Sorption of micropollutant estrone to a water treatment ion exchange resin†
Peta A.
Neale
a,
Maibritt
Mastrup
b,
Thomas
Borgmann
b and
Andrea I.
Schäfer
*a
aSchool of Engineering, The University of Edinburgh, Edinburgh, UK EH9 3JL. E-mail: Andrea.Schaefer@ed.ac.uk; Fax: +44 (0)131 650 6781; Tel: +44 (0)131 650 7209
bIngeniørhøjskolen Odense Teknikum, Niels Bohrs Alle 1, 5230, Odense M, Denmark
First published on 29th October 2009
Abstract
Micropollutants occur in natural waters from a range of sources. Estrogenic compounds are naturally excreted by humans and hence stem predominantly from wastewater effluents. Due to their small molecular weight and concentration their effective control is a challenge. In this study magnetic ion exchange (MIEX®), which was developed to remove natural organic matter (NOM) from surface water, was investigated for such a micropollutant, estrone. The interaction of estrone with the resin occurs as a side effect when NOM is removed. This interaction results in some degree of removal. However, the accumulation of those hazardous materials on the resin, which can be associated with accidental release, as well as the concentration in the regeneration brine of the process, is environmentally more significant. For this reason a thorough investigation of interaction phenomena was undertaken. Estrone and polymeric materials (such as ion exchange resins or membranes) interact through a number of mechanisms including specific and non-specific interactions. Sorption and desorption of estrone were studied as a function of pH, temperature, natural organic matter concentration, sulfate concentration and ionic strength to elucidate possible mechanisms. The results demonstrated that the resin removed around 70% estrone at high pH conditions (>10.4) when estrone was predominantly negatively charged. However, below pH 10.4, when estrone was neutral, approximately 40% of estrone still sorbed due to hydrogen bonding. The optimum temperature for estrone sorption was observed to be in the 15 to 35 °C range, while the presence of other anions, including natural organic matter reduced estrone removal due to competition for anion exchange sites. Desorption of estrone was most effective with 2 M NaCl regeneration brine concentration when estrone was negatively charged (98% desorption). However, when estrone was neutral there was no significant difference between 1 M and 2 M NaCl. The results presented in this study indicate that polar non-ionic micropollutants were removed by magnetic ion exchange resin due to sorption to the resin polymer. This has implications for treatment, however, the accumulation of micropollutants on polymeric materials in water treatment as well as the abundance of such micropollutants in the regeneration brine are risks that warrant monitoring.
Environmental impactThe variable removal of micropollutants by conventional water treatment processes has led to increased interest in new removal technologies. In this study the interaction of an ion exchange resin with the steroidal hormone estrone in both charged and uncharged states was investigated. There are two important implications of this work, namely removal capacity of the technology and secondly accumulation potential and resulting risk of accidental release. Estrone removal was 40–70%, with pH, temperature and presence of competing anions influencing removal. Removal of estrone can be improved by coupling the resin with membrane filtration. Micropollutant desorption from the resin is significant, which has environmental consequences for both brine and resin disposal. |
Introduction
Water treatment processes are increasingly designed to remove dissolved contaminants and micropollutants that reach water supplies from various sources. Natural organic matter (NOM), for example, poses a significant problem in water supplies as disinfection processes transforms these natural compounds into hazardous disinfection by-products. In consequence, a strong base anion exchange resin (AER) that can remove such NOM (weak acidic ions predominantly) has been developed. An example of an AER is magnetic ion exchange resin (MIEX®) which is a polyacrylate macroporous polymer with strong base quaternary ammonium functional groups for ion exhange1 and a magnetic iron oxide core to assist with resin agglomeration and recovery.2 This resin can also be used to remove other contaminants, such as bromide, sulfate, nitrate, arsenic and chromium,3,4 and the process can further be used to control fouling in membrane filtration, where NOM plays an important role. The process