Discrimination between diffuse and point sources of arsenic at Zimapán, Hidalgo state, Mexico†
Ondra
Sracek
ab,
María Aurora
Armienta
*c,
Ramiro
Rodríguez
c and
Guadalupe
Villaseñor
d
aOPV s.r.o. (Protection of groundwater Ltd), Bělohorská 31, 169 00, Praha 6, Czech Republic
bDepartment of Geology, Faculty of Science, Palacký University, 17. Listopadu 12, Olomouc, 771 46, Czech Republic
cUniversidad Nacional Autónoma de México, Instituto de Geofísica, Ciudad Universitaria, México, DF 04510, Mexico. Fax: +52 55 55502486; Tel: +52 5556224114
dUniversidad Nacional Autónoma de México, Instituto de Geología, Ciudad Universitaria, México, DF 04510, Mexico
First published on 30th October 2009
Abstract
There are two principal sources of arsenic in Zimapán. Point sources are linked to mining and smelting activities and especially to mine tailings. Diffuse sources are not well defined and are linked to regional flow systems in carbonate rocks. Both sources are caused by the oxidation of arsenic-rich sulfidic mineralization. Point sources are characterized by Ca–SO4–HCO3 ground water type and relatively enriched values of δD, δ18O, and δ34S(SO4). Diffuse sources are characterized by Ca–Na–HCO3 type of ground water and more depleted values of δD, δ18O, and δ34S(SO4). Values of δD and δ18O indicate similar altitude of recharge for both arsenic sources and stronger impact of evaporation for point sources in mine tailings. There are also different values of δ34S(SO4) for both sources, presumably due to different types of mineralization or isotopic zonality in deposits. In Principal Component Analysis (PCA), the principal component 1 (PC1), which describes the impact of sulfide oxidation and neutralization by the dissolution of carbonates, has higher values in samples from point sources. In spite of similar concentrations of As in ground water affected by diffuse sources and point sources (mean values 0.21 mg L−1 and 0.31 mg L−1, respectively, in the years from 2003 to 2008), the diffuse sources have more impact on the health of population in Zimapán. This is caused by the extraction of ground water from wells tapping regional flow system. In contrast, wells located in the proximity of mine tailings are not generally used for water supply.
Environmental impactArsenic is one of the most serious environmental contaminants and may originate from both natural and anthropogenic sources, under reducing/oxidizing and/or alkaline conditions. Determination of arsenic sources is important because source type has an impact on the choice of mitigation and remediation strategies. The article suggests an approach to discriminate between different arsenic groundwater sources in Zimapán on the basis of geochemical, statistical, and isotopic methods. We believe that this methodology is applicable not only in arid regions affected by mining such as Zimapán, but also at other arsenic-contaminated sites. The health impact of arsenic on the local population is also discussed. |
1. Introduction
High arsenic concentrations in ground water have been detected at many sites around the world.1 In many situations high arsenic contents have natural causes including processes like reductive dissolution of ferric oxide and hydroxides in Bangladesh and West Bengal in India,2,3 dissolution of volcanic glass and high mobility of arsenic under high pH conditions at the Pampean region of Argentina4,5 or recent geothermal activity in the Andes in Chile.6However, the highest reported arsenic concentrations are linked to mining activities.1,7 Arsenic in mining wastes is generally present in sulfidic minerals like As-rich pyrite, FeS2, arsenopyrite, FeAsS, and orpiment, As2S3, which release arsenic during their oxidation. There is arsenic contamination from arsenopyrite residue stockpile at Snow Lake, Manitoba, Canada8 with arsenic concentrations in ground water of adjacent monitoring wells > 20 mgL−1 of arsenic. At Kaňk site close to Kutna Hora in Czech Republic,9 controlled flooding of mine resulted in reductive dissolution of ferric minerals formed during dissolution of arsenopyrite and arsenic concentrations in mining shaft outflow reached 59 mgL−1. Extremely high arsenic concentrations (as high as 850 mgL−1) were reported from the Richmond Mine in California, USA, where acid mine drainage water is concentrated by evaporation.10 The world highest reported dissolved arsenic contents were from the Berikul gold mine, Siberia, Russia.11 Concentrations of arsenic in pore water of a high-sulfide pile reached 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mgL−1 and concentrations of sulfate were about 190
000 mgL−1 and concentrations of sulfate were about 190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mgL−1. At site Carnoulès in southern France12 up to 250 mgL−1 of As in pore water of mine tailings were reported.
000 mgL−1. At site Carnoulès in southern France12 up to 250 mgL−1 of As in pore water of mine tailings were reported.
Zimapán is an old mining town located in Hidalgo State north of Mexico City (Fig. 1). The Zimapán valley is placed in the middle of a Cretaceous shelf basin within the physiographic provinces of the Sierra Madre Oriental and the Transmexican Volcanic Belt. The oldest Jurassic age Trancas Formation is a thinly bedded, gray phylitic calcareous shale interbedded with dark gray limestone.13,14 This formation is also the basement of the mineralization zone. The Doctor Formation and the Upper Tamaulipas Formation consist of gray limestone and define the Cretaceous system. The Late Cretaceous Soyatal Formation is composed of thin layers of limestone with shale and sand interbedded with clayey limestone and occasionally with red shale. The Laramide Orogeny faulted and intensely fractured the Cretaceous rocks.
 | ||
| Fig. 1 Geographic location of Zimapán. | ||
The mining deposits are of replacement and skarn type. In the replacement deposits the mineralization took place after the limestone deformation. The skarn mineralization consists of mixture of silicates with sulfide disseminations. In these deposits the most important As-bearing mineral is arsenopyrite; scorodite, adamite, mimetite and olivinite have also been reported in the alteration zones. The Zimapán mining district has been divided in four mineralized zones: El Carrizal, El Monte, San Pascual-Santa Gorgonia and La Luz-La Cruz. Most productive mines are located in El Carrizal area.15,16
The local aquifer system is integrated by three main units. A deep fractured aquifer developed in Cretaceous limestones (Soyatal and Tamaulipas formations); a granular shallow aquifer formed by Quaternary sediments and alluvial deposits overlying the calcareous rocks; and a volcanic aquifer in the eastern part of the valley. The fractured limestones constitute the more productive aquifer formation. Its groundwater also contains the highest As concentrations. The wells exploiting this aquifer are located in the proximity of intrusive bodies and dikes where arsenic minerals are found.17 Groundwater with low (from non detectable up to 0.05 mgL−1) As concentration is found in the volcanic aquifer which, on the other hand, has a low productivity.
