Hydrotalcites of zinc and titanium as precursors of finely dispersed mixed oxide semiconductors for dye-sensitized solar cells
Laura
Teruel
,
Younes
Bouizi
,
Pedro
Atienzar
,
Vicente
Fornes
and
Hermenegildo
Garcia
*
Instituto Universitario de Tecnología Química, Av. De los Naranjos s/n, 46022, Valencia, Spain. E-mail: hgarcia@qim.upv.es; Fax: +34 06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 387
387![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7809; Tel: +34 06
7809; Tel: +34 06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 387
387![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7807
7807
First published on 12th November 2009
Abstract
Hydrotalcites are layered materials whose sheets are constituted by octahedra occupied by two different metals (one divalent and the other tri- or tetravalent) and having oxide or hydroxide at the corners. The excess of positive charge of the sheets is compensated by anions located at the interlamellar space. In the present work we have synthesized two hydrotalcites containing zinc and titanium (Zn/Ti atomic ratio in the gel synthesis 6.25) that differ on the absence or presence of sodium dodecyl sulfate in the interlamellar spaces. Calcination of these hydrotalcites leads to a film of intimately dispersed mixed oxide semiconductor that was used as semiconductor for dye sensitised solar cells. Using a ruthenium polypyridyl complex as dye, two photovoltaic cells constructed by films of mixed oxides derived from hydrotalcite calcination were prepared. The highest efficiency parameters were VOC = 0.63 V, JSC = 2.18 mA cm−2, FF = 0.465, η = 0.64%. These efficiency values are not far from those obtained for an analogous photovoltaic cell prepared using P25 titania.
Broader contextDye-sensitized solar cells transforming solar light into electricity can serve to alleviate the shortage of fossil fuels, can contribute to the reduction of CO2 emissions and in the long term can form part of the pool of renewable, sustainable energy resources. However, there are still important problems with this type of cell that need to be solved in order to facilitate the widespread application of this technology. In the current state of the art, the maximum overall efficiency for the conversion of solar energy into electrical power is about 10% and there is still much room for improvement. In this context, most of the dye-sensitized solar cells are based on conventional titania nanoparticles as the active semiconductor. The efficiency of solar cells based on titania nanoparticles has been continuously improved in a large number of contributions, but, in spite of the intensive research in this area, the increment in the efficiency is very minor. The approach described in our contribution is to develop novel semiconductor materials that derive from layered hydrotalcites as semiconductors. The synthesis of these materials is simple and advantageous because they can be reliably prepared in large quantities by precipitation from aqueous solutions. In this paper we show that the efficiency of solar cells derived from hydrotalcites is similar to that of titania. Considering the wide range of hydrotalcites that can be prepared with various di- and trivalent metals, our report can serve to develop new semiconductors that eventually can overcome the efficiency of titania nanoparticles. |
Introduction
Hydrotalcites (HTs) are layered double metal hydroxides (LDH) in which two metal ions, one with +2 and the other with +3 oxidation states, are octahedrically coordinated in the sheet.1–3 Besides divalent and trivalent metal ions, the combination of di- and tetravalent metal ions has also been shown to be feasible.4,5 The presence of the trivalent or tetravalent cation creates a net positive charge in the layer that is compensated by the presence of a charge balancing anion that is located in the intergallery space. Typical anions are carbonate and nitrate. The structure of HTs is generally reflected in the morphology of the solid particles that tend to form small crystalline plates.HTs are used as poly(vinyl chloride) stabilizers as well as solid bases in catalysis.6–10 However the large variety of transition metals that can form HTs opens also the way to other applications in nanotechnology. In particular there are multitude of reports showing that titanium and zinc oxides behave independently as semiconductors and show a photoresponse upon illumination with photons having a higher energy than the semiconductor bandgap.11,12 Although doping of titania, as well as zinc oxide, with metallic and non-metallic elements is a well-known procedure to increase the photoresponse of the bare oxides, as far as we know mixed titania/zinc oxides are almost unexplored with respect to their photoresponse. Considering the versatility of HTs to prepare finely dispersed mixed oxides by calcination,13–18 it is remarkable that these types of LDH solids remain unexplored in photocatalysis.19–23 A particular case relevant to the present work has been reported by Seftel et al.23 This authors have described the synthesis of an HT(ZnTi), but the photocatalytic activity of this interesting material was not determined. In addition HTs offer the possibility to incorporate in the intergallery space some dye or metallic complexes that could act as a light harvester to effect the sensitization of the HT layers acting as semiconductor.9,24 The present work reports the photovoltaic activity of mixed oxides derived from calcination of HT having zinc and titanium. As far as we know there are no precedents describing the use of HTs as precursors of the semiconductor film in dye sensitized solar cells.
