; ± = SEM value; — = no activity. | |||||||||
| C. indica | HE | 10.0 ± 0.16 | — | — | — | — | — | — | 9.3 ± 0.33 |
| EA | 9.5 ± 0.16 | — | — | — | — | — | — | 9.7 ± 0.33 | |
| ME | 8.7 ± 0.05 | — | — | — | — | — | — | — | |
| AQ | 9.3 ± 0.05 | — | — | — | — | — | — | — | |
| P. sativum | HE | 10.5 ± 0.00 | — | — | — | — | — | — | 9.0 ± 0.00 |
| EA | 12.8 ± 0.33 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| ME | 9.7 ± 0.17 | — | — | — | — | — | — | — | |
| AQ | 11.7 ± 0.17 | — | — | — | — | — | — | — | |
| V. faba | HE | 10.3 ± 0.47 | — | — | — | — | — | — | 9.3 ± 0.33 |
| EA | 10.3 ± 0.15 | — | — | — | — | — | — | 9.7 ± 0.33 | |
| ME | 9.5 ± 0.17 | — | — | — | — | — | — | — | |
| AQ | 9.5 ± 0.17 | — | — | — | — | — | — | — | |
| V. mungo | HE | 8.7 ± 0.17 | — | — | — | — | — | — | 10.0 ± 0.00 |
| EA | 10.3 ± 0.60 | — | — | — | — | — | — | 10.0 ± 0.00 | |
| ME | 8.0 ± 0.29 | — | — | — | — | — | — | — | |
| AQ | 8.1 ± 0.23 | — | — | — | — | — | — | — | |
| V. radiata | HE | 8.5 ± 0.00 | — | — | — | — | — | — | 9.0 ± 0.00 |
| EA | 9.3 ± 0.17 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| ME | 8.0 ± 0.29 | — | — | — | — | — | — | — | |
| AQ | 9.8 ± 0.17 | — | — | — | — | — | — | — | |
| V. unguiculata | HE | 9.5 ± 0.00 | — | — | — | — | — | — | 9.0 ± 0.00 |
| EA | 11.0 ± 0.29 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| ME | 9.5 ± 0.00 | — | — | — | — | — | — | — | |
| AQ | 9.7 ± 0.17 | — | — | — | — | — | — | — | |
| Plant name | Solvents | Zone of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | ||||||||
| BC | BS | BM | CR | PA | PS | PP | KA | ||
| a HE = hexane; EA = ethyl acetate; ME = methanol; AQ = aqueous; BC = Bacillus cereus; BS = Bacillus subtilis; BM = Bacillus megaterium; CR = Corynebacterium rubrum; PA = Pseudomonas aeruginosa; PS = Pseudomonas stutzeri; PP = Pseudomonas pictoruim; KA = Klebsiella aerogenes; ± = SEM value; — = no activity. | |||||||||
| C. indica | HE | 14.3 ± 0.17 | — | — | — | — | — | — | 9.0 ± 0.00 |
| EA | 14.7 ± 0.44 | 9.0 ± 0.00 | 12.0 ± 0.0 | 9.3 ± 0.33 | — | — | 11.7 ± 0.33 | 9.7 ± 0.33 | |
| ME | 13.3 ± 0.17 | 9.0 ± 0.00 | 10.3 ± 0.33 | 10.7 ± 0.33 | — | — | 12.0 ± 0.00 | 9.3 ± 0.33 | |
| AQ | 8.5 ± 0.00 | — | — | — | — | — | — | 10.0 ± 0.00 | |
| P. sativum | HE | 13.0 ± 0.29 | 11.0 ± 0.00 | — | — | — | — | — | 9.7 ± 0.33 |
| EA | 13.0 ± 0.29 | 11.0 ± 0.00 | — | — | — | — | — | 9.0 ± 0.00 | |
| ME | 9.0 ± 0.29 | — | — | — | — | — | — | — | |
| AQ | 9.5 ± 0.00 | — | — | — | — | — | — | — | |
| V. faba | HE | 9.5 ± 0.29 | — | — | — | — | — | — | 9.0 ± 0.00 |
| EA | 12.7 ± 0.17 | 10.0 ± 0.00 | 12.0 ± 0.00 | 11.7 ± 0.33 | — | — | 10.7 ± 0.33 | 9.7 ± 0.33 | |
| ME | 11.7 ± 0.60 | — | — | — | — | — | — | 9.3 ± 0.33 | |
| AQ | 10.0 ± 0.29 | — | — | — | — | — | — | 10.0 ± 0.00 | |
| V. mungo | HE | 9.3 ± 0.44 | — | — | — | — | — | — | 9.0 ± 0.00 |
| EA | 10.3 ± 0.44 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| ME | 11.0 ± 0.29 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| AQ | 11.7 ± 0.17 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| V. radiata | HE | 10.0 ± 0.29 | — | — | — | — | — | — | 9.0 ± 0.00 |
