Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum’s ability to attenuate macrophage cholesterol accumulation
Mira
Rosenblat
,
Nina
Volkova
,
Judith
Attias
,
Riad
Mahamid
and
Michael
Aviram
*
The Lipid Research Laboratory, Technion-Israel Institute of Technology Faculty of Medicine, The Rappaport Family Institute for Research in the Medical Sciences, Rambam Medical Center, Haifa, 31096, Israel. E-mail: aviram@tx.technion.ac.il; Fax: +972-4-8542130; Tel: +972-4-8542970
First published on 15th September 2010
Abstract
The present study analyzed the antioxidative effects of various beverages, in vitro, and also the effect of short term consumption of beverages richest in polyphenols by healthy subjects on serum anti-atherogenic properties. Healthy subjects consumed 250 mL of the selected beverages for 2 h, or daily, for up to 1 week.
We hypothesized that differences in the anti-atherogenic properties of the studied beverages could be related, not only to the quantity of polyphenols, but also to their quality. Furthermore, we hypothesized that consumption of these juices by healthy subjects for just a short-term, will increase their serum anti-atherogenic properties, as was demonstrated previously in long-term consumption studies.
Of 35 beverages studied, both 100% Wonderful-variety pomegranate and 100% black currant juices were the most potent antioxidants in vitro, as they inhibited copper ion-induced LDL oxidation by up to 94% and AAPH-induced serum lipid peroxidation by up to 38%. Furthermore, they increased in vitro serum paraoxonase 1 (PON1) lactonase activity by up to 51%. Consumption of five selected polyphenol rich beverages by healthy subjects increased serum sulfhydryl group (SH) levels and serum PON1 activities after 2 h, and more so after 1 week of drinking these beverages. These effects were most pronounced after the consumption of 100% Wonderful-variety pomegranate and 100% black currant juices. In conclusion, polyphenolic-rich juices with impressive in vitro antioxidant properties, also demonstrate antioxidant effects in vivo when analyzed for short term consumption. In this respect, 100% Wonderful-variety pomegranate and 100% black currant juices were most the potent.
Introduction
Macrophage cholesterol accumulation and foam cell formation are hallmarks of early atherogenesis.1 Oxidative stress contributes to the development and progression of atherosclerosis.2 Cholesterol accumulation in macrophages can result from increased uptake of oxidized LDL and/or a decreased rate of HDL-mediated cholesterol efflux from the cells.1,2Dietary polyphenols, such as those present in some beverages, were shown to be potent antioxidants3 and cardioprotective agents.4–7 Grape juice consumption by hypertensive individuals for 8 weeks increased serum antioxidant potential,8 and exerts hypolipidemic and anti-inflammatory effects in both hemodialysis patients and in healthy subjects.9 Red wine consumption by healthy subjects inhibited LDL oxidation10 and decreased monocyte migration.11 A more potent cardioprotector than grape juice or red wine was found to be 100% Wonderful-variety pomegranate juice (WPJ), as it protected atherosclerotic patients from further atherosclerosis development.12,13 Consumption of WPJ by healthy subjects for at least 2 weeks, significantly reduced the oxidation of both LDL and HDL and increased HDL-associated paraoxonase 1 (PON1) activity.14 Studies in patients with carotid artery stenosis (CAS) that consumed WPJ for 3 years, clearly demonstrated reduced serum oxidative stress, increased serum PON1 activity, and most importantly - a reduction in atherosclerotic lesion size.12 Furthermore, in diabetic patients, WPJ consumption decreased serum and macrophage oxidative stress, decreased oxidized LDL uptake by their cells,13 and increased PON1 association with HDL.15 Similarly, WPJ in vitro was shown to increase PON1 binding to HDL,16 and also to up-regulate PON1 expression in hepatocytes.17 In all the above studies, the effects of beverages were noted after relatively long periods of consumption (weeks, months, and years). There are a limited number of studies which analyzed the acute effects of beverage consumption on serum oxidative stress and atherogenicity. Plasma antioxidant power was increased postprandially after red wine consumption by healthy subjects.18 Black currant juice consumption for 1 week decreased serum lipid peroxidation and increased urinary excretion of quercetin.19 Similarly, twelve hours after consumption of acai juice by healthy subjects, the plasma antioxidant capacity was significantly increased.20 Finally, consumption of a mixture of berry juices, which include acai and palm fruit as the predominant ingredients, increased the serum antioxidant level, and inhibited lipid peroxidation, two hours post-consumption.21
The aim of the present study was to compare the antioxidative effects of polyphenolic-rich beverages (and especially, those of 100% Wonderful-variety pomegranate juice, 100% black currant juice, 100% Concord grape juice, acai juice blend and red wine) in vitro, and also to study the effects of their short term consumption by healthy subjects (either 2 h, or daily intake for 1 week) on serum anti-atherogenic properties.
Experimental
In vitro studies
Table 1 demonstrates the list of the 35 beverages used in the study.| Category | Brand | Product name |
|---|---|---|
| 100% Pomegranate juice | POM Wonderful | 100% Pomegranate Juice (Wonderful-variety) |
| Knudsen | Just Pomegranate (variety unknown) | |
| Langers | 100% Pomegranate Juice (variety unknown) | |
| Odwalla | Pomegranate Juice (variety unknown) | |
| Naked | Pomegranate (variety unknown) | |
| 100% Concord grape juice | Welch's | 100% Concord Grape Juice |
| Knudsen | Concord Grape | |
| Lakewood | Pure Concord Grape | |
| 100% Black cherry juice | Knudsen | Just Black Cherry |
| Lakewood | Pure Black Cherry | |
| 100% Black currant juice and blends | Knudsen | Just Black Cherry |
| Currant C | Black Currant Nectar | |
| 100% Blueberry juice | Knudsen | Just Blueberry |
| Wyman's | 100% Wild Blueberry | |
| 100% Yumberry | Frutzzo | 100% Yumberry |
| Acai juice blends | Bossa Nova | Acai Juice- The Original |
| Naked | Acai Machine | |
| Sambazon | Original Blend | |
| “Superfruit” blends | Naked | Red Machine |
| Green Machine | ||
| Odwalla | Red Rhapsody | |
| Lakewood | Mangosteen/antioxidant fruit | |
| Born Dia | Acai Mangosteen | |
| Acai Blueberry | ||
| Bossa Nova | Mangosteen Dragonfruit | |
| Goji Lania | Goji Cherry | |
| Goji Acai | ||
| Goji Mangosteen | ||
| Minute Maid | Pomegranate Blueberry | |
| Tropicana | Pomegranate Blueberry | |
| Green tea | Nestea | Green Tea with natural flavor |
| Lipton | Green Tea with citrus | |
| Red wine | Beaulieu | BV Napa Cabernet Sauvignon |
| Mondavi | Robert Mondavi Napa Merlot | |
| Cline | Zinfandel (California) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v
10, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v. Aliquots of 20 μL from the diluted beverages were mixed with 1 mL of 0.1 mmol DPPH/L in ethanol, and the change in optical density at 517 nm was monitored after 5 min.