Since 1993 after its detection in drinking water, various studies have been performed in the area to determine the concentration levels, distribution, and possible sources of As in groundwater.17,18 Consumption of As-tainted water has produced health affectations such as hyperkeratosis, hypopigmentation and hyperpigmentation.19 High As contents are also present in soils.20,21 Studies developed to identify As provenance have been supported by chemical analyses of water, rocks, soils, and mine wastes, jointly with mineralogical determinations and geochemical modeling.17,18,22 Results indicate that groundwater As proceeds mainly from natural mineralization in the limestone aquifer. However, anthropogenic sources related with ore processing have also been identified.23,24 Stable isotopes may give an additional signature to discriminate among groundwater As related to natural processes and ore processing. This knowledge may be generalized throughout Zimapán valley. Besides, the main methodological framework developed in this area may also be applied at other mining zones. Principal objective of the research was to develop criteria and methodology for discrimination between different sources of arsenic in areas affected by mining activities.
2. Material and methods
Water samples were taken from 2003 to 2008 in shallow wells close to tailings, deep wells, and from a shallow river; sampling points are shown in Fig. 2. Field determinations included pH, T, redox potential Eh, and electrical conductivity EC. Buffer solutions were submerged in the water coming out from the wells, and allowed to equilibrate with the water temperature before calibrating the pH-meter prior to each reading. Electrical conductivity was measured using Conductronic PC18. Calibration was made with a solution containing 1000 mgL−1 NaCl. Redox potential was determined with an ORP meter Instrulab with a combined platinum electrode; Zobell solution potential was measured after every five samples. Results were corrected with respect to standard hydrogen electrode (SHE). At each sampled point an aliquot of 1 L of water in a polyethylene bottle was taken for the determination of main anions. Two bottles of 500 mL were taken for the analysis of cations and As, and acidified immediately by adding 50 drops of 14.8 N HNO3 per 500 mL of sample. Previous sampling campaigns showed a negligible difference between filtered and unfiltered groundwater samples and for this reason samples were not filtered in the field. Samples were preserved at 4 °C, until their analyses within 48 h at the Geophysics Institute, UNAM, México. Chemical analyses were performed following standard methods as described in APHA (1995). Arsenic was determined by hydride generation atomic absorption spectroscopy with a Perkin Elmer 2380 MHS-10, and a detection limit of 0.0005 mg L−1. Calibration curve was constructed using certified commercial standards “High Purity” traceable to NIST Standard Reference Material No. 3103a. Accuracy was checked against Multi-Element Quality Control Standard 19, giving a recovery of 105%. Besides, As concentrations of groundwater samples collected in 2008 obtained with the MHS-10 were checked against results measured with a FIAS 100 connected to a Perkin Elmer AAnalyst 100 giving differences less than 10 percent.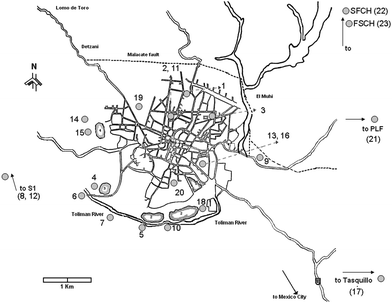 | ||
| Fig. 2 Location of sampling points. Numbers correspond to those of Table 1. | ||
Univariate and multivariate statistics calculations were performed using program PAST.26 Speciation calculations were performed using the program PHREEQC27 and thermodynamic data for As were compiled from databases of minteq.dat and llnl.dat.
Isotopic determinations of D and 18O were performed at the laboratory “Geoquímica de isótopos estables de C, O, N, H” of the Geology Institute, UNAM, using a Finnigan Delta Plux XP–Isotopic Ratio Mass Spectrometer (IRMS) with continuous flow inlet techniques. In 2H/H measurement pure hydrogen gas is equilibrated with water sample in the presence of platinum catalyst. Measurements were carried out after an equilibration time of one hour. Water samples were equilibrated overnight with purified tank CO2 before the 18O/16O ratio determination. Results were converted to δ-notation with respect to VSMOW (Vienna Standard Mean Ocean Water). The analytical reproducibility was ±1.20‰ for δ2H measurement and ±0.05‰ in the case of oxygen isotopes measurement.
Analyses of 34S were performed at the isotopic laboratory of the University of Waterloo. Sulfate was precipitated with BaCl2 after sampling and concentration of 34S(SO4) was determined using the SO2 gas produced by thermal decomposition of BaSO4. Values were reported as δ34S(SO4) ‰ with respect to Canyon Diablo Trolite (CDT) standard with a precision of ±0.3‰.