| EA | 11.7 ± 0.44 | — | — | — | — | — | — | 9.3 ± 0.33 | |
| ME | 10.3 ± 0.60 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| AQ | 10.0 ± 0.29 | — | — | — | — | — | — | 9.3 ± 0.33 | |
| V. unguiculata | HE | 10.7 ± 0.17 | — | — | — | — | — | — | 9.0 ± 0.00 |
| EA | 10.7 ± 0.17 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| ME | 9.7 ± 0.44 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| AQ | 9.5 ± 0.00 | — | — | — | — | — | — | 9.0 ± 0.00 | |
| Antibiotic | Zone of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gram positive | Gram negative | |||||||
| BC | BS | BM | CR | PA | PS | PP | KA | |
| a — = No activity; BC = Bacillus cereus; BS = Bacillus subtilis; BM = Bacillus megaterium; CR = Corynebacterium rubrum; PA = Pseudomonas aeruginosa; PS = Pseudomonas stutzeri; PP = Pseudomonas pictoruim; KA = Klebsiella aerogenes. | ||||||||
| Carbennicillin | 10 | 20 | — | 22 | 12 | 28 | 20 | — |
| Imipenem | 36 | 37 | 41 | 24 | 27 | 38 | 27 | 30 |
| Ceftazidimine | 18 | 25 | — | 19 | 17 | 30 | 12 | — |
| Ciprofloxacin | 19 | 30 | 27 | 16 | 28 | 30 | 23 | 10 |
| Cefaclor | 20 | 35 | 14 | 27 | — | — | 23 | 16 |
| Amikacin | 11 | 15 | 23 | 16 | 22 | 28 | 20 | — |
| Tetracycline | 17 | 21 | 19 | 20 | 15 | 25 | 25 | 10 |
| Piperacillin | 16 | 17 | — | 19 | — | 22 | 15 | 11 |
| Methicillin | — | 16 | 14 | 15 | — | — | 14 | — |
| Chloramphenicol | 10 | 16 | 14 | 15 | 34 | 22 | 25 | 13 |
| Azithromycin | 11 | 14 | 12 | 14 | 12 | 32 | 18 | — |
The antibacterial activities of C. indica and V. faba were more pronounced on Gram positive bacteria than Gram negative bacteria. The reason for this difference in sensitivity might be ascribed to differences in the morphological constitution of these microorganisms; Gram negative bacteria have an outer phospholipidic membrane with a lipopolysaccharide component that makes the cell wall impermeable to plant extracts. Gram positive bacteria, on the other hand, are more susceptible, having only an outer peptidoglycan layer that is not as effective a permeability barrier. There are many reports in the literature that Gram positive bacteria are more susceptible to herbal extracts26,16 and Gram negative bacteria are resistant.27,15 The fruit rind of C. indica showed the best antibacterial activity, and therefore it can be further explored as a novel source of natural antibacterial drugs.
The present investigation suggests that fruit rind, usually a waste product that is discarded into the environment, possesses potential antibacterial and antioxidant properties. However, further isolation and preparation of bioactive compounds of the fruit rind of C. indica are required to establish in vivo antioxidant activity using different animal models. These are novel, natural and economic sources of antioxidants that can be used in the prevention of diseases caused by free radicals. However, they need to be explored as a viable, alternative source to commercially available synthetic and antibiotic drugs. This is the best use of such waste material.