v. Aliquots of 20 μL from the diluted beverages were mixed with 1 mL of 0.1 mmol DPPH/L in ethanol, and the change in optical density at 517 nm was monitored after 5 min.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v:v. LDL (100 μg of protein/mL) was then pre-incubated for 1 h at room temperature with 25 μL/mL of the samples of diluted beverages. Then, 5 μmol/L of CuSO4 was added and the tubes were incubated for 2 h at 37 °C. At the end of the incubation, the degree of LDL oxidation was determined by measuring the generated amount of thiobarbituric acid reactive substances (TBARS). The TBARS assay was performed at 532 nm, using malondialdehyde (MDA) for the standard curve.26
100, v:v. LDL (100 μg of protein/mL) was then pre-incubated for 1 h at room temperature with 25 μL/mL of the samples of diluted beverages. Then, 5 μmol/L of CuSO4 was added and the tubes were incubated for 2 h at 37 °C. At the end of the incubation, the degree of LDL oxidation was determined by measuring the generated amount of thiobarbituric acid reactive substances (TBARS). The TBARS assay was performed at 532 nm, using malondialdehyde (MDA) for the standard curve.26
In vivo studies
Serum oxidative stress parameters
Serum PON1 activities
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with “activity buffer” (1 mmol/L CaCl2 in 50 mmol/L Tris HCl, pH 8.0) and then 5 μL were taken for a total reaction volume of 200 μL. Initial rates of hydrolysis were determined spectrophotometrically at 270 nm for 3 min (every 15 s). The assay mixture included 1.0 mmol/L phenyl acetate in “activity buffer”. One unit of arylesterase activity equals 1 μmol of phenyl acetate hydrolyzed/min/mL.29
10 with “activity buffer” (1 mmol/L CaCl2 in 50 mmol/L Tris HCl, pH 8.0) and then 5 μL were taken for a total reaction volume of 200 μL. Initial rates of hydrolysis were determined spectrophotometrically at 270 nm for 3 min (every 15 s). The assay mixture included 1.0 mmol/L phenyl acetate in “activity buffer”. One unit of arylesterase activity equals 1 μmol of phenyl acetate hydrolyzed/min/mL.29
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with “activity buffer” (1 mM CaCl2 in 50 mmol/L Tris HCl, pH 8.0), and 3 μL were then taken for the assay. Lactonase activity was measured using dihydrocoumarin (DHC) as the substrate. Initial rates of hydrolysis were determined spectrophotometrically at 270 nm, for 10 min (every 15 s). The assay mixture included 1 mmol/L DHC in “activity buffer”. Non-enzymatic hydrolysis of DHC was subtracted from the total rate of hydrolysis. One unit of lactonase activity equals 1 μmol of DHC hydrolyzed/min/mL.29
10 with “activity buffer” (1 mM CaCl2 in 50 mmol/L Tris HCl, pH 8.0), and 3 μL were then taken for the assay. Lactonase activity was measured using dihydrocoumarin (DHC) as the substrate. Initial rates of hydrolysis were determined spectrophotometrically at 270 nm, for 10 min (every 15 s). The assay mixture included 1 mmol/L DHC in “activity buffer”. Non-enzymatic hydrolysis of DHC was subtracted from the total rate of hydrolysis. One unit of lactonase activity equals 1 μmol of DHC hydrolyzed/min/mL.29
Macrophage cholesterol metabolism
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; v
2; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). The hexane phase was collected and dried under N2. The amount of cellular cholesterol was determined using a kit (CHOL, Roche Diagnostics GMbH, Mannheim, Germany). After extraction of cellular lipids, the cells were dissolved in 0.1 mol/L NaOH for measurement of cellular protein by the Lowry assay.25
v). The hexane phase was collected and dried under N2. The amount of cellular cholesterol was determined using a kit (CHOL, Roche Diagnostics GMbH, Mannheim, Germany). After extraction of cellular lipids, the cells were dissolved in 0.1 mol/L NaOH for measurement of cellular protein by the Lowry assay.25
Results
In vitro studies
| Category | Product brand | Product name | Total polyphenols (mg GAE/mL) | Free radical scavenging capacity (% O.D. reduction of controla) | CuSO4-induced LDL oxidation (TBARS, % reduction of controla) |
|---|---|---|---|---|---|
| a Control represents samples with no beverage added. | |||||
| 100% Pomegranate juice | POM Wonderful | 100% Pomegranate juice (Wonderful-variety) | 4.80 + 0.30 | 76 ± 4 | 93 ± 9 |
| Knudsen | Just Pomegranate (variety unknown) | 4.70 + 0.10 | 75 ± 2 | 93 ± 9 | |
| Langers | 100% Pomegranate Juice (variety unknown) | 3.70 + 0.20 | 62 ± 3 | 57 ± 6 | |
| Odwalla | Pomegranate juice (variety unknown) | 4.20 + 0.20 | 71 ± 4 | 84 ± 7 | |
| Naked | Pomegranate (variety unknown) | 4.30 + 0.20 | 70 ± 4 | 92 ± 8 | |
| 100% Concord grape juice | Welch's | 100% Concord Grape | 2.70 ± 0.20 | 23 ± 2 | 26 ± 3 |