Results and discussion
HT containing zinc as the divalent cation and titanium as the tetravalent metal was prepared by precipitation of a mixture of zinc chloride and titanium tetraethoxide in aqueous phase upon heating the solution at 100 °C using urea as an “in situ” generator of ammonium. The general procedure to form HTs consists of the precipitation at basic pH and moderate temperatures of appropriate mixtures of di- and trivalent metal ions.4,23,25 One alternative procedure gradually increases the basicity of the solution and the concentration of the carbonate in the solution by using the urea decomposition.26–29 Upon heating in the aqueous phase, urea decomposes slowly giving ammonium and carbonate. This in situ generation of ammonium carbonate increases the solution pH to 9 and produces HT precipitation.28,30 Also in the case considered here, and taking into account that the oxidation state of titanium is +IV, it is more convenient to use the tetraethoxide instead of a metal salt. Under the synthesis conditions titanium tetraethoxide will undergo hydrolysis and the corresponding titanium hydroxide will be the actual species forming the resulting HT(ZnTi). For the synthesis, the initial Zn+2/Ti+4 atomic ratio of the solution was 6.25. Scheme 1 illustrates the preparation procedure followed to obtain HT(ZnTi). | ||
| Scheme 1 Preparation procedure followed for the synthesis of HT(ZnTi). | ||
The rationale behind the synthesis of HT(ZnTi) is to prepare a material that upon calcination will afford intimately dispersed mixed oxides that can behave as semiconductors for dye sensitized solar cells. HTs have a well defined structure constituted by ZnO6 and TiO6 octahedra with coordination spheres similar to those of zinc oxide and titanium dioxide, both being the most widely used photoactive semiconductors. Therefore it can be anticipated that after calcination, the resulting mixed oxides derived from HT(ZnTi) will exhibit some photovoltaic activity in solar cells. The versatility of hydrotalcites as precursors of the semiconductor film derive from their easy and reliable preparation in the aqueous phase at moderate temperatures with a well defined structure that upon calcination form finely dispersed mixed oxide. This contrasts with other high-temperature solid-state syntheses of mixed oxides.
To exemplify the advantages of this approach, we have prepared another hydrotalcite in which during the synthesis dodecyl sulfate (DS) becomes incorporated in the material (HT(ZnTi/DS)). There are precedents in the literature showing that the crystal growth, composition and even the Coulombic interaction and polarity among the sheets in layered materials varies by a large extent when surfactants are present in the synthesis.32–34 Thus, proceeding in a similar way as previously indicated for HT(ZnTi) (Scheme 1), but in the presence of a large excess of DS we obtained an analogous material HT(ZnTi/DS) in which DS as counter anion (rather than carbonate as in HT(ZnTi)) becomes incorporated into the intergallery spaces of the layered material. This synthetic procedure to include DS is advantageous due to its simplicity and economy in the number of steps. The conventional procedures to include anionic guests in the interlamellar space of HTs consist either of the intercalation of the anion from aqueous solution using pre-synthesized HTs or thermal treatment of pre-synthesized HTs to decompose the anion and reconstitution of the structure in the presence of the desired guest. In contrast to these common two-steps procedures, herein we have obtained a HT(ZnTi/DS) in a single step from zinc chloride and titanium tetraethoxide by precipitation in a solution containing dodecyl sulfate.