can be retrofitted to existing water treatment plants that were not designed to remove NOM and may be environmentally and energetically beneficial over more advanced treatment.Micropollutants, such as natural and synthetic hormones, pesticides and pharmaceutically active products, are ubiquitous in the aquatic environment, and can be considered a cause of concern for human and environmental health.5 Estrone is a naturally excreted steroidal hormone, which can also be formed through the oxidation of estradiol.6 Natural hormones such as estrone have the potential to disrupt the endocrine system and consequently have implications for fertility and reproductive health.7,8 Studies have shown that low concentrations of estrone (3 ng L−1) can cause behavioural changes in some aquatic organisms.9 Removal of estrone by conventional sewage treatment plants is variable. A study by Carballa et al.10 found higher concentrations of estrone in wastewater effluent compared to the influent concentration due to estradiol degradation, while other studies have observed average removals of 53–61%.11–13 As a result, estrone is regularly detected in surface waters and sewage effluents at low ng L−1 concentrations.14,15
As conventional treatment cannot completely remove micropollutants such as estrone, other removal technologies have been explored including ozonation, advanced oxidation processes and membrane filtration, particularly nanofiltration/reverse osmosis (NF/RO). Studies have demonstrated that estrone can be removed to below detectable limits using ozonation16–18 and ClO2 oxidation,19 while Nghiem et al.20 found estrone retention by NF/RO membranes varied from 13 to >90% due to differences in membrane properties. While very few studies exist on the removal of hormones by ion exchange (IX) resins, Zhang and Zhou21 found moderate removal of estrone from solution using a styrene IX resin, with 71 h required to reach equilibrium. Environmental effects like temperature or water quality such as pH have not been considered.
Micropollutant removal mechanisms of IX resins other than ion exchange of charged molecules are to date not well understood. Humbert et al.4 studied the removal of pesticides atrazine and isoproturon using a range of strong AERs. The study showed pesticide removal ranged from 5–37% for all AERs, however, MIEX® could only remove 5–7% of pesticides.4 The low removal was attributed to the non-ionic nature of the pesticides in the experimental conditions. Despite this, specific and non-specific interactions, such as hydrophobically assisted ion exchange and hydrogen bonding, can occur between neutral acidic species such as phenolic groups (at pH 7) and the polymer.22,23 The porous nature of the resin can assist with the removal of neutrally charged contaminants.24 It is also likely that non-ionic micropollutants can interact with the resin polymer through hydrogen bonding,25 increasing potential non-ionic micropollutant uptake. Consequently, micropollutant removal is related to chemical structure. This was also indicated by Choi et al.26 who found the removal of sulfonamides ranged from 15 to 90% depending on their structure. While micropollutant removal is generally a desired phenomenon in water treatment, it can also occur as a side effect with accumulation of micropollutants on the resin and concentration in the brine.
The resin is regenerated using a NaCl brine which exchanges any anions sorbed to the quaternary ammonium functional group for chloride ions.27 Caustic compounds such as NaOH can be added to the NaCl brine to enhance desorption. It is to date not understood if regeneration only reverses micropollutants removed by ion exchange or also other sorption processes.
The purpose of the study is to investigate the removal as well as partitioning mechanisms of estrone and to assess the influence of water chemistry parameters such as pH and ionic strength. As estrone is predominately negatively charged at pH > 10.4, studying the influence of pH on estrone removal can improve understanding of both ion exchange and specific and non-specific interaction mechanisms. Temperature effects as well as estrone removal in the presence of competing ions, such as NOM and sulfate, will be studied to understand the influence of competition. Finally, the release of estrone from the resin during regeneration will be investigated in order to understand any associated treatment liabilities and risks concerning accidental contamination of water as well as the potential presence of micropollutants in brines following their accumulation on the resin.