| Knudsen | Concord Grape | 3.60 + 0.20 | 32 ± 3 | 11 ± 2 | |
| Lakewood | Pure Concord Grape | 3.30 + 0.20 | 32 ± 3 | 15 ± 2 | |
| 100% Black cherry juice | Knudsen | Just Black Cherry | 2.40 + 0.10 | 18 ± 2 | 14 ± 1 |
| Lakewood | Pure Black Cherry | 1.90 + 0.10 | 15 ± 1 | 5 ± 1 | |
| 100% Black currant juice and blends | Knudsen | Just Black Currant | 6.80 + 0.10 | 48 ± 4 | 94 ± 5 |
| Currant C | Black Currant Nectar | 3.40 + 0.10 | 31 ± 3 | 11 ± 2 | |
| 100% Blueberry juice | Knudsen | Just Blueberry | 2.80 + 0.20 | 23 ± 2 | 8 ± 2 |
| Wyman's | 100% Blueberry | 2.60 + 0.20 | 15 ± 1 | 5 ± 1 | |
| 100% Yumberry | Frutzzo | 100% Yumberry | 1.90 + 0.10 | 23 ± 2 | 15 ± 2 |
| Acai juice blends | Bossa Nova | Acai Juice- The Original | 1.60 + 0.18 | 11 ± 1 | 5 ± 1 |
| Naked | Acai Machine | 4.40 + 0.40 | 18 ± 2 | 5 ± 1 | |
| Sambazon | Original Blend | 1.60 + 0.14 | 7 ± 1 | 3 ± 1 | |
| “Superfruit” blends | Naked | Red Machine | 2.00 + 0.10 | 11 ± 2 | 10 ± 2 |
| Green machine | 1.20 + 0.14 | 4 ± 1 | 4 ± 1 | ||
| Odwalla | Red Rhapsody | 1.90 + 0.10 | 11 ± 1 | 5 ± 1 | |
| Lakewood | Mangosteen/antioxidant fruit | 1.10 + 0.10 | 10 ± 1 | 3 ± 1 | |
| Born Dia | Acai Mangosteen | 1.80 + 0.10 | 10 ± 1 | 1 ± 1 | |
| Acai Blueberry | 1.70 + 0.10 | 10 ± 1 | 2 ± 1 | ||
| Bossa Nova | Mangosteen Dragonfruit | 1.00 + 0.06 | 5 ± 1 | 1 ± 1 | |
| Goji Lania | Goji Cherry | 1.20 + 0.16 | 8 ± 1 | 1 ± 1 | |
| Goji Acai | 0.80 + 0.03 | 5 ± 1 | 3 ± 1 | ||
| Goji Mangosteen | 0.60 + 0.06 | 8 ± 2 | 1 ± 1 | ||
| Minute Maid | Pomegranate Blueberry | 1.25 + 0.01 | 8 ± 2 | 8 ± 2 | |
| Tropicana | Pomegranate Blueberry | 1.50 + 0.06 | 26 ± 3 | 11 ± 2 | |
| Green tea | Nestea | Green Tea with natural flavor | 0.20 + 0.02 | 2 ± 1 | 2 ± 1 |
| Lipton | Green Tea with citrus | 0.90 + 0.02 | 20 ± 2 | 8 ± 2 | |
| Red wine | Beaulieu | BV Cabernet Sauvignon | 3.70 + 0.30 | 29 ± 3 | 21 ± 3 |
| Mondavi | Robert Mondavi Napa Merlot | 4.70 + 0.40 | 37 ± 4 | 37 ± 4 | |
| Cline | Zinfandel (California) | 3.30 + 0.20 | 27 ± 3 | 16 ± 2 |
100% Pomegranate juice showed very potent free radical scavenging capacity, decreasing the optical density of the DPPH solution by as much as 62%–76% (Table 2). 100% Black currant juice decreased the optical density by 48%, while the red wines and 100% Concord grape juice decreased it by 23%–37% (Table 2). The Tropicana blend, 100% blueberry (Knudsen), 100% Concord grape (Welch's), 100% Yumberry (Frutzo) and green tea (Lipton) exhibited very similar, limited free radical scavenging capacity, as demonstrated by only 20%–26% reduction in the DPPH optical density (Table 2). Most beverages from the “Super fruit” blends category, and green tea (Nestea) were very limited in their free radical scavenging abilities (Table 2).
All beverages, when used at a concentration of 0.25 μL/mL, inhibited copper ion-induced LDL oxidation, but the extent of inhibition was very different. 100% Pomegranate and 100% black currant juices were the most potent antioxidants (Table 2). Red wines and 100% Concord grape juices were somewhat less potent than the 100% pomegranate and 100% black currant juices (Table 2). Red wine (Mondavi) was the best one among all the tested wine brands, with a reduction in LDL oxidation of as high as 37%. Acai juice blends and some of the superfruit blends were very weak inhibitors of LDL oxidation (reductions of only 3–11%) (Table 2). Green tea (Nestea) was the weakest inhibitor of LDL oxidation, as it decreased TBARS formation by only 2% (Table 2). Many of the superfruit blends reduced TBARS by only 1%. These results indicate that all pomegranate beverages except Langers and 100% black currant juice have superior total polyphenol concentration, free radical scavenging capacity and are the most potent inhibitors of LDL oxidation.
In vitro system. According to the results obtained in the in vitro studies (Table 2), we chose the five beverages containing the highest polyphenol concentrations i.e., 100% Wonderful-variety pomegranate juice (POM Wonderful), 100% black currant juice (Knudsen), red wine (Mondavi), acai juice blend (Naked) and 100% Concord grape juice (Knudsen) for further in vitro study. All five beverages decreased the serum susceptibility to AAPH-induced lipid peroxidation (as compared to control serum, with no beverage addition), as measured by the TBARS assay (Fig. 1A). 100% Wonderful-variety pomegranate juice, 100% black currant juice and red wine were the most potent antioxidants in this respect, with reduction rates of serum lipid peroxidation of 38%, 38% or 31%, respectively (Fig. 1A). 100% Concord grape juice decreased serum oxidation by 23%, and acai juice blend was the least potent antioxidant juice in this respect, with only 8% inhibition of AAPH-induced serum lipid peroxidation (Fig. 1A).