3. Results
3.1. Water chemistry
Water chemistry data are presented in Table 1. A classification of samples based on their relation to the principal sources of contamination (diffuse, i.e., regional and point, i.e., tailings) is also included. Samples considered affected by diffuse sources are from deep wells (> 15 m) located upgradient from tailings, samples considered affected by point sources are collected from shallow wells (< 15 m) located downgradient from tailings. However, in some cases it is difficult to classify the samples because they may be affected by both sources. Groundwater samples belong to Ca–HCO3 type or Ca–Na–HCO3 type in regional flow system and Ca–SO4–HCO3 type in wells affected by drainage from mine tailings. Samples were plotted in the Piper graph shown in Fig. 3. In the triangle for cations samples from wells close to mine tailings or samples collected directly at mine tailings (tailings leachate) are close to Ca corner and samples from the regional flow system are displaced to the right, forming a transition between Ca corner and Na + K corner. There is a clear distinction between both groups also in the anion triangle, where samples from wells close to mine tailings are clustered close to SO4 corner and samples from regional flow system are close to CO3 + HCO3 corner, with some samples shifted towards SO4 corner. Both groups are separated clearly in the upper diamond. Samples collected in Toliman River are between both groups and their position depends on the level of contamination.| Identification | pH | EC μS cm−1 | HCO3 | SO4 | Cl | Na | K | Ca | Mg | As | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 P2-08 | 6.87 | 527 | 281.1 | 30.4 | 7.5 | 17.5 | 2.5 | 91.2 | 6.7 | 0.05 | regional |
| 2 Pb-PV | 6.78 | 545 | 270.6 | 58.1 | 6.7 | 14.6 | 1.3 | 92.2 | 12.2 | 0.50 | regional |
| 3 P3-old | 6.90 | 475 | 287.1 | 23.9 | 4.0 | 7.6 | 1.2 | 100.2 | 4.9 | 0.05 | regional |
| 4 SMVJ-08 | 6.86 | 645 | 369.3 | 744.9 | 67.9 | 81.0 | 9.1 | 360.7 | 48.6 | 0.28 | tailings |
| 5 CMZ | 6.64 | 2880 | 412.7 | 1323.6 | 55.8 | 132.1 | 6.2 | 541.1 | 60.8 | 0.38 | tailings |
| 6 SMV-08 | 6.58 | 1928 | 278.1 | 99.9 | 12.6 | 15.6 | 2.1 | 124.2 | 7.3 | 0.06 | tailings? |
| 7 Toliman | 8.12 | 1153 | 452.3 | 218.7 | 37.9 | 60.0 | 7.9 | 176.3 | 24.3 | 0.15 | river |
| 8 ZCMPZ | 7.73 | 1233 | 208.3 | 485.8 | 43.2 | 65.2 | 10.0 | 176.8 | 36.3 | 0.42 | river |
| 9 PozoZLP6 | 7.57 | 528 | 267.8 | 52.5 | 9.6 | 17.2 | 2.0 | 79.6 | 16.4 | 0.32 | regional? |
| 10 J-4-5 | 6.84 | 2600 | 499.5 | 1178.8 | 73.6 | 130.5 | 9.0 | 513.9 | 78.2 | 0.30 | tailings |
| 11 NORIA_Pb | 7.72 | 2670 | 272.2 | 422.3 | 160.6 | 194.2 | 35.3 | 321.4 | 55.3 | 1.30 | tailings? |
| 12 SI | 7.99 | 1848 | 143.5 | 913.1 | 27.0 | 66.7 | 7.4 | 396.8 | 43.3 | 0.04 | river |
| 13 ISA05 | 7.67 | 562 | 233.3 | 70.7 | 25.0 | 42.0 | 4.5 | 58.7 | 17.1 | 0.22 | regional |
| 14 SMNP_I | 7.25 | 1464 | 228.0 | 564.6 | 49.2 | 50.0 | 9.3 | 267.2 | 25.6 | 0.42 | tailings |
| 15 SMNP_II | 7.57 | 1849 | 174.7 | 914.6 | 77.7 | 79.8 | 16.8 | 331.5 | 37.8 | 0.40 | tailings |
| 16 Casa | 7.61 | 556 | 237.2 | 67.2 | 17.0 | 34.2 | 3.5 | 64.7 | 18.3 | 0.39 | regional? |
| 17 Tasquillo | 7.86 | 638 | 206.3 | 94.6 | 37.5 | 70.0 | 7.3 | 41.8 | 19.8 | 0.01 | regional |
| 18 Pila_Jales | 7.37 | 2710 | 65.4 | 1738.4 | 122.0 | 81.3 | 33.3 | 668.0 | 1.0 | 0.79 | leachate |
| 19 Agua_Mpal_CM | 7.72 | 527 | 193.8 | 79.7 | 17.4 | 56.3 | 5.6 | 37.7 | 15.0 | 0.57 | regional |
| 20 Agua_Mpal_Penoles | 7.63 | 527 | 177.4 | 86.7 | 20.6 | 51.2 | 5.5 | 42.6 | 15.6 | 0.19 | regional |
| 21 PLF | 7.10 | 661 | 288.1 | 42.3 | 12.6 | 32.0 | 3.5 | 70.4 | 30.4 | 0.08 | regional |
| 22 SFCH1 | 6.20 | 1613 | 24.3 | 1072 | 4.1 | 9.6 | 2.7 | 341.3 | 21.6 | 0.04 | leachate |
| 23 FSCH2 | 2.32 | 5170 | 0.0 | 498 | 27.0 | 31.4 | 7.8 | 396.8 | 222.6 | 77.50 | leachate |
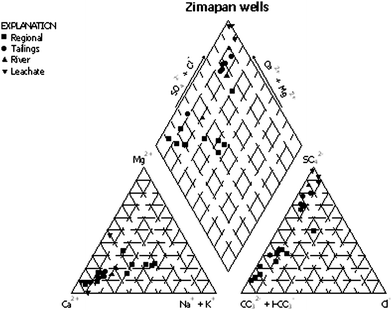 | ||
| Fig. 3 Piper diagram for water samples from Zimapán. | ||
Basic statistical characteristics for samples affected by different arsenic sources are presented in Table 2. Only samples, which can be clearly classified, were included here. For example, well Noria_Pb, which cannot be attributed clearly to either source, was omitted. There are clear differences between regional flow system wells and wells affected by mine drainage from mine tailings for some species. Maximum difference is in sulfate concentration (mean 60.8 mgL−1 compared to 804.4 mg L−1, i.e. more than 13-fold difference), smaller difference is for Ca (about 5-fold) and there only is a small difference for bicarbonate (about 1.3-fold). For As, mean values are 0.21 mgL−1 and 0.31 mgL−1 respectively (about 1.5-fold difference), indicating that As concentrations in regional flow systems can be significant. Using statistical t-test for comparison of the impacts of both arsenic sources, the differences at p = 0.01 are statistically significant for SO42−and Ca2+, but not for HCO3− and As.