Experimental
Chemicals
All chemicals used are of analytical grade. Radiolabelled [2,4,6,7-3H]estrone was purchased from Sigma Aldrich (Saint Louis, US). Estrone is a bipolar hormone, with an acid dissociation constant (pKa) of 10.4.28 The background electrolyte was 1 mM NaHCO3. The pH was adjusted using 1 M HCl and 1 M NaOH. Natural organic matter (NOM), from Brisbane Water National Park in Australia, was concentrated using microfiltration and RO, and was extensively characterised by Schäfer.29Magnetic ion exchange resin (MIEX®)
The resin was supplied by Orica Watercare (Melbourne, Australia). The concentration of resin used in the experiment was 10 mL L−1. Industrial applications typically use doses ranging from 2 to 20 mL L−1,30 however, previous studies have indicated that a dose of 8–10 mL L−1 is optimal for NOM and inorganic anion removal.4,27Sorption experiments
The sorption experiments were conducted in 100 mL Erlenmeyer flasks containing 50 mL of MilliQ water with 1 mM NaHCO3 background electrolyte. The initial estrone concentrations were 1, 5, 10, 50, 100 and 500 ng L−1, which are in the range of typical surface and wastewaters. Erlenmeyer flasks were placed in a temperature controlled Bioline incubator shaker (Alexandria, Australia). Before each experiment 1 mL from each flask was removed for analysis, and 0.5 mL (0.113 g) of resin slurry was added to each flask using 5 mL single use syringes, giving a total concentration of 10 mL L−1. Flasks were shaken for 2 h at 200 rpm, and a sample of 1 mL was collected. Adsorption kinetics reached equilibrium within 25 min. In the pH sorption experiments the pH was adjusted to 3, 5, 8, 9, 10, 11 and 12 using 1 M HCl and NaOH and the temperature was kept constant at 25 °C. In the temperature experiments the temperature was adjusted to 15, 25, 35, 45 and 55 °C at pH 8. The NOM, ionic strength and sulfate experiments were studied at pH 8 and 11 and a constant temperature of 25 °C. The NOM concentrations studied were 0, 10, 30 and 100 mg L−1 (0, 0.63, 1.89, 6.3 mg C L−1), while in the ionic strength sorption experiments NaCl concentrations of 0, 20, 50, 100 and 200 mM were studied. For the sulfate experiments the SO42− concentrations were 0, 5 and 20 mM.The desorption experiments were also conducted in 100 mL Erlenmeyer flasks following the 100 ng L−1 estrone sorption experiments at pH 7, 8, 9, 10 and 11. The resin was allowed to settle and the supernatant was decanted and 50 mL of MilliQ water were added. The effect of ionic strength using NaCl concentrations of 0, 1 and 2 M was studied. The pH was adjusted to 7, 8, 9, 10 and 11. 1 mL of initial solution was removed for analysis. The flasks were shaken in the incubator shaker at 200 rpm for 1 h and samples of 1 mL were collected at 2, 5, 10, 15, 30 and 60 min.
Analysis
Scintillation counting was used to detect estrone at very low concentrations. Samples from sorption and desorption experiments (1 mL) were added to 9 mL of Ultima Gold LLT scintillation cocktail (Perkin Elmer, Boston, US) and shaken until clear. The samples were taken after the resin had settled and analysed using a Packard Instruments Tri-Carb® liquid scintillation counter (2100 TR, Boston, US) where they were left to count for 5 min each. The activity in counts per minute (cpm) was converted to estrone concentration in ng L−1. The percentage of estrone removal by sorption is calculated using eqn (1), while the mass of estrone removed per mass of resin is calculated using eqn (2). The sorption isotherms are calculated as the mass of estrone removed per mass of resin as a function of the estrone equilibrium concentration. This represents the freely dissolved hormone concentration in solution at equilibrium. The percentage of estrone desorbed with the addition of NaCl brine can also be calculated using eqn (1).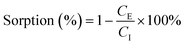 | (1) |
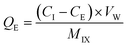 | (2) |
A field emission scanning electron microscope (Hitachi s-900 FESEM) at 20 kV was used to image the resin.