![The effect of polyphenol-rich beverages on in vitro AAPH-induced serum lipid peroxidation, and on serum PON1 lactonase activity. The serum from healthy subjects was pre-incubated for 1 h with no addition (control), or with 2 μL of the concentrated beverages [POM Wonderful, black currant (Knudsen), red wine (Mondavi), grape (Lakewood), acai (Naked)]. (A) The extent of AAPH-induced serum lipid peroxidation was determined by the TBARS assay. (B) Serum PON1 lactonase activity. Results are expressed as mean ± SEM of three different experiments. *p <0.01 vs. control.](/image/article/2010/FO/c0fo00011f/c0fo00011f-f1.gif) | ||
| Fig. 1 The effect of polyphenol-rich beverages on in vitro AAPH-induced serum lipid peroxidation, and on serum PON1 lactonase activity. The serum from healthy subjects was pre-incubated for 1 h with no addition (control), or with 2 μL of the concentrated beverages [POM Wonderful, black currant (Knudsen), red wine (Mondavi), grape (Lakewood), acai (Naked)]. (A) The extent of AAPH-induced serum lipid peroxidation was determined by the TBARS assay. (B) Serum PON1 lactonase activity. Results are expressed as mean ± SEM of three different experiments. *p <0.01 vs. control. | ||
Paraoxonase 1 (PON1) is associated in serum with HDL, and possesses anti-atherogenic properties.31,32 Antioxidants have been shown to preserve PON1 activity.33 Thus, we next analyzed the effects of these selected beverages on serum PON1 catalytic activities. PON1 lactonase activity (DHC hydrolysis) was significantly increased by 48%, 51% or by 41% on serum incubation with 100% Wonderful-variety pomegranate juice, 100% black currant juice or red wine, respectively, as compared to activity in non-treated (control) serum (Fig. 1B). Grape juice or acai juice were less potent, as they increased serum PON1 lactonase activity by 35% or 29%, respectively (Fig. 1B). A similar pattern was observed upon measuring serum PON1 arylesterase activity (phenyl acetate hydrolysis, data not shown).
In vivo studies
All the beverages used contain high concentrations of naturally occurring sugar (which can possibly increase serum glucose and triacylglycerol concentrations, as well as enhance serum oxidative stress). Nevertheless, they also possess potent in vitro antioxidative properties. Thus, we questioned the in vivo effects of the beverages on healthy subjects. In the present study, we analyzed the effects of the five selected polyphenol-rich beverages when consumed for a short period of time (2 h after consumption, or a daily intake for 1 week), on serum glucose and lipid profiles, on serum oxidative stress, on serum PON1 activities and on serum ability to affect cholesterol accumulation in macrophages.| Serum biochemical parameter | Acai juice blend (Naked) | 100% Concord grape juice (Lakewood) | 100% Black currant juice (Knudsen) | 100% Wonderful-variety pomegranate juice (POM Wonderful) | Red wine (Merlot/Mondavi) | |
|---|---|---|---|---|---|---|
| Cho (mg/dL) | 0 | 152 ± 7 | 159 ± 7 | 158 ± 6 | 154 ± 7 | 159 ± 4 |
| 2 h | 157 ± 8 | 157 ± 8 | 162 ± 7 | 157 ± 9 | 159 ± 5 | |
| 1 w | 157 ± 7 | 157 ± 7 | 159 ± 7 | 154 ± 3 | 164 ± 3 | |
| TG (mg/dL) | 0 | 71 ± 14 | 89 ± 17 | 80 ± 11 | 92 ± 17 | 86 ± 18 |
| 2 h | 67 ± 13 | 81 ± 12 | 74 ± 11 | 78 ± 15 | 89 ± 14 | |
| 1 w | 71 ± 15 | 84 ± 10 | 76 ± 11 | 84 ± 16 | 84 ± 17 | |
| HDL-C (mg/dL) | 0 | 53 ± 4 | 51 ± 5 | 54 ± 3 | 49 ± 4 | 51 ± 4 |
| 2 h | 53 ± 4 | 52 ± 4 | 55 ± 3 | 51 ± 4 | 52 ± 3 | |
| 1 w | 52 ± 4 | 51 ± 4 | 53 ± 4 | 50 ± 3 | 55 ± 4 | |
| Glucose (mg/dL) | 0 | 80 ± 2 | 78 ± 4 | 87 ± 4 | 86 ± 4 | 85 ± 3 |
| 2 h | 77 ± 2 | 76 ± 3 | 78 ± 2 | 75 ± 4 | 77 ± 2 | |
| 1 w | 81 ± 2 | 81 ± 3 | 83 ± 4 | 85 ± 2 | 84 ± 3 | |
| Sodium/nmol/L | 0 | 145.0 ± 0.5 | 145.0 ± 1.0 | 144.0 ± 1.2 | 146.0 ± 0.6 | 144.0 ± 0.7 |
| 2 h | 144.0 ± 0.4 | 144.0 ± 1.6 | 144.0 ± 1.1 | 145.0 ± 0.8 | 144.0 ± 0.7 | |
| 1 w | 145.0 ± 1.2 | 144.0 ± 0.9 | 145.0 ± 1.0 | 145.0 ± 0.5 | 144.0 ± 1.4 | |
| Potassium/nmol/L | 0 | 4.50 ± 0.10 | 4.80 ± 0.24 | 4.30 ± 0.09 | 4.40 ± 0.10 | 4.50 ± 0.11 |
| 2 h | 4.60 ± 0.10 | 4.60 ± 0.13 | 4.60 ± 0.02 | 4.60 ± 0.08 | 4.60 ± 0.14 | |
| 1 w | 4.50 ± 0.07 | 4.50 ± 0.12 | 4.60 ± 0.12 | 4.40 ± 0.07 | 4.40 ± 0.04 | |
b. The effects of beverage consumption by healthy subjects on serum oxidative stress
Measurement of TBARS levels in the serum samples (basal oxidative status) before and after 2 h or 1 week of beverage consumption, revealed that all of the five beverages used, did not significantly impact serum basal oxidative stress, which is already low in healthy subjects (Table 4). However, consumption of the five selected beverages increased serum antioxidant status, as measured by the serum concentrations of sulfhydryl (-SH) groups (Fig. 2). Two hours after consumption of 100% Wonderful-variety pomegranate juice, the concentration of serum SH groups significantly increased by 6% (Fig. 2A). After 1 week of consumption, 100% black currant juice or 100% Wonderful-variety pomegranate juice, significantly increased the concentration of serum SH groups by 11% or 8%, respectively (Fig. 2B). In contrast, consumption of all other beverages for 2 h or for one week had no statistically significant effect on the concentration of serum SH groups (Fig. 2B).| Beverage | TBARS/nmol/mL before beverage consumption | TBARS/nmol/mL 2 h after beverage consumption | TBARS/nmol/mL after 1 week of beverage consumption |