| System/parameter | Ca | HCO3 | SO4 | As | |
|---|---|---|---|---|---|
| Regional | N | 8 | 8 | 8 | 8 |
| Range | 37.7–100.2 | 174.7–288.1 | 23.9–94.6 | 0.01–0.57 | |
| Mean | 66.85 | 242.21 | 60.78 | 0.21 | |
| Stand. dev. | 25.32 | 45.26 | 26.46 | 0.21 | |
| Tailings | N | 6 | 6 | 6 | 6 |
| Range | 124.2–541.1 | 174.7–499.5 | 99.9–1323.6 | 0.06–0.42 | |
| Mean | 356.4 | 327 | 804.4 | 0.31 | |
| Stand. Dev. | 155.8 | 121.9 | 442.5 | 0.13 | |
3.2. Multivariate statistics
Results of Principal Component Analysis (PCA) are in Fig. 4. Just first two principal components are plotted. PC1 and PC2 explain 90.5% and 8.7% (sum 99.2%), respectively, variability in the data set. PC1 has high positive loadings for electrical conductivity (EC), SO42−, and Ca2+. It is clearly linked to the oxidation of sulfides in mine tailings and neutralization of acid mine drainage. PC2 is less clearly defined; it has positive loadings for Ca2+, SO42−, HCO3−, Cl−, and Na+, but negative loading for EC. It seems to be linked to ground water, which is neutralized, but does not have very high mineralization. | ||
| Fig. 4 Results of Principal Component Analysis (PCA), regional – circle, well downgradient of tailings – square, tailings leachate – cross, river – star. | ||
In Fig. 4, shift of samples to the right indicates increasing influence of mine drainage. Sample FSCH2 at complete bottom right represents leachate collected directly at mine tailings with very low pH and very high sulfate concentration. Samples clustered together at the left represent regional flow system ground water. Samples from Toliman River are located between both end-members. Samples of leachate from tailings and samples from wells downgradient from tailings at the top of the plot are generally well-neutralized by the dissolution of carbonates.
Results of Hierarchical Cluster Analysis (HCA) in Ward's mode are shown in Fig. 5. There are two principal clusters. The cluster at the left with wells from SMVJ-08 to PLF (14 samples) includes wells from regional flow system, but also wells SMVJ-08 and SMNP_I, which are located downgradient from mine tailings, but are less contaminated. Furthermore, samples Riv_Tol and ZCMPZ, representing River Toliman, also belong to the group. These last samples (next to mine tailings and from the river) form a separate cluster within the left branch. Second cluster at the right includes sample from FSCH2 to J-4-5 (9 samples). All samples from this cluster are strongly affected by mine drainage. Sample SI collected from River Toliman in the close proximity of mine tailings also belongs to this group. In contrast, there are no wells from regional flow system in this cluster. Sample FSCH2 has a special status in this cluster and is located separately because represents acid mine drainage, which was not neutralized.
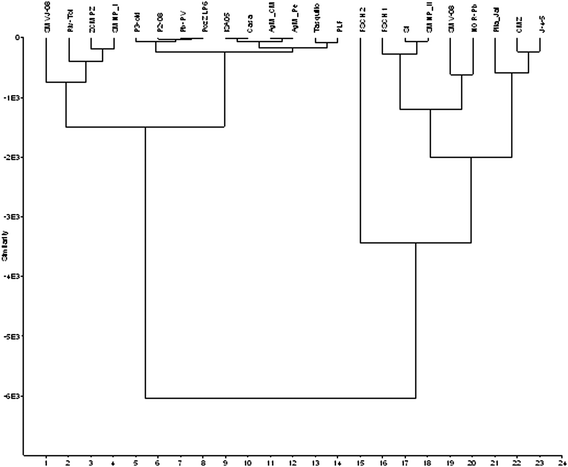 | ||
| Fig. 5 Results of Hierarchical Cluster Analysis (HCA). | ||
3.3. Speciation calculations
Selected results of speciation modeling calculations performed for samples collected in April, 2008, are shown as saturation indices (SI) values in Table 3.| Mineral/well | calcite | dolom. | gypsum | Fe(OH)3(a) a | goeth. a | scorodite | CaHAsO4·H2O | SiO2(a) | fluorite |
|---|---|---|---|---|---|---|---|---|---|
| a Fe in all samples was below detection limit of 0.3 mg L−1, concentration at half of the detection limit was used for calculations. | |||||||||
| P2-08 | −0.27 | −1.33 | −1.99 | 1.96 | 7.71 | −4.96 | −4.75 | −1.30 | −2.14 |
| P3-old | −0.30 | −1.64 | −2.05 | 1.95 | 7.66 | −4.86 | −4.74 | −1.42 | −2.13 |
| Pb-PV | −0.20 | −0.93 | −1.74 | 1.78 | 7.67 | −4.15 | −3.74 | −1.41 | −1.30 |
| SMVJ-08 | −0.25 | −1.55 | −1.40 | 2.29 | 7.80 | −4.64 | −4.61 | −1.15 | −1.78 |
| SMV-08 | −0.07 | −0.77 | −0.41 | 1.98 | 7.59 | −4.03 | −3.87 | −0.90 | −1.05 |
| CMZ | −0.06 | −0.87 | −0.10 | 2.11 | 7.58 | −3.77 | −3.73 | −0.84 | −1.01 |
| Toliman | 1.21 | 1.76 | −1.05 | 2.81 | 8.24 | −6.25 | −4.12 | −0.96 | −0.87 |
Samples P2-08, P3-old, and Pb-PV, representing regional flow system, are all undersaturated with respect to calcite, dolomite, and gypsum. Samples SMVJ, SMV, and CMZ, representing ground water affected by mine tailings, are also undersaturated with respect to these minerals, but especially sample CMZ is very close to saturation. This suggests that saturation with respect to these minerals is probably reached in mine tailings located up-gradient from these wells. Water in Toliman River is supersaturated with respect to calcite and dolomite due to degassing of CO2. All samples are supersaturated with respect to amorphous Fe(OH)3 and goethite. However, the values are only approximate due to default dissolved Fe concentrations used for calculations. All samples are undersaturated with respect to As(V) mineral scorodite, FeAsO4·2H2O, and As(V)-carbonate minerals like CaHAsO4·H2O. This confirms ferric oxide and hydroxides as a principal sink for dissolved arsenic. Saturation is not reached with respect to amorphous silica and fluorite.