Both HT(ZnTi) and HT(ZnTi/DS) were characterized by their XRD pattern. Fig. 1 shows the XRD patterns of the as-synthesized materials. As it can be seen, the crystallinity of HT(ZnTi/DS) is significantly better than that of the HT(ZnTi). Although the exact reasons why the crystallinity increases in the presence of dodecyl sulfate are unknown, a similar observation on the positive influence of dodecyl sulfate on the crystallinity of hydrotalcite has been previously reported.31 We notice however that dodecyl sulfate acting as surfactant can increase the solubility and the dispersion of the small clusters present when the material is nucleating, this effect being beneficial for the final crystallinity of the resulting HT(ZnTi/DS). This higher crystallinity is reflected also by the presence of d(006), d(009), d(0012), d(0015), d(0018) and d(0021) diffraction peaks in the XRD of HT(ZnTi/DS). From the characteristics d(003) peak the interlayered distance was estimated as 0.207 nm and 3.219 nm for HT(ZnTi) and HT(ZnTi/DS), respectively. This expanded interlayer distance indicates that two layers of DS with the polar head interacting with the upper and lower HT sheet have become incorporated inside the HT(ZnTi/DS) material.
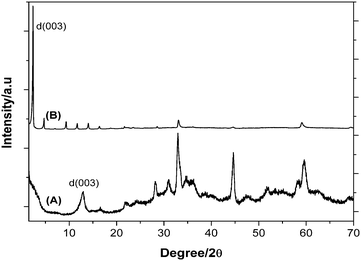 | ||
| Fig. 1 XRD diffractograms of HT(ZnTi) (A) and HT(ZnTi/DS) (B). | ||
The band gap of HT(ZnTi) and HT(ZnTi/DS) as semiconductors was estimated from the optical spectrum of these materials recorded by diffuse reflectance mode. Fig. 2 shows the diffuse reflectance UV-visible spectrum of HT(ZnTi) and HT(ZnTi/DS) compared to those of two samples of titanium oxide anatase and zinc oxide. As it can be seen there, the band gap of HT(ZnTi) and HT(ZnTi/DS) were 3.43 and 3.52 eV, that are similar to those of titanium oxide and zinc oxide. These spectra constitute an evidence supporting our claim that mixed double oxides constituted by zinc oxide and titania should not be far from pure titanium oxide and zinc oxide, particularly considering that these semiconductors exhibit similar band gap of about 3.2 eV.
 | ||
| Fig. 2 Normalized diffuse reflectance UV-Vis optical spectra of HT(ZnTi) (A), HT(ZnTi/DS) (B), P25 (C) and ZnO (D). | ||
Chemical analysis upon dissolution of the solids by ICP of the resulting liquids establishes that the Zn+2/Ti+4 atomic ratio of the materials were 5.33 and 7.23 for HT(ZnTi) and HT(ZnTi/DS) respectively. These atomic ratios differ significantly from the Zn+2/Ti+4 atomic ratio of the synthesis solution that was in both cases 6.25. The differences in the chemical composition between HT(ZnTi) and HT(ZnTi/DS) arise from the effect of the DS surfactant controlling the mobilization and incorporation of titanium species from the solution into the growing HT particle.32 It is anticipated that this different titanium content can be later reflected in a different performance of the resulting mixed oxides for dye sensitized solar cells.