|---|---|---|---|
| Acai juice blend (Naked) | 1.50 ± 0.16 | 1.60 ± 0.18 | 1.60 ± 0.07 |
| 100% Concord grape juice (Lakewood) | 1.60 ± 0.07 | 1.70 ± 0.11 | 1.50 ± 0.09 |
| 100% Black currant juice (Knudsen) | 1.60 ± 0.09 | 1.70 ± 0.11 | 1.60 ± 0.12 |
| 100% Wonderful-variety pomegranate juice (POM Wonderful) | 1.50 ± 0.18 | 1.50 ± 0.12 | 1.50 ± 0.08 |
| Red wine (Merlot/Mondavi) | 1.60 ± 0.07 | 1.60 ± 0.08 | 1.60 ± 0.11 |
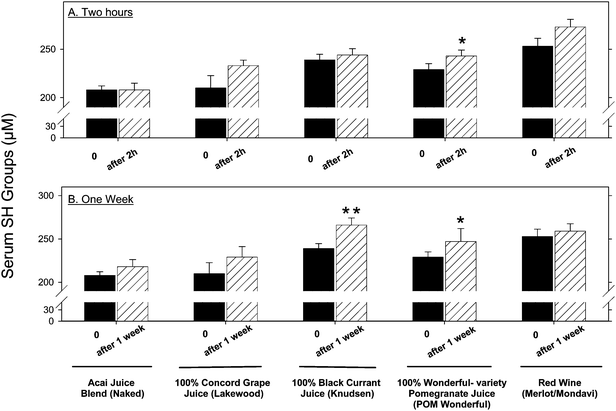 | ||
| Fig. 2 The effect of polyphenol rich beverages consumed by healthy subjects on the concentration of their serum SH groups. Six healthy subjects consumed POM Wonderful, black currant (Knudsen), red wine (Mondavi), grape (Lakewood), and acai (Naked). Blood samples were collected before and after 2 h or 1 week of beverage consumption. Serum SH group concentrations were determined as described under the methods section. (A) 2 h after consumption of beverages. (B) 1 week after of consumption of beverages. Results are expressed as mean ± SEM. *p <0.05 vs. 0 time, **p <0.01 vs. 0 time. | ||
c. The effects of consumption of beverages by healthy subjects on serum paraoxonase 1 (PON1) activities
PON1 has been shown to be inactivated under oxidative stress.33 Since nutritional antioxidants increase its expression and activities,17,33 we next analyzed the effects of the selected beverages (2 h or 1 week consumption) on serum PON1 catalytic activities (Fig. 3). Two hours after consumption of the selected beverages, serum PON1 lactonase activity was not significantly affected (Fig. 3A). After 1 week of consumption however, 100% black currant juice significantly increased serum PON1 lactonase activities by 20%, and 100% Wonderful-variety pomegranate juice increased it by 5% (Fig. 3B). The other beverages had no significant impact on PON1 lactonase activity after one week of consumption (Fig. 3B). Similar results were also found for serum PON1 arylesterase activity (data not shown).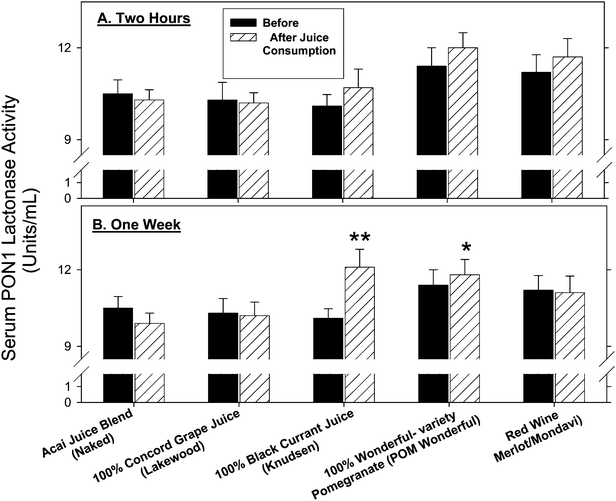 | ||
| Fig. 3 The effects of consumption of polyphenol-rich beverages by healthy subjects on their serum paraoxonase 1 (PON1) catalytic activity. Six healthy subjects consumed POM Wonderful, black currant (Knudsen), red wine (Mondavi), grape (Lakewood), and acai (Naked). Blood samples were collected before and after 2 h or 1 week of beverage consumption. Serum PON1 lactonase activity was determined. (A) 2 h after beverage consumption. (B) 1 week after beverage consumption. Results are expressed as mean ± SEM. *p <0.05 vs. 0 time, **p <0.01 vs. 0 time. | ||
d. The effects of beverage consumption by healthy subjects on serum-induced changes in J774A.1 macrophage cholesterol content
Serum lipoproteins can be taken up by macrophages, leading to cellular accumulation of cholesterol.2,34 Under oxidative stress, lipoproteins undergo oxidation and the oxidized lipoproteins can be taken up by macrophages at an enhanced rate, further contributing to cellular cholesterol accumulation.2 As 100% Wonderful-variety pomegranate and 100% black currant juices were the most potent antioxidants among all the beverages studied, we further analyzed them for their effects on serum-induced macrophage cholesterol accumulation. Serum samples collected 2 h after juice consumption did not significantly affect cellular cholesterol content, but consumption of these juices for 1 week resulted in a significant decrement (8%) in macrophage cholesterol content compared to baseline (Fig. 4A).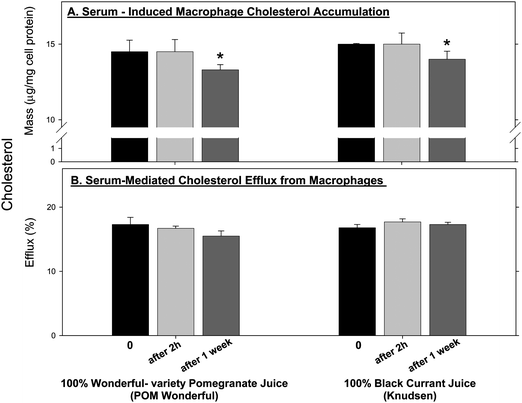 | ||
| Fig. 4 The effect of pomegranate juice and black currant juice consumption by healthy subjects on serum-induced cholesterol accumulation in J774A.1 macrophages. J774A.1 macrophages were incubated with 20 μL/mL of the above serum samples for 20 h at 37 °C. (A) The cellular total cholesterol content was then determined in the cells' lipid extract. (B) The extent of serum-mediated cholesterol efflux from the cells was determined as described in the Methods section. Results are expressed as mean ± SEM. *p <0.05 vs. 0 time. | ||
Finally, we questioned whether the above serum-induced decrement in macrophage cholesterol content could be associated with increased serum-mediated cholesterol efflux from cell cultured macrophages. As shown in Fig. 4B, consumption of 100% Wonderful-variety pomegranate and 100% black currant juices for 2 h or for 1 week, had no significant effect on the extent of the human serum-induced cholesterol efflux rate from the cells, compared to serum obtained at 0 time (before juice consumption).