3.4. Stable isotopes
Results of isotopic analyses are in Table 4 and in Fig. 6. Local meteoric water line for Central Mexico δD = 7.97 δ18O + 11.0328 is included for comparison. All sampled waters fall on evaporation line with slope of about 4. This is similar to the data collected in the aquitard at the Chalco Plain in the Valley of Mexico.29 This corresponds to evaporation within porous media, which was observed in waste rock pile at Mine Doyon in Québec, Canada, by Sracek et al.30 The evaporation line originates at values of δD about −79‰ and δ18O −11.3‰, indicating a common origin of all groundwater samples. Contrary to expectations, these values indicate similar altitude of recharge. Samples from mine tailings and sample from the Toliman River are at generally to the right, i.e. they are more enriched by evaporation. Their deuterium excess values are from 0.9 to 3.9‰ lower than values 8.1 to 9.4‰ of samples from the regional aquifer (Table 4). Deuterium excess values decrease with increasing humidity of air.31 This indicates that samples in tailings were probably influenced by evaporation in unsaturated porous media under high humidity of air conditions. Samples from regional wells out of mine tailings are at the left, close to the isotopic composition of recharge water. This suggests relatively slow recharge influenced by evaporation in fine grained tailings and faster recharge without much evaporation in regional flow system. Isotopic composition of water in the Toliman River is close to the isotopic composition of samples from the wells located in the proximity of mine tailings.| Indication | δ 18OVSMOW (‰) | δ 2HVSMOW (‰) | D-excess(‰) | δ 34S(SO4)CDT (‰) | Name |
|---|---|---|---|---|---|
| a n.a. – not available. | |||||
| SMVJ-08 | −9.35 | −71.2 | 3.5 | −2.05 | San Miguel Viejo Jales |
| CMZ | −8.85 | −69.9 | 0.9 | −0.65 | Compañía Minera Zimapán |
| Toliman | −9.51 | −72.2 | 3.9 | −2.59 | Toliman River |
| SMV-08 | −10.52 | −76.1 | 8.1 | −8.26 | San Miguel Viejo |
| Pb-PV | −10.74 | −77.8 | 8.2 | −12.14 | Plomo |
| P3-old | −10.85 | −77.4 | 9.4 | n.a. | P3 Viejo |
| P2-08 | −10.65 | −76.8 | 8.4 | −9.86 | P2 |
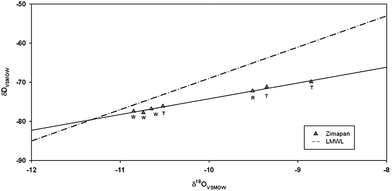 | ||
| Fig. 6 δD and δ18O data with local meteoric water line (LMWL) and evaporation line. W: wells tapping regional flow, T: wells close to tailings, R: Toliman River. Sample T to the left, close to W corresponds to SMV. | ||
Results of 34S(SO4) analyses are in Table 4 and in plot with concentrations of SO42− in Fig. 7. Values of δ34S(SO4) fall into two groups: samples from mine tailings including the sample from the Toliman River have values from −0.65 to −2.59‰ and samples from regional flow system have more negative values from −9.86 to −12.14‰. An exception is sample SMV, with value of −8.26‰, which is considered to be influenced by tailings (Table 1), but has regional flow system isotopic fingerprint (see later). Both ranges indicate the oxidation of sulfides as a source of sulfate, but sulfides seem to correspond to two different types of mineralization or, as stated by Hoefs,32 they are released from distinct zones within an isotopically zoned vein deposit. According to findings of Camprubí et al.33 from Zacatecas north of Zimapán, δ34S(SO4) values may cover a large range from −33‰ to 0‰ with decreasing values in the sequence pyrite-sphalerite-galena. In Zimapán deposits, there is a vertical zonality where sphalerite and galena with expected more negative δ34S(SO4) values are found at relatively shallower depth.34 However, no sulfur isotopic data on solid phase sulfides are available from Zimapán. Sulfate cannot originate from evaporate minerals in sedimentary rocks, which typically have δ34S(SO4) values about +20‰.31 When sulfate reduction takes place, there is an enrichment of residual sulfate in heavier isotope 34S and there is inverse correlation between δ34S(SO4) values and sulfate concentrations.31 However, in Fig. 7 an opposite trend is observed, i.e. more positive δ34S(SO4) values correspond to higher sulfate concentrations. This means that sulfate reduction is not in operation at the site.