Photovoltaic activity
With the two hydrotalcites previously characterized we proceeded to build dye sensitized solar cells. In the first place we prepared a paste containing about 10% of HT that was deposited on a FTO transparent electrode. After drying, the film was calcined at 450 °C to promote transformation of HTs into the corresponding mixed oxides as well as sintering and electric contact among the particles. After calcination, XRD showed that HTs have been converted into finely dispersed zinc oxide and mixed zinc titanium oxide (Fig. 3). This phase transformation is in agreement with the well known calcination behaviour of HTs.35–38 We notice that the resulting material derived from HT(ZnTi/DS) has broader diffraction lines than HT(ZnTi). This reflects the smaller particle size of the mixed oxides and zinc oxide formed in the calcination of HT(ZnTi/DS) as compared to HT(ZnTi). The difference in the particle size of the resulting mixed oxides must derive from the different crystallinity of the HT(ZnTi) and HT(ZnTi/DS). We also notice that this smaller particle size can play a beneficial influence on the photovoltaic activity, favouring HT(ZnTi/DS) over HT(ZnTi). | ||
| Fig. 3 XRD diffractograms of HT(ZnTi) (A) and HT(ZnTi/DS) (B) after sintering. | ||
The resulting thin films (8 μm) formed by mixed oxides resulting from HT calcination were immersed into a 0.5 mM tert-butanol solution of commercial ruthenium dye N3. After adsorption of the dye the film was washed and the cell was finalized using a platinized FTO cathode and an acetonitrile I3−/I− solution as electrolyte.39–41 The process of cell formation is summarized in Scheme 2.
For the sake of comparison we have prepared following the same procedure a dye sensitized solar cell using P25 as semiconductor.
After cell preparation we proceeded to determine the photovoltaic activity of the three cells. Fig. 4 shows the current density vs. voltage plot for the cells based on HT(ZnTi), HT(ZnTi/DS) and P25 materials while Table 1 lists the main efficiency parameters for these hydrotalcites cells as well as that obtained with P25 titania.
 | ||
| Fig. 4 Current density–voltage plot for the dye sensitized solar cells prepared by calcination of HT(ZnTi)(A), HT(ZnTi/DS)(B) and P25(C). | ||
| Materiala | V OC/V | J SC/mAcm−2 | FF | Efficiency (%) | Dye content/mol mg−1 × 108 |
|---|---|---|---|---|---|
| a The corresponding mixed oxides derived from HT calcination. | |||||
| HT(ZnTi) | 0.63 | 2.18 | 0.465 | 0.64 | 2.52 |
| HT(ZnTi/DS) | 0.62 | 1.16 | 0.528 | 0.38 | 3.70 |
| P25 | 0.79 | 4.98 | 0.520 | 2.08 | 2.32 |
As can be deduced from Fig. 4 and the values from Table 1, although the open circuit voltages of the cells with HTs are similar, the main difference were observed in the current density and fill factor (FF) of the photovoltaic response of HT(ZnTi) and HT(ZnTi/DS). The highest overall efficiency of HTs was achieved for HT(ZnTi).
To put the values of the photovoltaic response for HTs in context it has to be commented that the efficiency of the HT cells is about three times lower than that measured for P25.42–45 It has to be noted, however, that for titania based solar cells the highest efficiency value has been obtained after much optimization of the physicochemical properties of the semiconductor and cell preparation. In this regard it is very reasonable to assume that the efficiency of HTs can also be substantially improved by control of parameters such as the atomic ratio of the metals, optimization of the calcination temperature, the nature of the charge balancing anion, the nature of the dye, as well as the dye adsorption among different parameters. Also it is known that dye sensitized solar cells based on zinc oxide have lower efficiencies than those prepared with titania. Therefore the data presented in Fig. 4 and Table 1 are encouraging since they can trigger new research exploiting HTs as precursors of the semiconductor film in dye sensitized cells.
Fig. 5 shows the photocurrent spectrum obtained for the dye sensitized solar cells using HT(ZnTi) as precursor. This spectrum is the one expected based on the optical spectrum of ruthenium polypyridyl complex N3 showing that the photovoltaic response arises from the photochemical excitation of the electron donor dye. Probably, as in the case of titania, for HTs the excited state of N3 will also inject one electron into the conduction band of the mixed oxides formed in the calcination process of HT(ZnTi).
With respect to the relative efficiency of HT(ZnTi) and HT(ZnTi/DS) the purpose was, as noted earlier, to determine the influence of the presence of surfactant on the semiconductor performance of the resulting mixed oxides. As can be seen in Table 1 the amount of dye adsorbed per mass unit is larger in HT(ZnTi/DS) than in HT(ZnTi). Actually these two materials have a larger adsorption capacity than P25. However, we have also indicated that the titanium content of HT(ZnTi/DS) is lower than that of HT(ZnTi). Apparently this lower titanium content overcompensates the benefits of having a high dye content in the cell.
