DOI:
10.1039/C0CE00077A
(Paper)
CrystEngComm, 2011,
13, 138-144
A new family of 3D heterometallic 3d–4f organodisulfonate complexes based on the linkages of 2D [Ln(nds)(H2O)]+ layers and [Cu(ina)2]− chains†
Received
10th April 2010
, Accepted 22nd June 2010
First published on 27th August 2010
Abstract
Seven novel 3D heterometallic coordination compounds, LnCu(nds)(ina)2·H2O [Ln = Eu (1), Ce (2), Er (3), Gd (4), Sm (5), Tb(6), Dy(7); nds = 2,6-naphthanlenedisulfonate; ina = isonicotinic acid], have been synthesized under hydrothermal conditions and characterized by elemental analysis, infrared spectra, thermogravimetric analysis and single-crystal X-ray diffraction analysis. These compounds are isostructural and constructed by the linkages of 2D [Ln(nds)(H2O)]+ layers and Cu(ina)2− chains, which represent the first examples of 3D heterometallic coordination polymers created by the organodisulfonate ligands. Moreover, the complexes exhibit a novel (3,6)-connected net. The luminescence properties of complexes 1, 5 and 6 and the magnetic property of 4 were also investigated.
Introduction
Organosulfonates are typical weak-bonded ligands1 and the coordination compounds with them are rarely reported. However, coordination compounds with organosulfonate ligands have been of great interest due to the following reasons: (i) the functionality of organosulfonate ligands is regarded with respect to multiple binding abilities of the coordinating sites and various topologies, as demonstrated by J. W. Cai.2 (ii) Metal organosulfonate families show great potential for new functional materials with regards to absorption,3 catalysis,4 magnetism, optics, single-crystal to single-crystal conversion,5 and as potential analogues of metal phosphonates.6 (iii) The proton of the sulfonic group is easily dissociable at very low pKa, which causes the flexible coordination modes of SO3−, ranging from μ1 to μ6, making structural characterization, a hallmark property of MOFs, a challenge.7 The supramolecular chemistry of the organosulfonate group in extended solids built up by cooperative coordination and other weak intermolecular interactions has been reviewed by Côté and Shimizu.8 There are some examples in the literature with main group and transition metal arenesulfonates since their structural chemistry and properties have been broadly discussed in a review.9 Lanthanide ions have high coordination numbers and can afford enough coordination sites for different ligands, which can lead to the syntheses of lanthanide coordination polymers with unprecedented structures. Up to now, metal–organic frameworks constructed from arenedisulfonates and rare earth elements have been examined to a considerably small extent.4a,10 However, there is only one report on 2,6-naphthalenedisulfonate coordinated to lanthanide ions by directional covalent bonds.4a With this in mind, lanthanide s disulfonates, incorporating secondary ligands, are particularly attractive.
As is known to all, lanthanide (Ln) and transition metal (TM) ions posses different affinities for N and O donors on the basis of the hard-soft acid/base classification.11 Such a characteristic provides us the impetus to construct interesting Ln–TM heterometallic coordination polymers.12Isonicotinic acid is a rigid ligand with carboxylate preferring to coordinate to Ln ions, the N atom on the opposite side has a strong tendency to coordinate to TM ions. Thus, it is an excellent candidate to construct high-dimensional heterometallic coordination frameworks. A characteristic of the high-dimensional frameworks is that they are entangled and the individual units cannot be separated without breaking coordination bonds.13 As far as we know, a 3D heterometallic organodisulfonate coordination polymer has not been reported elsewhere.
Herein, we report the synthesis, crystal structure, and characterization of seven novel heterometallic naphthanlenedisulfonate compounds, EuCu(nds)(ina)2·H2O (1), CeCu(nds)(ina)2·H2O (2), ErCu(nds)(ina)2·H2O (3), GdCu(nds)(ina)2·H2O (4), SmCu(nds)(ina)2·H2O (5), TbCu(nds)(ina)2·H2O (6) and DyCu(nds)(ina)2·H2O (7), which represent the first examples of 3D coordination frameworks with unique (3,6)-connected topology constructed from the linkages of 2D layers and Cu(ina)2− chains.
Experimental
Materials and methods
Solvents and starting materials were purchased commercially and used without further purification unless otherwise noted. The FT-IR spectra was recorded from KBr pellets in the 4000–400 cm−1 range on a Nicolet 5DX spectrometer. The C, H, N elemental analysis was performed on a Perkin-Elmer 2400 elemental analyzer. Thermogravimetric analysis was performed on Perkin-Elmer TGA7 analyzer with a heating rate of 10 °C min−1 in flowing air atmosphere. Fluorescence spectra were recorded with an F-2500 FL spectrophotometer. The magnetic susceptibility measurement was carried out on polycrystalline samples on a Quantum Design MPMS-XL5 SQUID magnetometer. Powder X-ray diffraction (PXRD) patterns were recorded on a X-pert diffractometer or Rigaku D M−1-2200T automated diffractometer for Cu-Kα radiation (λ = 1.54056 Å), with a scan speed of 4° min−1 and a step size of 0.02° in the 2θ range of 5–50°.