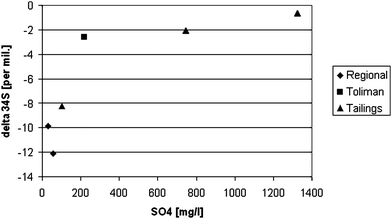 | ||
| Fig. 7 Plot of δ34S(SO4) vs. concentration of SO42−(mgL−1). | ||
In Fig. 8 there is plot of δ34S(SO4) vs. δ18O in water. Two groups of samples are evident again: samples from mine tailings and Toliman River, which have enriched δ18O values and also enriched δ34S(SO4) values and samples from regional flow system, which have depleted both δ34S(SO4) values and δ18O values. Isotopic analyses of sample SMV considered influenced by tailings in Table 1, plot near the regional samples in Fig. 6, 7 and 8. This implies that although a strong influence of tailings leachates was expected in this shallow well due to its location near a tailings pile, it is fed by water from the regional flow system explaining its relatively low concentrations of SO42− (100 mgL−1) and As (0.06 mg L−1).
 | ||
| Fig. 8 Plot of δ34S(SO4) vs. δ18Owater. | ||
4. Population affected by diffuse As sources vs. population affected by point As sources
Epidemiological studies developed at Zimapán have revealed arsenic-related health effects in a significant proportion of the studied inhabitants. A study carried out by Armienta et al.19 showed various degrees of skin affectation in 80% of the studied population. The average arsenic content in hair was 8.55 ± 3.56 mgkg−1 (almost six times the international standard) in people drinking water from less than 0.014 mgL−1 up to 1.0 mgL−1. Ninety seven, out of 120 sampled individuals showed some degree of skin affectation. Hypopigmentation was observed in 19.66% of the studied group, hyperpigmentation in 12.82%, 26.49% presented hyperkeratosis, and 21.36% had hypopigmentation and hyperpigmentation. The average As hair content for the affected group was 9.22 ± 3.13 mgkg−1. Results revealed relations among water arsenic levels, hair contents, and dermal effects. Reséndiz and Zúñiga35 carried out a similar study including another 71 individuals; an average of 9.74 mgkg−1 As was determined in that group. More than half (56.1%) of the studied population consuming water containing from less than 0.025 mg L−1 (the Mexican drinking water standard) to 0.50 mg L−1 showed dermal damage, with a higher proportion of affected women than men. The highest As content in hair (average of 29.25 mgkg−1) was measured in people ranging from 50.1 to 60 years old. An average of a 15 year period was estimated for the appearance of hyperkeratosis. Due to the unknown variation in drinking water source it was not possible to establish a correlation between As in hair and in water. Nevertheless, one factor ANOVA test showed correlation between dermal health effects and As content in hair. Valenzuela et al.,36 in a cross sectional study conducted with 72 female residents, observed at least one sign of arsenicism in about half of a high-As (0.10 mgL−1 on average) exposed group. Besides, association of arsenic presence with the increment of transforming growth factor alpha (TGF-α) levels in bladder urothelial cells was shown by multivariate linear regression analyses. TGF-α levels in urothelial cells were significantly associated with As in urine in those individuals presenting skin lesions. The genetic damage potential of Zimapán well water was experimentally studied by Gómez-Arroyo et al.37 Root tip meristems from the broad bean Vicia faba were treated with water from Zimapán wells to determine sister chromatide exchanges (SCE). This plant assay constitutes a sensitive bioindicator of genetic injures. Significantly higher frequencies in those specimens treated with arsenic-contaminated water of Zimapán than the negative control were obtained for all samples (except in a well with an arsenic concentration of 0.02 mg L−1) employing the Tukey–Kramer multiple comparisons test_p-0.001. A concentration–response relationship was observed between SCE and As contents in water.5. Discussion
In mine tailings, the process generating mine drainage is the oxidation of sulfides like pyrite by oxygen,38| FeS2(s) + 3.5O2(g) + H2O = Fe2+ + 2SO42− + 2H+ | (1) |
When arsenopyrite is present in mine tailings, it can also be oxidized by oxygen,39
| FeAsS(s) + 2.75O2 + 1.5H2O = Fe2+ + H3AsO3 + SO42− | (2) |
Oxidation of arsenopyrite by Fe(III), which is important in low pH water,40 is not significant at Zimapán mining site because Fe(III) concentrations are very low due to high pH values. The reaction above does not generate acidity directly, but acidity is produced by the formation of ferric hydroxide, which precipitates on the surface of arsenopyrite grains,
| Fe2+ + 0.25O2 + 2.5H2O = Fe(OH)3(s) + 2H+ | (3) |
Simultaneously, in the presence of oxygen, As(III) is oxidized to As(V),
| H3AsO3 + 0.5O2 = HAsO42− + 2H+ | (4) |
| CaCO3(s) + 2H+ + SO42− + 2H2O = CaSO4·2H2O(s) + H2CO3 | (5) |
When reactions (1) and (3) are combined, resulting reaction is
| FeS2(s) + 3.75O2(g) + 3.5H2O = Fe(OH)3(s) + 2SO42− + 4H+ | (6) |
These processes result in close to neutral pH water with high concentration of Ca and sulfate, but low concentrations of iron. Ferric oxide and hydroxides coatings limit the sulfide oxidation rate because they represent a barrier for penetration of oxygen and they also are efficient adsorbents of contaminants. However, alkaline pH conditions may result in mobilization of elements forming oxyanions such as As.41 Under oxidizing and alkaline conditions As present as HAsO42− is desorbed from negatively charged surfaces of ferric oxide and hydroxides.42
In Zimapán mine drainage is generally neutralized. However, high sulfide and carbonate contents in mine tailings result in high sulfate and bicarbonate concentrations. In contrast, in recharge areas of regional flow system there also is oxidation of sulfides, which may release As, but reaction rate is limited compared to fine-grained and relatively homogeneous mine tailings and carbonates may or may not be available for neutralization.
It is evident that multivariate statistics cannot discriminate arsenic contaminated wells and wells without arsenic in Zimapán. However, the impact of mine drainage is clearly evident in PCA results (Fig. 4) and HCA results (Fig. 5). In wells affected by mine drainage high arsenic concentrations are more probable.
Results of δD and δ18O analyses indicate slow recharge in mine tailings affected by evaporation and faster recharge in regional aquifer with less evaporation. Altitude of recharge seems to be similar for both, mine tailings and regional flow system, because all points fall on the same evaporation line, which intersects LMWL. Results of δ34S(SO4) exclude evaporate minerals as a source of sulfate and indicate two types of sulfidic mineralization in Zimapán or, more probably, zonality of isotopic composition within mineralization. Also, reduction of sulfate does not occur because there is no enrichment of samples with low sulfate concentrations by heavier isotope 34S. This means that both types of arsenic sources can be distinguished by their isotopic fingerprints.