Experimental
Titanium P25 was a commercial sample from Degussa. Ruthenium polypyridyl dye N3 was purchased from Solaronix. Iodine 99.99%, lithium iodide powder 99.9%, 4-tert-butylpyridine 99% were commercial samples from Aldrich.XRD diffractions were obtained using a Philips X'Pert diffratometer using the copper radiation (Cu-Kα = 1.54178 Å) and measuring at 2° min−1 from 2° to 70°.
Diffuse reflectance UV-Visible spectra were recorded using a Varian Cary 5G spectrophotometer coupled with an integrating sphere. BaSO4 was used as reference.
Chemical analysis were measured by inductively coupled plasma-emission atomic spectrometry (ICP-EAS). A known amount of the solids was dissolved using concentrated HF and the resulting solution weight and analyzed by ICP-AES.
Preparation of HT(ZnTi)
The HT(ZnTi) samples were prepared by precipitation of salts of zinc and titanium from aqueous solutions. Thus zinc chloride (59.4 mmol) and titanium tetraethoxide (9.5 mmol) were added to an aqueous solution (100 ml) of urea (0.11 mol) and vigorous stirring and the mixture was heated at 100 °C for 48 h. After this time the solid formed was filtered and washed exhaustively with distilled water. Finally the solid was dried at 60 °C in an oven for 12 h.46 The resulting HT(ZnTi) exhibits the same XRD diffraction pattern as that reported by Saber et al. for the same material.Preparation of HT(ZnTi/DS)
The preparation of HT(ZnTi/DS) containing dodecyl sulfate intercalated into the layers was carried out following the same procedure and using the same amounts as for the synthesis of HT(ZnTi) except that the synthesis solution contains also 25 g of sodium dodecyl sulfate.Preparation of the dye-sensitized photovoltaic solar cells
A paste of HT(ZnTi), HT(ZnTi/DS) or P25 was obtained by dispersing the corresponding semiconductor (10% wt) in a ethylcellulose (6%)–α-terpineol solution. The paste was spread onto freshly-cleaned transparent conductive glass (FTO) and the layer was sintered in an oven at 450 °C. The programme temperature for calcination starts at room temperature increasing at a rate of 14 °C min−1 to 450 °C which temperature is maintained for 30 min. After cooling to 50 °C, the films were immersed in a 0.5 mM solution of commercial N3 dye (Solaronix) in tert-butanol for two days. The electrode was assembled with the platinized (sputtering) transparent conducting glass counter-electrode using a double-sided adhesive polymer film (Surlyn, DuPont) that acts as the separator and sealing element. The electrolyte (0.5 M lithium iodide, 0.05 M iodine and 0.4 M 4-tert-butylpyridine in acetonitrile) was added between the two electrodes by capillarity. The two electrodes were held together by hot melting the Surlyn at 100 °C while applying pressure.Characterization of the photovoltaic response of dye-sensitized solar cells
To measure the photovoltaic response, the cells were connected to an Amel potentiostat (MOD. 7050) by using metallic clamps covered with gold. This was done to determine the current density vs. voltage plots measuring the current generated for the cell upon illumination with an Oriel solar simulator (MOD. 91192). The data from the potentiostat is automatically transferred to a PC that controls the experiment at the same time as provide data storage capability to the system.The measurement time was shorter than 2 min, depending on the maximum voltage to the open circuit, avoiding long illumination times. The solar simulator was adapted to the AM 1.5G filter and the nominal power for the measurements was 100 mW cm−2. Efficiency was calculated from eqn (1). The values of VOC, JSC and FF present in the equation are those indicated in Table 1.
 | (1) |
The same cells were used to record the photocurrent spectra by placing them in a spectrofluorimeter sample chamber in front of the excitation monochromator. The sample was excited with a 75 W xenon lamp through a Czerny–Turner monochromator. The current output at short circuit was measured by the potentiostat that transferred the data through the A/D converter to the PC controlling the spectrofluorimeter apparatus.