Discussion
In the present study we have demonstrated, for the first time, that 100% Wonderful-variety pomegranate juice or 100% black currant juice, and to a lesser extent 100% Concord grape juice, or red wine, but not acai juice blend, consumed by healthy subjects in the short term (2 h, or daily consumption for 1 week) improved serum anti-atherogenic properties, i.e. increment in serum antioxidative status (SH groups, PON1 activity), and decrement in serum-induced macrophage cholesterol accumulation.Among the 35 beverages analyzed in vitro, 100% Wonderful-variety pomegranate juice and 100% black currant juice were the most potent antioxidants (free radical scavenging capacity, and inhibition of copper ion-induced LDL oxidation). 100% Black currant juice, which contains the highest polyphenol concentration among all the beverages studied, inhibited LDL oxidation to the same degree as 100% pomegranate juice, but its free radical scavenging capacity was lower than that of 100% pomegranate juice, suggesting that pomegranate polyphenols are more potent antioxidants than black currant juice polyphenols. However, we cannot exclude the effects of other potent antioxidants in these beverages. Red wine and 100% Concord grape juice were both less potent than 100% pomegranate juice and 100% black currant juice, while acai juice blend was the weakest antioxidant among those studied in vivo. A previous in vitro comparison of the antioxidant potency of commonly consumed polyphenol-rich beverages in the United States, demonstrated the following order of antioxidant potency: 100% Wonderful-variety pomegranate juice > red wine > 100% Concord grape juice > 100% blueberry juice > 100% black cherry juice, acai juice blend, 100% cranberry juice > 100% orange juice, iced tea beverages, and 100% apple juice.3 This pattern is similar to that shown in the present study, where additional beverages and brands were tested, including 100% black currant juice. In comparison to Knudsen Black Currant juice, Currant C (another black currant juice blend) contains only one-half of the total polyphenol concentration, and demonstrated much lower antioxidative properties than the Knudsen 100% black currant juice.
We chose for the in vivo study, the five juices which contained the highest polyphenol concentration: 100% black currant (Knudsen), 100% Wonderful-variety pomegranate (POM Wonderful), red wine (Merlot/Mondavi), acai juice blend (Naked) and 100% Concord grape (Knudsen). The present study is the first to compare the acute effects of the above beverages on serum anti-atherogenic properties. We chose 1 week of beverage consumption since we have previously observed a significant reduction in serum oxidative stress after two weeks of 100% Wonderful-variety pomegranate juice or red wine consumption by healthy subjects.14,10 Furthermore, in the current study we evaluated the acute effects 2 h after beverage consumption. Polyphenols in the above beverages were probably absorbed to some extent, as the levels of urinary quercetin,19 or anthocyanins,20,35 or those of plasma catechin,18 were all shown to be significantly increased dose- and time-dependently after acai juice blend, 100% black currant juice or red wine consumption.
The bioavailability of the pomegranate active components and metabolites has been demonstrated previously, with ellagic acid detected in the blood and dimethylellagic acid glucuronide (DMEAG), as well as urolithin A and B, found in the urine of most subjects after consumption of 100% pomegranate juice.36–39
An important issue in the current study is that consumption of the beverages (which contain sugars) by healthy subjects for 2 h or for 1 week, did not increase serum glucose levels and did not affect serum lipids or electrolytes (potassium and sodium).
Serum PON1 activities (arylesterase and lactonase) were analyzed as an additional marker for oxidative stress. In vitro all the five beverages used significantly increased serum PON1 activities. The mechanism responsible for the PON1 activity increase could be related to the binding of the beverages’ polyphenols to PON1. Such binding may change the enzyme conformation, thus affecting PON1 active site interactions with its substrates. Furthermore, certain polyphenols increase PON1 binding to the HDL, thus stabilizing PON1, as was shown previously upon incubation of serum in vitro with pomegranate juice or its most potent unique polyphenol punicalagin.15,16 Furthermore, 100% Wonderful-variety pomegranate juice consumption over longer periods of time (weeks, months, or years) has been shown to increase PON1 binding to the HDL particles, and as a result, stabilize PON1 and enhance its catalytic and biological activities.12,13,15,16 We evaluated also serum sulfhydryl (SH) group concentrations, as they have been shown to be inversely related to oxidative stress, and positively correlated with PON1 activity.40 Although all five selected polyphenol-rich juices inhibited AAPH-induced serum lipid peroxidation in vitro, in vivo their consumption for a short period of time (2 h or 1 week) did not significantly affect AAPH-induced serum lipid peroxidation. Serum SH group concentration and PON1 activity however, were modestly, but significantly increased following beverage consumption, with 100% Wonderful-variety pomegranate and 100% black currant juices being the most potent beverages after 1 week of consumption. Nevertheless, this protection by SH groups and PON1 may be insufficient to protect the serum from free radical-induced oxidative stress. PON1 was shown to protect serum from oxidative stress, by its ability to hydrolyze specific oxidized lipids in lipoproteins (such as specific oxidized phospholipids, lipid peroxides, cholesteryl linoleate hydroperoxides).41–43 Longer periods of beverage consumption (up to months) may be needed to achieve more impressive effects on serum oxidative stress in healthy subjects. Among the in vivo studied beverages, acai juice blend was the least potent, even though it has a similar concentration of polyphenols. This suggests that acai juice blend polyphenols are weak antioxidants, in comparison to the polyphenols in 100% pomegranate juice, 100% black currant juice or 100% Concord grape juice (and also red wine). Similar to the present study, plasma antioxidant capacity increased twelve hours after consumption of acai juice blend,20 or 2 h post-consumption of a mixture of berries, by healthy subjects.21 In a recent study, 100% black currant juice consumption for 1 week, substantially decreased serum lipid peroxidation,19 but this impressive effect is likely related to the high volume of juice consumed (1500 mL).