People at Zimapán has been exposed to distinct As concentrations in drinking water along the years. The highest content (1.11 mgL−1 of As) was measured at El Muhi well in 1994. Furthermore, this well was the main source of potable water to Zimapán, but it was mixed with less polluted water before delivering to the potable water pipeline system. However, dwellers living close to El Muhi well consumed the water directly from the well outflow until the end of 1995, when it was closed due to its high As concentration. The Detzaní well that provided water to other small community in the Zimapán municipality had also high concentrations (up to 0.75 mg L−1 As). It was also closed around the year 2000 as a consequence of operation problems. Drinking water supply to most of the population has showed strong concentration variations along the time due to variations in the proportion coming from each one of several supplying wells.
It must be emphasized that all arsenic-related health effects related with drinking water are linked to the consumption of ground water from wells tapping the regional flow system. Wells in the proximity of mine tailings, contaminated by point pollution sources are mainly used for agriculture, house cleaning and industrial applications. Currently, although part of the population is supplied with good quality water, the central area of Zimapán still has As present, since water from one of the deep wells sampled in this study (containing 0.50 mgL−1 of As) is mixed with good quality water, resulting in As concentration of 0.1 mg L−1 in November 2008.
6. Conclusions
Two principal sources of arsenic contamination in Zimapán have been identified. Point sources are linked to the mining and smelting activities and especially to mine tailings located close to the River Toliman. Diffuse sources are not well defined and are linked to the regional flow system in carbonate rocks. However, both sources (point and diffuse) are caused by the oxidation of arsenic-rich sulfide minerals. Point sources are characterized by Ca–SO4–HCO3 ground water type and relatively enriched values of δD, δ18O, and δ34S(SO4) isotopes. Diffuse sources are characterized by Ca–Na–HCO3 type of ground water and more depleted values of δD, δ18O, and δ34S(SO4) isotopes. Values of δD and δ18O indicate similar altitude of recharge for both arsenic sources and stronger impact of evaporation for point sources in mine tailings. Evaporation seems to occur within porous media, just like at the Chalco Plain southeast of Mexico City. There also are different values of δ34S(SO4) for both sources, with samples from regional flow system exhibiting more depleted values, presumably due to two different types of mineralization or from the release from distinct zones of the deposit. In Principal Component Analysis (PCA), the principal component 1 (PC1), which describes the impact of sulfide oxidation and neutralization by the dissolution of carbonates, has higher values in samples from point sources, i.e. mine tailings. In spite of similar concentrations of As in ground water from diffuse sources and point sources (mean values 0.21 mgL−1 and 0.31 mgL−1, respectively), the diffuse sources have more impact on the health of population in Zimapán. This is caused by the extraction of ground water from wells tapping regional flow system. In contrast, wells located in the proximity of mine tailings are not generally used for water supply. The advantage of point sources is their generally easy identification and elimination of contaminated wells. On the other hand, diffuse sources cannot be identified easily and new wells in and around Zimapán have to be screened carefully for arsenic. Use of multivariate statistical methods jointly with isotopic signatures proved to be effective to discriminate between point to diffuse sources of arsenic.Acknowledgements
The authors are grateful to Olivia Cruz, Nora Ceniceros and Alejandra Aguayo of the Laboratorio de Química Analítica, IGEF, UNAM, for their skillful chemical analytical determinations, to P. Morales and E. González of the Geology Institute, UNAM, for analyses of δD and δ18O, and to R. Drimmie of University of Waterloo, Canada, for 34S(SO4) determinations. Funding was provided by Consejo Nacional de Ciencia y Tecnología (project CONACYT-SEMARNAT C01-0017-2002), Instituto de Geofísica and Coordinación de Estudios de Posgrado (Ciencias de la Tierra), UNAM.References
- P. L. Smedley and D. G. Kinniburgh, Appl. Geochem., 2002, 17, 517–568 CrossRef CAS.
- R. T. Nickson, J. McArthur, P. Ravenscroft, W. G. Burgess and K. M. Ahmed, Appl. Geochem., 2000, 15, 403–413 CrossRef CAS.
- K. M. Ahmed, P. Bhattacharya, M. A. Hasan, S. H. Akhter, S. M. M. Alam, M. A. H. Bhuyian, M. B. Imam, A. A. Khan and O. Sracek, Appl. Geochem., 2004, 19, 181–200 CrossRef CAS.
- P. L. Smedley, D. G. Kinniburgh, D. M. J. Macdonald, H. B. Nicolli, A. J. Barros, J. O. Tullio, J. M. Pearce and M. S. Alonso, Appl. Geochem., 2005, 20, 989–1016 CrossRef CAS.
- P. Bhattacharya, M. Claesson, J. Bundschuh, O. Sracek, J. Fagerberg, G. Jacks, R. A. Martin, A. del Storniolo and J. M. Thir, Sci. Total Environ., 2006, 358, 97–120 CrossRef CAS.
- L. Romero, H. Alonso, P. Campano, L. Fanfani, R. Cidu, C. Dadea, T. Keegan, I. Thornton and M. Farago, Appl. Geochem., 2003, 18, 1399–1416 CrossRef CAS.
- D. K. Nordström, Science, 2002, 296(5576), 2143–2145 CrossRef CAS.
- K. A. Salzsauer, N. V. Sidenko and B. L. Sheriff, Appl. Geochem., 2005, 20, 2303–2314 CrossRef.
- A. Kopřiva, J. Zeman, O. Sracek, in Natural Arsenic in Groundwater: Occurrence, Remediation and Management, ed. J. Bundschuh, P. Bhattacharya, D. Chandrasekharam, A.A. Balkema Publishers, Rotterdham, 2005, pp. 49–56 Search PubMed.
- D. K. Nordström and C. N. Alpers, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3455–3462 CrossRef CAS.
- R. Gieré, N. V. Sidenko and E. V. Lazareva, Appl. Geochem., 2003, 18, 1347–1359 CrossRef CAS.