Results and discussion
Characteristics of MIEX®
Within the literature, the resin beads are suggested to have an average diameter of 150 to 180 µm, which is approximately 2 to 5 times smaller than other commonly used IX resins.31 As a result, it has a greater surface area to volume ratio compared to larger IX resins thus increasing micropollutant removal.32 However, the average diameter of the resin in Fig. 1 appears to be considerably less than what is suggested in the literature, with particles as small as 5 to 10 µm observed. Therefore, it is likely that the resin is susceptible to mechanical break-up. The charge of the resin is unlikely to be influenced by solution pH, as the quaternary ammonium functional groups give the resin a positive charge in both acidic and alkaline solutions. | ||
| Fig. 1 Field emission scanning electron microscope (FESEM) image of the magnetic ion exchange resin. | ||
Sorption
To understand the sorption mechanisms of charged and uncharged estrone the influence of pH is studied. pH can influence the speciation and charge of estrone as it has a pKa of 10.4. This has implications for the transport and behaviour of estrone within natural and engineered systems as charge can influence hormone removal.28 The removal of uncharged estrone from pH 3 to 10 is similar (Fig. 2a), with approximately 40% removal. At pH 11 and 12 when estrone is negatively charged, estrone removal increases substantially to 60–70%. Fig. 2b shows the mass of estrone sorbed per mass of resin as a function of pH, and this also indicates a significantly increased isotherm slope at pH 11 and 12.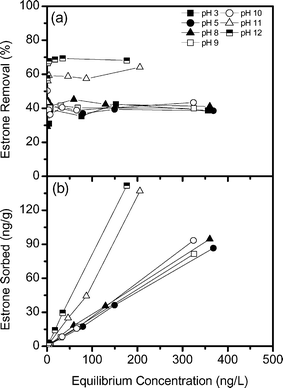 | ||
| Fig. 2 (a) Removal of estrone and (b) sorption isotherms for estrone as a function of pH (background electrolyte 1 mM NaHCO3). | ||
Due to the strong base quaternary ammonium functional groups, the resin has a strong pH tolerance,33 and hence observed changes in estrone removal can be attributed to the dissociation of estrone. Estrone is 99.5% protonated at pH 8 and is 82–97% dissociated at pH 11 and 12. As the dominant removal mechanism for the resin is ion exchange, estrone removal is greatest when estrone is predominantly negatively charged at pH > 10.4 (Fig. 3). However, at pH values lower than the pKa, moderate estrone removal was also observed (40%). The removal of non-ionic estrone can be explained with physical interactions such as hydrophobically assisted ion exchange and hydrogen bonding with the polyacrylate polymer. The mechanism of interaction is likely to be absorption, compared to adsorption, due to the resin material. The polyacrylate coating is similar to the coating of some fibres used for solid-phase microextraction (SPME), and studies have indicated that micropollutants are removed by such SPME fibres through absorption due to hydrogen bonding.34 Further, the linearity of the sorption isotherms in Fig. 2b suggests that absorption is indeed the dominant mechanism.35
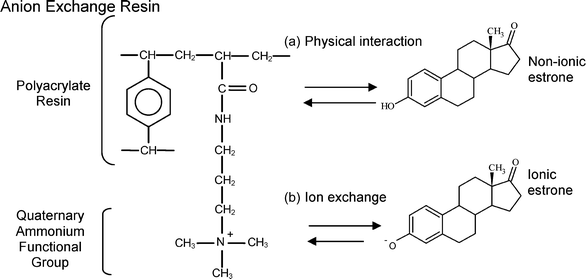 | ||
| Fig. 3 Primary mechanisms of estrone removal by anion exchange resin as (a) physical interactions and (b) ion exchange (adapted from Li and SenGupta46 and Hubicka and Kolodynska47). | ||
The significant removal of non-ionic estrone suggests that hydrogen bonding is the dominant physical sorption mechanism. Estrone contains a phenolic hydroxyl group which acts as a hydrogen donor and acceptor, hence it is bipolar, and a ketone group which is a strong hydrogen acceptor.36 The polyacrylate resin also contains polar functional groups such as ketone and ammonium moieties, and therefore hydrogen bonding is indeed possible. Previous studies have observed strong estrone sorption to membrane polymers and it is suggested that this is due to hydrogen bonding, as well as non-specific interactions.28,37 Schäfer et al.28 found estrone sorption to the membrane polymers significantly decreased as the pH exceeded the pKa and hydrogen bonding capacity decreased. In the case of the resin, the dissociation of estrone adds the potential of ion exchange, which is indeed observed.
Temperature is of interest as it may have implications for micropollutant removal kinetics. As estrone is non-ionic at pH 8, removal by the resin is due to physical sorption. The removal of estrone as a function of temperature is shown in Fig. 4a. The removal of estrone is similar from 15 to 35 °C, with average removals ranging from 36–40%. As the temperature increases to 45 °C the average estrone removal decreases to 31% and to 23% at 55 °C. The sorption isotherms (Fig. 4b) indicate a similar mass of estrone removed per mass of resin from 15 to 35 °C.