As 100% Wonderful-variety pomegranate juice and 100% black currant juice were the most potent antioxidants in the in vitro and in the in vivo studies, we chose them to assess an important feature of atherogenesis, i.e., macrophage cholesterol accumulation and foam cell formation, the hallmark of early atherogenesis.1,2 Consumption of 100% Wonderful-variety pomegranate juice or 100% black currant juice for 1 week, modestly, but significantly, decreased serum-induced cholesterol accumulation in macrophages. The reduction in macrophage cholesterol mass, however, was not the result of increased cholesterol efflux from the cells. In fact, it is probably the result of inhibition of cholesterol-rich lipoprotein uptake by the cells, mediated by serum associated polyphenols and/or polyphenol metabolites.44,45
In conclusion, we showed in the present study that 100% Wonderful-variety pomegranate juice and 100% black currant juice have potent antioxidant properties, both in vitro and in vivo.
These findings were observed even after short periods of daily juice consumption (two hours, or for one week), though the antioxidant and anti-atherogenic effects were modest, in comparison to previous studies conducted over longer periods of juice consumption.12–15
Acknowledgements
The financial support for this research was obtained from “POM Wonderful” Ltd., Los Angeles, California, USA. All authors declare that they have no conflict of interest.References
- A. J. Lusis, Atherosclerosis, Nature, 2000, 407, 233–241 CrossRef CAS.
- M. Aviram, M. Rosenblat, Oxidative stress in cardiovascular disease: role of oxidized lipoproteins in macrophage foam cell formation and atherosclerosis, in Redox-Genome Interactions in Health and Disease, Dekker, New York, 2003, ch. 25, pp. 557–590 Search PubMed.
- N. P. Seeram, M. Aviram, Y. Zhang, S. M. Henning, L. Feng, M. Dreher and D. Heber, Comparison of antioxidant potency of commonly consumed polyphenols-rich beverages in the United States, J. Agric. Food Chem., 2008, 56, 1415–1422 CrossRef CAS.
- T. L. Zerm and M. L. Fernandez, Cardioprotective effects of dietary polyphenols, J. Nutr., 2005, 135, 2291–2294.
- W. R. Leifert and M. Y. Abeywardena, Cardioprotective actions of grape polyphenols, Nutr. Res., 2008, 28, 729–737 CrossRef CAS.
- A. A. Bertelli and D. K. Das, Grapes, Wines, Resveratrol and Heart Health, J. Cardiovasc. Pharmacol., 2009, 54, 468–476 CrossRef CAS.
- A. Basu and K. Penugonda, Pomegranate juice: a heart-healthy fruit juice, Nutr. Rev., 2009, 67, 49–56 Search PubMed.
- Y. K. Park, S. H. Lee, E. Park, J. S. Kim and M. H. Kang, Changes in antioxidant status, blood pressure, and lymphocyte DNA damage from grape juice supplementation, Ann. N. Y. Acad. Sci., 2009, 1171, 385–390 CrossRef CAS.
- P. Castilla, R. Echarri, A. Dávalos, F. Cerrato, H. Ortega, J. L. Teruel, M. F. Lucas, D. Gómez-Coronado, J. Ortuño and M. A. Lasunción, Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects, Am. J. Clin. Nutr., 2006, 84, 252–262 CAS.
- B. Fuhrman, A. Lavy and M. Aviram, Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation, Am. J. Clin. Nutr., 1995, 61, 549–554 CAS.
- A. Imhof, R. Blagieva, N. Marx and W. Koenig, Drinking modulates monocyte migration in healthy subjects: a randomized intervention study of water, ethanol, red wine and beer with or without alcohol, Diabetes and Vascular Disease Research, 2008, 5, 48–53 Search PubMed.
- M. Aviram, M. Rosenblat, D. Gaitini, S. Nitecki, A. Hoffman, L. Dornfeld, N. Volkova, D. Presser, J. Attias, H. Liker and T. Hayek, Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation, Clin. Nutr., 2004, 23, 423–433 CrossRef CAS.
- M. Rosenblat, T. Hayek and M. Aviram, Antioxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages, Atherosclerosis, 2006, 187, 363–371 CrossRef CAS.
- M. Aviram, L. Dornfeld, M. Rosenblat, N. Volkova, M. Kaplan, R. Coleman, T. Hayek, D. Presser and B. Fuhrman, Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice, Am. J. Clin. Nutr., 2000, 71, 1062–1076 CAS.
- W. Rock, M. Rosenblat, R. Miller-Lotan, A. P. Levy, M. Elias and M. Aviram, Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities, J. Agric. Food Chem., 2008, 56, 8704–8713 CrossRef CAS.
- B. Fuhrman, N. Volkova and M. Aviram, Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: Studies in vitro and in diabetic patients, Nutrition, 2010, 26, 359–366 CrossRef CAS.
- J. Khateeb, A. Gantman, A. J. Kreitenberg, M. Aviram and B. Fuhrman, Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-gamma pathway, Atherosclerosis, 2010, 208, 119–125 CrossRef CAS.
- D. Modun, I. Music, J. Vukovic, I. Brizic, V. Katalinic, A. Obad, I. Palada, Z. Dujic and M. Boban, The increase in human plasma antioxidant capacity after red wine consumption is due to both plasma urate and wine polyphenols, Atherosclerosis, 2008, 197, 250–256 CrossRef CAS.