- C. Casiot, S. Lebrun, G. Morin, O. Bruneel, J. C. Personné and F. Elbaz-Poulichet, Sci. Total Environ., 2005, 347, 122–130 CrossRef CAS.
- M. M. Carrillo, M. Suter, Excursión a la Región de Zimapán y areas circundantes. Libro Guía Conv. Geol. Nacional 6. Soc. Geol. Méx., México, D.F., 1982 Search PubMed.
- S. F. Simons, V. E. Mapes-Vázquez, ( 1956). Geology and ore deposits of the Zimapán mining district, State of Hidalgo, Mexico. US Geological Survey Professional Paper 284. U.S. Geological Survey, Washington, D.C. Search PubMed.
- G. G. García, S. Querol, in Economic Geology, Mexico, ed. G. P. Salas, Geological Society of America, Boulder, 1991, P-3, pp. 295–313 Search PubMed.
- M. G. Villaseñor, E. U. Petersen, S. Avendaño-Cano, J. A. Gomez-Caballero, J. Sousa and A. M. Reyes-Salas, Actas INAGEQ, 1996, 2, 129–134 Search PubMed.
- M. A. Armienta, G. Villaseñor, L. K. Ongley and H. Mango, Environ. Geol., 2001, 40, 571–581 CrossRef CAS.
- M. A. Armienta, R. Rodríguez, A. Aguayo, N. Ceniceros and G. Villaseñor, Hydrogeol. J., 1997, 5, 39–46 CrossRef.
- M. A. Armienta, R. Rodríguez and O. Cruz, Bull. Environ. Contam. Toxicol., 1997, 59, 583–589 CrossRef CAS.
- L. K. Ongley, A. Armienta and H. Mango, J. Phys. IV, 2003, 107, 983–986 Search PubMed.
- L. K. Ongley, L. Sherman, A. Armienta, A. Concilio and C. Ferguson-Salinas, Environ. Pollut., 2007, 145, 793–799 CrossRef CAS.
- M. A. Armienta and R. Rodríguez, Revista MAPFRE Seguridad, 1996, 63, 33–43 Search PubMed.
- M. Méndez and M. A. Armienta, Geofis.Int., 2003, 42, 131–140 Search PubMed.
- F. M. Romero, M. A. Armienta, G. Villaseñor and J. L. González, Int. J. Environ. Pollut., 2006, 26, 23–40 Search PubMed.
- APHA, AWWA, WPCF Standard Methods for the Examination of Water and Wastewater, American Public Health Association, the American Water Works Association and the Water Pollution Control Federation, Washington, D.C., 1995 Search PubMed.
- O. Hammer, D. A. T. Harper and P. D. Ryan, Palaeontologia Electronica, 2001, 4, 1–9 Search PubMed.
- D. L. Parkhurst, C. A. J. Appelo, User's guide to PHREEQC: A computer program for speciation, reaction-path, 1-D transport, and inverse geochemical calculations. U.S. Geological Survey Water-Resources Investigations Report 99-4259. U.S. Geological Survey, Washington, D.C., 1999 Search PubMed.
- A. Cortés, J. Durazo and R. N. Farvolden, J. Hydrol., 1997, 198, 346–376 CrossRef CAS.
- G. A. Ortega, J. A. Cherry and R. Aravena, J. Hydrol., 1997, 197, 47–69 CrossRef.
- O. Sracek, M. Choquette, P. Gelinas, R. Lefebvre and R. V. Nicholson, J. Contam. Hydrol., 2004, 69, 45–71 CrossRef CAS.
- I. Clark, P. Fritz, Environmental Isotopes in Hydrogeology, Lewis Publishers, New York, 1997 Search PubMed.
- J. Hoefs, Stable Isotope geochemistry, Springer, Berlin, 1997 Search PubMed.
- A. Camprubí, E. Gonzáles-Partida, D. Saldívar, P. Alfonso and C. Canet, J. Geochem. Explor., 2009, 101, 19 CrossRef CAS.
- E. Gonzáles-Partida, A. Carrillo-Chávez, G. Levresse, J. Tritoly and A. Camprubí, Ore Geol. Rev., 2003, 23, 91–96 CrossRef.
- R. M. I. Reséndiz, L. J. C. Zúñiga, Bachelor's Thesis on Engineering Chemistry, Universidad Tecnológica de México, México, D.F., 2003 Search PubMed.
- O. L. Valenzuela, D. R. Germolec, V. H. Borja-Aburto, J. Contreras-Ruiz, G. G. García-Vargas and L. M. Del Razo, Toxicol. Appl. Pharmacol., 2007, 222, 264–270 CrossRef CAS.
- S. Gómez-Arroyo, M. A. Armienta, J. Cortés-Eslava and R. Villalobos-Pietrini, Mutat. Res., 1997, 394, 1–7 CAS.
- D. W. Blowes, C. J. Ptacek, J. L. Jambor, C. G. Weisener, in Treatise on Geochemistry, ed. B. S. Lollar, Elsevier, Amsterdam, 2003, vol. 9, pp. 149–204 Search PubMed.
- F. P. Walker, M. E. Schreiber and J. D. Rimstidt, Geochim. Cosmochim. Acta, 2006, 70, 1668–1676 CrossRef CAS.
- Y. Yunmei, Z. Yongsuan, A. F. Williams-Jones, G. Zhenmin and L. Dexian, Appl. Geochem., 2004, 19, 435–444 CrossRef.
- D. Langmuir, Aqueous Environmental Geochemistry, Prentice Hall, New Jersey, 1997 Search PubMed.
- K. G. Stollenwerk, in Arsenic in Ground Water: Geochemistry and Occurrence, eds. A. H. Welch, K. G. Stollenwerk, Kluwer Academic Publishers, The Netherlands, 2003, pp 67–100 Search PubMed.
Footnote |
| † Part of a themed issue dealing with water and water related issues. |
| This journal is © The Royal Society of Chemistry 2010 |