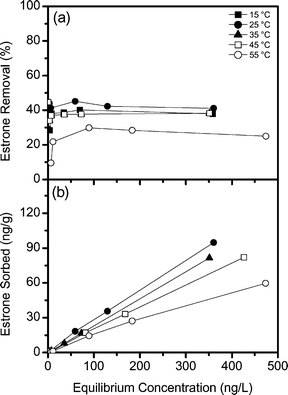 | ||
| Fig. 4 (a) Removal of estrone and (b) sorption isotherms for estrone as a function of temperature (background electrolyte 1 mM NaHCO3, pH 8). | ||
The effect of temperature on NOM removal by magnetic ion exchange resin has been studied previously. Humbert et al.4 studied NOM removal from 6 to 36 °C and found no difference in removal between 6 and 26 °C, however, removal increased as the temperature increased at 36 °C due to accelerated kinetics. In contrast, Semmens et al.38 compared NOM removal by the resin in summer (24 °C) and winter (6 °C) conditions and found a 12% reduction in NOM removal in colder conditions due to slower NOM uptake. Chen39 found that NOM removal was similar at 25 °C and 35 °C with a polyacrylic IX resin. The variations in the literature are due to different experimental conditions, such as equilibrium time and resin doses. However, at pH 8 estrone is not interacting with the resin through ion exchange, as NOM does, therefore it is difficult to compare these results or identify a coherent mechanism. Similar to this study, a decrease in micropollutant uptake by polyacrylate SPME fibres has previously been observed at high temperatures.40 As sorption to polyacrylate is diffusion controlled, the temperature accelerates removal kinetics.41 However, as the temperature increases to 45 and 55 °C the polyacrylate–water partition coefficient decreases,40 and this may lead to a reduction in estrone removal at high temperatures.
The sorption of estrone is also studied in the presence of varying NOM, ionic strength and sulfate concentrations. NOM and sulfate are selected as they may compete with estrone for ion exchange binding sites, while ionic strength has implications for charge shielding and hence can affect the ion exchange process. Experiments are conducted at pH 8 and 11 to study the influence of estrone dissociation on removal. In Fig. 5a the isotherm slope decreases as NOM concentration increases at pH 8, indicating a reduction in estrone removal with increasing NOM concentration. However, there appears to be no significant difference in sorption at pH 11 (Fig. 5b). This change in sorption is related to estrone charge. As estrone is neutrally charged at pH 8 it can only be removed through physical interactions, while the anionic NOM can be removed through ion exchange, which is a stronger interaction. Therefore, as more NOM is removed less estrone can be sorbed by the resin. It is likely this is due to the significantly higher NOM concentration (10–100 mg L−1 compared to 1–500 ng L−1) which can block estrone sorption sites. However, at pH 11 when estrone is negatively charged it can out-compete NOM for binding sites, therefore there is no significant difference in estrone sorption with increasing NOM concentration. Ion exchange to the macroporous IX resin is diffusion controlled,42 therefore estrone out-competes NOM due to the significantly lower molecular weight of estrone (270.4 g mol−1versus <1000 g mol−1) which results in a greater diffusion constant.
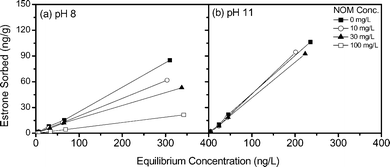 | ||
| Fig. 5 Sorption isotherms for estrone as a function of natural organic matter (NOM) concentration at (a) pH 8 and (b) pH 11 (background electrolyte 1 mM NaHCO3). | ||
The influence of ionic strength (NaCl) is studied as it may shield electrostatic interactions between estrone and the resin functional groups.43Fig. 6a shows for pH 8 a slight decrease in estrone sorption in the presence of 20 mM NaCl, however, there is no difference in isotherm slope as the ionic strength increases from 20 mM to 200 mM NaCl. For pH 11 a significant reduction in isotherm slope with increasing ionic strength is observed, indicating decreasing estrone sorption (Fig. 6b). At high ionic strength the charge of estrone and resin functional groups is shielded reducing the ion exchange capability, resulting in decreasing sorption as a function of ionic strength at pH 11. Below the pKa of estrone the high ionic strength did not influence the sorption, as ion exchange is not the removal mechanism.