- J. F. Young, S. E. Nielsen, J. Haraldsdóttir, B. Daneshvar, S. T. Lauridsen, P. Knuthsen, A. Crozier, B. Sandström and L. O. Dragsted, Effect of fruit juice intake on urinary quercetin excretion and biomarkers of antioxidative status, Am. J. Clin. Nutr., 1999, 69, 87–94 CAS.
- S. U. Mertens-Talcott, J. Rios, P. Jilma-Stohlawetz, L. A. Pacheco-Palencia, B. Meibohm, S. T. Talcott and H. Derendorf, Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers, J. Agric. Food Chem., 2008, 56, 7796–7802 CrossRef CAS.
- G. S. Jensen, X. Wu, K. M. Patterson, J. Barnes, S. G. Carter, L. Scherwitz, R. Beaman, J. R. Endres and A. G. Schauss, In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study, J. Agric. Food Chem., 2008, 56, 8326–8333 CrossRef CAS.
- V. L. Singleton and J. A. Rossi, Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents, Am J Enol Viti, 1965, 16, 144–58 Search PubMed.
- M. S. Blois, Antioxidant determination by the use of a stable free radical, Nature, 1958, 181, 1199–1200 CAS.
- M. Aviram, Plasma lipoprotein separation by discontinuous density gradient ultracentrifugation in hyperlipoproteinemic patients, Biochem. Med., 1983, 30, 111–118 CrossRef CAS.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, Protein measurement with the folin phenol reagent, J. Biol. Chem., 1951, 193, 265–275 CAS.
- J. A. Buege and S. D. Aust, Microsomal lipid peroxidation, Methods Enzymol., 1978, 52, 302–310 Search PubMed.
- B. Frei, R. Stocker and B. N. Ames, Antioxidant defenses and lipid peroxidation in human blood plasma, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 9748–9752 CrossRef CAS.
- M.-L. Hu, C. J. Dillard and A. L. Tappel, In vivo effects of aurothioglucose and sodium thioglucose on rat tissue sulfhydryl levels and plasma sulfhydryl reactivity, Agents Actions, 1988, 25, 132–138 Search PubMed.
- L. Gaidukov and D. S. Tawfik, The development of human sera tests for HDL-bound serum PON1 and its lipolactonase activity, J. Lipid Res., 2007, 48, 1637–1646 CAS.
- X. Gu, K. Kozarsky and M. Krieger, Scavenger receptor class B, type I-mediated [3H] cholesterol efflux to high and low density lipoproteins is dependent on lipoprotein binding to the receptor, J. Biol. Chem., 2000, 275, 29993–30001 CrossRef CAS.
- L. Gaidukov and D. S. Tawfik, High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I, Biochemistry, 2005, 44, 11843–11854 CrossRef CAS.
- M. Rosenblat and M. Aviram, Paraoxonases role in the prevention of cardiovascular diseases, BioFactors, 2009, 35, 98–104 Search PubMed.
- M. Aviram, M. Rosenblat, S. Billecke, J. Erogul, R. Sorenson, C. L. Bisgaier, R. S. Newton and B. N. La Du, Human serum paraoxonase (PON1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants, Free Radical Biol. Med., 1999, 26, 892–904 CrossRef CAS.
- A. M. Palmer, E. Nova, E. Anil, K. Jackson, P. Bateman, E. Wolstencroft, C. M. Williams and P. Yaqoob, Differential uptake of subfractions of triglyceride-rich lipoproteins by THP-1 macrophages, Atherosclerosis, 2005, 180, 233–244 CrossRef CAS.
- I. L. Nielsen, L. O. Dragsted, G. Ravn-Haren, R. Freese and S. E. Rasmussen, Absorption and excretion of black currant anthocyanins in humans and watanabe heritable hyperlipidemic rabbits, J. Agric. Food Chem., 2003, 51, 2813–2820 CrossRef CAS.
- N. P. Seeram, Y. Zhang, R. McKeever, S. M. Henning, R. P. Lee, M. A. Suchard, Z. Li, S. Chen, G. Thames, A. Zerlin, M. Nguyen, D. Wang, M. Dreher and D. Heber, Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects, J. Med. Food, 2008, 11, 390–394 CrossRef CAS.
- N. P. Seeram, S. M. Henning, Y. Zhang, M. Suchard, Z. Li and D. Heber, Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 h, J. Nutr., 2006, 136, 2481–2485 CAS.
- S. G. Kasimsetty, D. Bialonska, M. K. Reddy, G. Ma, S. I. Khan and D. Ferreira, Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins, J. Agric. Food Chem., 2010, 58, 2180–2187 CrossRef CAS.
- D. Bialonska, S. G. Kasimsetty, S. I. Khan and D. Ferreira, Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay, J. Agric. Food Chem., 2009, 57, 10181–10186 CrossRef CAS.
- U. Çakatay, R. Kayali and H. Uzun, Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population, Clin. Exp. Med., 2008, 8, 51–57 CrossRef CAS.
- Z. Ahmed, A. Ravandi, G. F. Maguire, A. Emili, D. Draganov, B. N. La Du, A. Kuksis and P. W. Connelly, Apolipoprotein A-I promotes the formation of phosphatidylcholine core aldehydes that are hydrolyzed by paraoxonase (PON-1) during high density lipoprotein oxidation with a peroxynitrite donor, J. Biol. Chem., 2001, 276, 24473–24481 CrossRef CAS.
- M. I. Mackness, S. Arrol and P. N. Durrington, Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein, FEBS Lett., 1991, 286, 152–154 CrossRef CAS.
- M. Aviram, M. Rosenblat, C. L. Bisgaier, R. S. Newton, S. L. Primo-Parmo and B. N. La Du, Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase, J. Clin. Invest., 1998, 101, 1581–1590 CrossRef CAS.
- B. Fuhrman, N. Volkova, R. Coleman and M. Aviram, Grape powder polyphenols attenuate atherosclerosis development in apolipoprotein E deficient (E0) mice and reduce macrophage atherogenicity, J. Nutr., 2005, 135, 722–728 CAS.
- B. Fuhrman, N. Volkova and M. Aviram, Pomegranate juice inhibits oxidized LDL uptake and cholesterol biosynthesis in macrophages, J. Nutr. Biochem., 2005, 16, 570–576 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