Conclusion
From the above study, it can be concluded that the total phenol content, DPPH free radical scavenging activity, superoxide anion radical scavenging activity and also the reducing capacity assessment are at their maximum in the methanolic extract of the fruit rind of C. indica. The best antibacterial activity was shown by the fruit rind of C. indica. It appears that the fruit rind of C. indica could be a potential source of antibacterial and antioxidant agents. However, further studies are needed to isolate and characterize the active compounds that are responsible for the antioxidant and antibacterial activities.Acknowledgements
The authors thank Prof. S. P. Singh, Head, Department of Biosciences, Saurashtra University, for providing excellent research facilities. M. K. is thankful to the University Grants Commission, New Delhi, India for providing financial support.References
- M. A. Al Bari, M. A. Sayeed, M. S. Rahman and M. A. Mossadik, Characterization and antimicrobial activities of a phenolic and derivative produced by Streptomyces bangladeshiensis a novel species collected in Bangladesh, Resp. J. Med. Sci., 2006, 1, 77–81 Search PubMed.
- H. Alho and J. Leinonen, Total antioxidant activity measured by chemiluminescence methods, Methods Enzymol. 399, 1999 Search PubMed.
- B. M. Ames, Dietary carcinogens and anticarcinogens: oxygen radical and degenerative diseases, Science, 1983, 221, 1256–1263 CrossRef CAS.
- J. Anjaria, M. Parabia and S. Dwivedi, Ethnovet Heritage Indian Ethnoveterinary Medicine—An Overview, Pathik Enterprise, Ahmedabad, India, 2002 Search PubMed.
- H. L. Aref, K. B. H. Salah, J. P. Chaumont, A. W. Fekih, M. Aouni and K. Said, In vitro antimicrobial activity of four Ficus carica latex fractions against resist human pathogens, Pak. J. Pharm. Sci., 2010, 23, 53–58 Search PubMed.
- Y. Athukorala, K. N. Kim and Y. J. Jeon, Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga Ecklonia cava, Food Chem. Toxicol., 2006, 44, 1065–1074 CrossRef CAS.
- P. Baardseth, Effect of selected antioxidants on the stability of dehydrated mashed potatoes, Food Add. Contam., 1989, 6, 201–207 CAS.
- Y. Baravalia, M. Kaneria, Y. Vaghasiya, J. Parekh and S. Chanda, Evaluation of antioxidant and antibacterial activity of Diospyros ebenum Roxb. Leaf (Ebenaceae), Turk. J. Biol., 2009, 33, 159–164 Search PubMed.
- J. Borowska, Fruits and vegetables as source of natural antioxidants, Przem. Ferm. Owok. Warz., 2003, 1, 11–12 Search PubMed.
- H. Hosein and D. Zinab, Phenolic compounds and antioxidant activities of heena leaves extracts (Lawsonia inermis), World J. Dairy Food Sci., 2007, 2, 38–41 Search PubMed.
- S. Itagaki, T. Kurokava, C. Nakata, Y. Saito, S. Oikawa, T. Kobayashi, T. Hirano and K. Iseki, In vitro and in vivo antioxidant properties of ferulic acid: a comparative study with other natural oxidation inhibitors, Food Chem., 2009, 114, 466–471 CrossRef CAS.
- N. Ito, S. Fukushima and A. Hagiwara, Carcinogenicity of butylated hydroxyanisole in F344 rats, J. Nat. Cancer Inst., 1983, 70, 343–352 Search PubMed.
- M. W. Iwu, A. R. Duncan and C. O. Okunji, New antimicrobials of plant origin, in Perspectives on new crops and new uses, ed. J. Janick, ASHS Press, Alexandria VA, 1999, pp. 457–462 Search PubMed.
- G. K. Jayaprakasha, L. J. Rao and K. K. Sakariah, Antioxidant activities of flavidin in different in vitro model systems, Bioorg. Med. Chem., 2004, 12, 5141–5146 CrossRef CAS.
- M. Kaneria, Y. Baravalia, Y. Vaghasiya and S. Chanda, Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra region, India, Indian J. Pharm. Sci, 2009, 71, 406–412 Search PubMed.