Conclusions
In conclusion we have shown that the mixed oxides derived from hydrotalcite calcination are suitable materials for photovoltaic applications. The results obtained for efficiency are in the same range as those that can be obtained using P25 titania and therefore they are promising materials as semiconductors. It can be expected that the present results will open new avenues on the use of hydrotalcites in dye sensitized solar cells aimed at improving and optimizing the efficiency.Acknowledgements
Financial support by the Spanish MICINN is gratefully ackwoledged.Notes and references
- A. Beres, I. Palinko, I. Kiricsi, J. B. Nagy, Y. Kiyozumi and F. Mizukami, Appl. Catal., A, 1999, 182, 237–247 CrossRef CAS.
- D. G. Evans and R. C. T. Slade, in Layered Double Hydroxides, 2006, vol. 119, pp. 1–87 Search PubMed.
- P. Nalawade, B. Aware, V. J. Kadam and R. S. Hirlekar, J. Sci. Ind. Res., 2009, 68, 267–272 Search PubMed.
- J. J. Bravo-Suarez, E. A. Paez-Mozo and S. T. Oyama, Quím. Nova, 2004, 27, 601–614 CAS.
- J. He, M. Wei, B. Li, Y. Kang, D. G. Evans and X. Duan, in Layered Double Hydroxides, 2006, vol. 119, pp. 89–119 Search PubMed.
- D. G. Evans and D. A. Xue, Chem. Commun., 2006, 485–496 RSC.
- A. A. Fedorov, Y. S. Checkryshkin, O. V. Rudometova and Z. A. Vnutskikh, Russ. J. Appl. Chem., 2008, 81, 1673–1685 CrossRef CAS.
- L. Feng and X. Duan, in Layered Double Hydroxides, 2006, vol. 119, pp. 193–223 Search PubMed.
- S. P. Newman and W. Jones, New J. Chem., 1998, 22, 105–115 RSC.
- F. Z. Zhang, X. Xiang, F. Li and X. Duan, Catal. Surv. Asia, 2008, 12, 253–265 CrossRef CAS.
- D. W. Bahnemann, Res. Chem. Intermed., 2000, 26, 207–220 CrossRef.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- M. del Arco, P. Malet, R. Trujillano and V. Rives, Chem. Mater., 1999, 11, 624–633 CrossRef.
- J. M. Fernandez, M. A. Ulibarri, F. M. Labajos and V. Rives, J. Mater. Chem., 1998, 8, 2507–2514 RSC.
- O. P. Ferreira, O. L. Alves, D. X. Gouveia, A. G. Souza, J. A. C. de Paiva and J. Mendes, J. Solid State Chem., 2004, 177, 3058–3069 CrossRef CAS.
- P. Kustrowski, D. Sulkowska, L. Chmielarz, A. Rafalska-Lasocha, B. Dudek and R. Dziembaj, Microporous Mesoporous Mater., 2005, 78, 11–22 CrossRef CAS.
- F. Rey, V. Fornes and J. M. Rojo, J. Chem. Soc., Faraday Trans., 1992, 88, 2233–2238 RSC.
- S. Velu, K. Suzuki, M. Okazaki, T. Osaki, S. Tomura and F. Ohashi, Chem. Mater., 1999, 11, 2163–2172 CrossRef CAS.
- Y. H. Guo, D. F. Li, C. W. Hu, E. Wang, Y. C. Zou, H. Ding and S. H. Feng, Microporous Mesoporous Mater., 2002, 56, 153–162 CrossRef CAS.
- V. Iliev, A. Ileva and L. Bilyarska, J. Mol. Catal. A: Chem., 1997, 126, 99–108 CrossRef CAS.
- L. Maretti, E. Carbonell, M. Alvaro, J. C. Scaiano and H. Garcia, J. Photochem. Photobiol., A, 2009, 205, 19–22 CrossRef CAS.