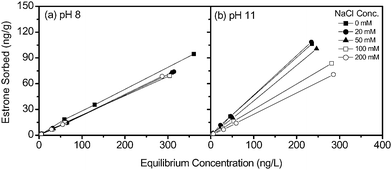 | ||
| Fig. 6 Sorption isotherms for estrone as a function of ionic strength (NaCl) at (a) pH 8 and (b) pH 11 (background electrolyte 1 mM NaHCO3). | ||
Sulfate is a multivalent anion (SO42−), and previous studies indicate that the presence of sulfate can reduce the removal of organic matter as it can compete for anion exchange sites.23,44 Consequently, the influence of estrone removal in the presence of sulfate is studied to determine if this influences estrone removal. Fig. 7a shows a reduction in isotherm slope in the presence of sulfate for pH 8, while in Fig. 7b little difference in estrone sorption at pH 11 is observed when ion exchange occurs. Similar to NOM, the concentration of sulfate is considerably higher (4–1850 × 10−9 mM of estrone versus 5–20 mM of sulfate) reducing estrone sorption sites at pH 8.
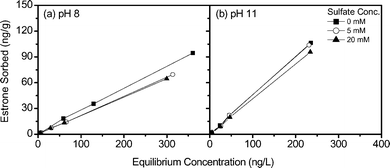 | ||
| Fig. 7 Sorption isotherms for estrone as a function of sulfate concentration at (a) pH 8 and (b) pH 11 (background electrolyte 1 mM NaHCO3). | ||
Desorption
IX resins are regenerated through reverse ion exchange, where contaminants are substituted for, in this case, chloride ions (Fig. 8).45 It is unknown if micropollutants, such as estrone, can be removed from the resin by regeneration, and for this reason the influence of ionic strength on estrone desorption as a function of pH is measured using MilliQ water (0 M), 1 M and 2 M NaCl (Fig. 9). Desorption is lowest in MilliQ water with 33–42% desorption, followed by 1 M NaCl with 59–67% estrone desorption and 58–98% at 2 mM. In both MilliQ water and 1 M NaCl the resin appears to demonstrate some degree of regeneration meaning that the contaminants are likely to desorb if the water quality varies, and that ion exchange is not the mechanism as there is no chlorine in MilliQ water. This indicates that pure water may be contaminated by micropollutants sorbed to the resin. At 2 M NaCl from pH 7 to 10 desorption is similar to 1 M NaCl with 58–62% desorption. At pH 11, when estrone is 82% negatively charged, there is 98% desorption of estrone, indicating that an ionic strength of 2 M is sufficient to regenerate the negatively charged estrone. No difference in desorption as a function of pH is observed for MilliQ water or 1 M NaCl, suggesting that regeneration is a NaCl concentration dependent process.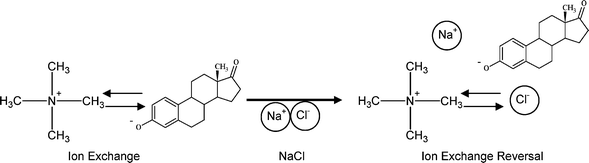 | ||
| Fig. 8 Resin regeneration mechanism using NaCl. | ||
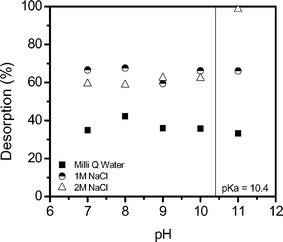 | ||
| Fig. 9 Desorption of estrone in MilliQ water, 1 M NaCl and 2 M NaCl as a function of pH (background electrolyte 1 mM NaHCO3). | ||
Conclusions
The interaction of micropollutants such as steroidal hormones with materials used in treatment of water and wastewater is of importance for both removal as well as environmental and health consequences. With regards to estrone removal, the performance of magnetic ion exchange resin (MIEX®) is influenced by the solution chemistry, temperature and the presence of competing anions. When estrone is negatively charged, the resin can remove as much as 70% of estrone through a combination of ion exchange and non-specific interactions. In this case up to 98% can be desorbed using NaCl brine. At neutral pH values removal is significantly less (40%), with desorption up to 67%. The interactions can be attributed in this case predominantly to hydrogen bonding. Results imply that the application of the resin combined with another water treatment technology, such as membrane filtration or advanced oxidation, may be a good and energy efficient option to ensure significant removal of charged micropollutants from water.An important implication of this study is that polymeric resins are likely to concentrate micropollutants resulting in an elevated concentration in the resin material and the brines from regeneration processes. The disposal and treatment of such brines require solid future investigation, as does the risk of accidental release of micropollutants if ionic strength or pH vary during treatment. Given that routine monitoring of the many micropollutants abundant in water is difficult, this issue presents a significant challenge to environmental monitoring.