- S. R. Kiran, P. Sita and K. Janardhan, Evaluation of in vitro antimicrobial activity leaf and stem essential oils of Chloroxylon swietenia Dc, World J. Microbiol. Biotechnol., 2008, 24, 1909–1914 CrossRef CAS.
- B. B. Li, B. Smith and M. M. Hossain, Extraction of phenolics from citrus peels: I. Solvent extraction method, Sep. Purif. Tech., 2006, 48, 182–188 Search PubMed.
- J. Loliger, The Use of Antioxidants in Food, in Free Radicals and Food Additives, ed. O. I. Aruoma and B. Halliwell, Taylor and Francis, London, 1991, pp. 129–150 Search PubMed.
- O. H. Lowery, N. J. Rosebrough, A. L. Farr and R. J. Randall, Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, 193, 265–275 CAS.
- H. L. Madsen and G. Bertelsen, Spices as antioxidants, Trends Food Sci. Tech., 1995, 6, 271–277 CrossRef CAS.
- P. Maisuthisakul, M. Suttajit and R. Pongasawatmanit, Assessment of phenolic content and free radical scavenging capacity of some Thai indigenous plants, Food Chem., 2007, 100, 1409–1418 CrossRef CAS.
- S. Mc Donald, P. D. Prenzler, M. Autolovich and K. Robards, Phenolic content and antioxidant activity of olive extracts, Food Chem., 2001, 73, 73–84 CrossRef CAS.
- L. M. McCune and T. Johns, Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest, J. Ethnopharmacol., 2002, 82, 197–205 CrossRef.
- G. L. Miller, Use of dinitrosalicylic acid reagent for determination of reducing sugar, Anal. Chem., 1959, 31, 426–428 CrossRef CAS.
- R. Nair and S. Chanda, Antimicrobial activity of Punica granatum in different solvents, Indian J. Pharm. Sci., 2005, 67, 239–243 Search PubMed.
- R. Nair and S. Chanda, Antimicrobial activities of some medicinal plants of the western region of India, Turk. J. Biol., 2007, 31, 231–236 Search PubMed.
- I. Oboh, J. Akerele and O. Obasuyi, Antimicrobial activity of the ethanol extracts of the aerial parts of Sida acuta burm.f. (Malvaceae), Trop. J. Pharma. Res., 2007, 6, 809–813 Search PubMed.
- F. Oke, B. Aslim, S. Ozturk and S. Altundag, Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten, Food Chem., 2009, 112, 874–879 CrossRef CAS.
- J. Parekh and S. Chanda, Evaluation of antimicrobial activity of Terminalia chebula Retz. Fruit in different solvents, J. Herbs Spices Med. Plant, 2007a, 13, 107–116 Search PubMed.
- J. Parekh and S. Chanda, In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants, Turk. J. Biol., 2007b, 31, 53–58 Search PubMed.
- J. Parekh, D. Jadeja and S. Chanda, Efficacy of aqueous and methanol extracts of some medicinal plants for potential antimicrobial activity, Turk. J. Biol., 2005, 29, 203–210 Search PubMed.
- C. Perez, M. Paul and P. Bazerque, An antibiotic assay by the agar well diffusion method, Acta Biol. Med. Exp., 1990, 15, 113–115 Search PubMed.
- A. Rajaei, M. Barzegar, A. M. Mobarez, M. A. Sahara and Z. H. Esfahani, Antioxidant, antimicrobial and antimutagenicity activities of pistachio (Pistachia vera) green hull extract, Food Chem. Toxicol., 2010, 48, 107–112 CrossRef CAS.
- J. Robak and R. J. Gryglewski, Flavonoids are scavengers of superoxide anions, Biochem. Pharmacol., 1988, 37, 83–88.
- J. J. Rojas, V. J. Ochoa, S. A. Ocampo and J. F. Monoz, Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: a possible alternative in treatment of non nosocomial infections, BMC Complementary Altern. Med., 2006, 6, 2–8 Search PubMed.
- D. Yang, Q. Wang, L. Ke, J. Jiang and T. Ying, Antioxidant activities of various extracts of lotus (Nelumbo nuficera Gaerth.) rhizome, Asia Pac. J. Clin. Nutr., 2007, 16, 158–163 CAS.
| This journal is © The Royal Society of Chemistry 2010 |