- K. M. Parida, N. Baliarsingh, B. S. Patra and J. Das, J. Mol. Catal. A: Chem., 2007, 267, 202–208 CrossRef CAS.
- E. M. Seftel, E. Popovici, M. Mertens, G. Tendeloo, P. Cool and E. F. Vansant, Microporous Mesoporous Mater., 2008, 111, 12–17 CrossRef CAS.
- L. Latterini, M. Nocchetti, G. G. Aloisi, U. Costantino and F. Elisei, Inorg. Chim. Acta, 2007, 360, 728–740 CrossRef CAS.
- Z. P. Liu, R. Z. Ma, Y. Ebina, N. Iyi, K. Takada and T. Sasaki, Langmuir, 2007, 23, 861–867 CrossRef CAS.
- Y. Arai and M. Ogawa, Appl. Clay Sci., 2009, 42, 601–604 CrossRef CAS.
- P. Benito, M. Herrero, C. Barriga, F. M. Labajos and V. Rives, Inorg. Chem., 2008, 47, 5453–5463 CrossRef CAS.
- P. P. Yang, J. F. Yu, Z. L. Wang, T. Wu and T. H. Wu, React. Kinet. Catal. Lett., 2004, 83, 275–282 CrossRef CAS.
- H. Y. Zeng, X. Deng, Y. J. Wang and K. B. Liao, AIChE J., 2009, 55, 1229–1235 CrossRef CAS.
- T. Hibino and H. Ohya, Appl. Clay Sci., 2009, 45, 123–132 CrossRef CAS; A. Naghash, T. H. Etsell and B. Lu, J. Mater. Chem., 2008, 18, 2562–2568 RSC.
- E. L. Crepaldi, P. C. Pavan, J. Tronto and J. B. Valim, J. Colloid Interface Sci., 2002, 248, 429–442 CrossRef CAS.
- B. Li and J. He, J. Phys. Chem. C, 2008, 112, 10909–10917 CrossRef CAS.
- M. Bouraada, M. Lafjah, M. S. Ouali and L. C. de Menorval, J. Hazard. Mater., 2008, 153, 911–918 CrossRef CAS.
- H. Zhang, X. Wen and Y. X. Wang, J. Solid State Chem., 2007, 180, 1636–1647 CrossRef CAS.
- X. Shu, J. He, D. Chen and Y. X. Wang, J. Phys. Chem. C, 2008, 112, 4151–4158 CrossRef CAS.
- C. G. Silva, Y. Bouizi, V. Fornes and H. Garcia, J. Am. Chem. Soc., 131(38), 13833–13839 Search PubMed.
- K. J. D. MacKenzie, R. H. Meinhold, B. L. Sherriff and Z. Xu, J. Mater. Chem., 1993, 3, 1263–1269 RSC.
- T. Moroz, L. Razvorotneva, T. Grigorieva, M. Mazurov, D. Arkhipenko and V. Prugov, Appl. Clay Sci., 2001, 18, 29–36 CrossRef CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef CAS.
- A. Hagfeldt and M. Grätzel, Chem. Rev., 1995, 95, 49–68 CrossRef CAS.
- M. Grätzel., J. Photochem. Photobiol., A, 2004, 164, 3–14 CrossRef CAS.
- M. Grätzel, Progr. Photovolt.: Res. Appl., 2006, 14, 429–442 Search PubMed.
- Z. Chena, Y. Tang, L. Zhang and L. Luo, Electrochim. Acta, 2006, 51, 5870–5875 CrossRef CAS.
- M. Saito and S. Fujihara, Energy Environ. Sci., 2008, 1, 280–283 RSC.
- Q. Zhang, T. P. Chou, B. Russo, S. A. Jenekhe and G. Cao, Angew. Chem., Int. Ed., 2008, 47, 2402–2406 CrossRef CAS.
- O. Saber and H. Tagaya, J. Inclusion Phenom. Macrocyclic Chem., 2003, 45, 109–116 CAS.
| This journal is © The Royal Society of Chemistry 2010 |