Acknowledgements
Orica Watercare (Melbourne, Australia) are thanked for provision of MIEX® sample. Menachem Elimelech (Yale University) is thanked for a critical review of the manuscript during his time in Edinburgh as Royal Academy of Engineering Distinguished Visiting Fellow.References
- C. J. Johnson and P. C. Singer, Water Res., 2004, 38, 3738–3750 CrossRef CAS.
- T. H. Boyer and P. C. Singer, Water Res., 2005, 39, 1265–1276 CrossRef CAS.
- A. K. Jha, A. Bose and J. P. Downey, Sep. Sci. Technol., 2006, 41, 3297–3312 CrossRef CAS.
- H. Humbert, H. Gallard, H. Suty and J.-P. Croué, Water Res., 2005, 39, 1699–1708 CrossRef CAS.
- R. P. Schwarzenbach, B. I. Escher, K. Fenner, T. B. Hofstetter, C. A. Johnson, U. von Gunten and B. Wehrli, Science, 2006, 313, 1072–1077 CrossRef CAS.
- G. G. Ying, R. S. Kookana and Y.-J. Ru, Environ. Int., 2002, 28, 545–551 CrossRef CAS.
- S. Jobling, M. Nolan, C. R. Tyler, G. Brighty and J. P. Sumpter, Environ. Sci. Technol., 1998, 32, 2498–2506 CrossRef CAS.
- S. Jobling, S. Coey, J. G. Whitmore, D. E. Kime, K. J. W. Van Look, B. G. McAllister, N. Beresford, A. C. Henshaw, G. C. Brightly, C. R. Tyler and J. P. Sumpter, Biol. Reprod., 2002, 67, 515–524 Search PubMed.
- C. A. Murphy, N. E. Stacey and L. D. Corkum, J. Chem. Ecol., 2001, 27, 443–470 CrossRef CAS.
- M. Carballa, F. Omil, J. M. Lema, M. Llompart, C. García-Jares, I. Rodríguez, M. Gómez and T. Ternes, Water Res., 2004, 38, 2918–2926 CrossRef CAS.
- A. C. Johnson, A. Belfroid and A. Di Corcia, Sci. Total Environ., 2000, 256, 163–173 CrossRef CAS.
- M. R. Servos, D. T. Bennie, B. K. Burnison, A. Jurkovic, R. McInnis, T. Neheli, A. Schnell, P. Seto, S. A. Smyth and T. A. Ternes, Sci. Total Environ., 2005, 336, 155–170 CrossRef CAS.
- G. D'Ascenzo, A. Di Corcia, A. Gentili, R. Mancini, R. Mastropasqua, M. Nazzari and R. Samperi, Sci. Total Environ., 2003, 302, 199–209 CrossRef CAS.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef CAS.
- C. Baronti, R. Curini, G. D'Ascenzo, A. Di Corcia, A. Gentili and R. Samperi, Environ. Sci. Technol., 2000, 34, 5059–5066 CrossRef CAS.
- T. A. Ternes, J. Stüber, N. Herrmann, D. McDowell, A. Ried, M. Kampmann and B. Teiser, Water Res., 2003, 37, 1976–1982 CrossRef CAS.
- M. M. Huber, T. A. Ternes and U. von Gunten, Environ. Sci. Technol., 2004, 38, 5177–5186 CrossRef CAS.
- H. Zhang, H. Yamada and H. Tsuno, Environ. Sci. Technol., 2008, 42, 3375–3380 CrossRef CAS.
- M. M. Huber, S. Korhonen, T. Ternes and U. von Gunten, Water Res., 2005, 39, 3607–3617 CrossRef CAS.
- L. D. Nghiem, A. Manis, K. Soldenhoff and A. I. Schäfer, J. Membr. Sci., 2004, 242, 37–45 CrossRef CAS.
- Y. Zhang and J. L. Zhou, Water Res., 2005, 39, 3991–4003 CrossRef CAS.
- X. Yang, J. Dai and P. W. Carr, J. Chromatogr., A, 2003, 996, 13–31 CrossRef CAS.
- Y. Tan and J. E. Kilduff, Water Res., 2007, 41, 4211–4221 CrossRef CAS.
- K. Shorrock and B. Drage, Water Environ. J., 2006, 20, 65–70 Search PubMed.
- B. Bolto, D. Dixon, R. Eldridge, S. King and K. Linge, Water Res., 2002, 36, 5057–5065 CrossRef CAS.
- K.-J. Choi, H.-J. Son and S.-H. Kim, Sci. Total Environ., 2007, 387, 247–256 CrossRef CAS.
- M. Kitis, B. I. Harman, N. O. Yigit, M. Beyhan, H. Nguyen and B. Adams, React. Funct. Polym., 2007, 67, 1495–1504 CrossRef CAS.
- A. I. Schäfer, L. D. Nghiem and T. D. Waite, Environ. Sci. Technol., 2003, 37, 182–188 CrossRef CAS.
- A. I. Schäfer, Natural Organics Removal using Membranes: Principles, Performance, and Cost, CRC Press, Boca Raton, 2001 Search PubMed.
- J. Y. Morran, M. Drikas, D. Cook and D. B. Bursill, Water Sci. Technol.: Water Supply, 2004, 4, 129–137 Search PubMed.
- P. C. Singer and K. Bilyk, Water Res., 2002, 36, 4009–4022 CrossRef CAS.
- A. Nel, T. Xia, L. Mädler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS.
- T. H. Boyer and P. C. Singer, Environ. Sci. Technol., 2008, 42, 608–613 CrossRef CAS.
- W. H. J. Vaes, C. Hamwijk, E. Urrestarazu Ramos, H. J. M. Verhaar and J. L. M. Hermens, Anal. Chem., 1996, 68, 4458–4462 CrossRef CAS.
- R. P. Schwarzenbach, P. W. Gschwend and D. M. Imboden, Environmental Organic Chemistry, John Wiley & Sons, Inc., Hoboken, 2nd edn, 2003 Search PubMed.
- K.-U. Goss and R. P. Schwarzenbach, J. Chem. Educ., 2003, 80, 450–455 Search PubMed.
- L. D. Nghiem, A. I. Schäfer and M. Elimelech, Environ. Sci. Technol., 2004, 38, 1888–1896 CrossRef CAS.
- M. J. Semmens, M. Burckhardt, D. Schuler, P. Davich, M. Slunjski, M. Bourke and H. Nguyen, American Water Works Association Conference, Denver, June 2000 Search PubMed.
- P. H. Chen, Environ. Int., 1999, 25, 655–662 CrossRef CAS.
- I. Citová, R. Sladkovský and P. Solich, Anal. Chim. Acta, 2006, 573–574, 231–241 CrossRef.
- H. Lord and J. Pawliszyn, J. Chromatogr., A, 2000, 902, 17–63 CrossRef CAS.
- P. Li and A. K. SenGupta, Environ. Sci. Technol., 2000, 34, 5193–5200 CrossRef CAS.
- L. D. Nghiem and A. I. Schäfer, Environ. Eng. Sci., 2002, 19, 441–451 CrossRef CAS.
- T. H. Boyer and P. C. Singer, Water Res., 2006, 40, 2865–2876 CrossRef CAS.
- B. Bolto, D. Dixon and R. Eldridge, React. Funct. Polym., 2004, 60, 171–182 CrossRef CAS.
- P. Li and A. K. SenGupta, React. Funct. Polym., 2004, 60, 27–39 CrossRef CAS.
- H. Hubicka and D. Kolodynska, Hydrometallurgy, 2001, 62, 107–113 CrossRef CAS.
Footnote |
| † Part of a themed issue dealing with water and water related issues. |
| This journal is © The Royal Society of Chemistry 2010 |