Preparation of compounds 1–7
A mixture of 2,6-naphthalenedisulfonate sodium salt (0.133 g, 0.4 mmol), isonicotinic acid (0.0984 g, 0.8 mmol), Eu(NO3)3·6H2O for 1 (0.135 g, 0.3 mmol), Ce(NO3)3·6H2O for 2 (0.131 g, 0.3 mmol), Er(NO3)3·6H2O for 3 (0.135 g, 0.3 mmol), Gd(NO3)3·6H2O for 4 (0.135 g, 0.3 mmol), Sm(NO3)3·6H2O for 5 (0.135 g, 0.3 mmol), Tb(NO3)3·6H2O for 6 (0.135 g, 0.3 mmol), Dy(NO3)3·6H2O for 7 (0.135 g, 0.3 mmol), CuCl2 (0.041 g, 0.3 mmol) and H2O (10 mL) was heated to 180 °C for 72 h in a 23 mL Teflon-lined stainless-steel autoclave and then cooled to room temperature at a rate of 5 °C h−1. Yellow block crystals were collected and dried in air.
EuCu(nds)(ina)2·H2O (1).
Yield: 65% based on Eu. Anal. calcd for C44H32Cu2N4Eu2O22S4: C 34.59, H 2.11, N 3.67%. Found: C 35.16, H 2.23, N 3.59%. IR frequencies (KBr, cm−1): 3508 w, 3205 w, 1601 s, 1539 s, 1417 s, 1241 s, 1181 m, 1156 m, 1091 m, 1031 vs, 897 w, 872 w, 856 w, 824 w, 786 w, 763 w, 705 m, 658 s, 621 m, 533 w.
CeCu(nds)(ina)2·H2O (2).
Yield: 60% based on Ce. Anal. calcd for C44H32Cu2N4Ce2O22S4: C 35.13, H 2.14, N 3.72%. Found: C 35.56, H 2.25, N 3.74%. IR frequencies (KBr, cm−1): 3445 w, 3213 w, 1592 s, 1539 s, 1416 s, 1337 w, 1244 s, 1179 m, 1144 m, 1091 m, 1028 vs, 898 w, 855 w, 788 w, 763 w, 706 m, 658 s, 623 m, 533 w.
ErCu(nds)(ina)2·H2O (3).
Yield: 39% based on Er. Anal. calcd for C44H32Cu2N4Er2O22S4: C 33.91, H 2.07, N 3.59%. Found: C 35.06, H 2.21, N 3.72%. IR frequencies (KBr, cm−1): 3445 w, 3212 w, 1594 s, 1539 s, 1419 s, 1242 s, 1144 m, 1091 m, 1039 m, 1029 vs, 897 w, 872 w, 856 w, 824 w, 787 w, 763 w, 706 m, 658 s, 621 m, 533 w.
GdCu(nds)(ina)2·H2O (4).
Yield: 70% based on Gd. Anal. calcd for C44H32Cu2N4Gd2O22S4: C 34.35, H 2.10, N 3.64%. Found: C 34.57, H 2.15, N 3.70%. IR frequencies (KBr, cm−1): 3510 w, 3213 w, 1603 s, 1594 m, 1541 m, 1419 s, 1377 w, 1241 s, 1181 m, 1156 m, 1144 m, 1092 w, 1042 w, 1032 vs, 897 w, 859 w, 824 w, 787 m, 713 w, 705 m, 658 s, 622 w, 563 w.
SmCu(nds)(ina)2·H2O (5).
Yield: 62% based on Sm. Anal. calcd for C44H32Cu2N4Sm2O22S4: C 34.66, H 2.12, N 3.67%. Found: C 35.12, H 2.14, N 3.69%. IR frequencies (KBr, cm−1): 3496 w, 3213 w, 1599 s, 1540 m, 1419 s, 1242 s, 1156 w, 1180 w, 1144 m, 1091 m, 1041 m, 1030 vs, 857 w, 824 w, 787 w, 706 m, 658 s, 623 m, 531 w.
TbCu(nds)(ina)2·H2O (6).
Yield: 61% based on Tb. Anal. calcd for C44H32Cu2N4Tb2O22S4: C 34.27, H 2.09, N 3.63%. Found: C 34.59, H 2.16, N 3.60%. IR frequencies (KBr, cm−1): 3521 w, 3212 w, 1605 s, 1541 s, 1419 s, 1241 s, 1156 m, 1144 m, 1092 m, 1032 vs, 897 w, 860 w, 824 w, 786 w, 764 w, 713 m, 658 s, 623 m, 533 w.
DyCu(nds)(ina)2·H2O (7).
Yield: 50% based on Dy. Anal. calcd for C44H32Cu2N4Dy2O22S4: C 34.12, H 2.08, N 3.62%. Found: C 34.36, H 2.19, N 3.70%. IR frequencies (KBr, cm−1): 3436 w, 3214 w, 1591 s, 1539 s, 1416 s, 1245 s, 1179 m, 1144 m, 1091 m, 1037 vs, 899 w, 872 w, 824 w, 787 w, 706 m, 659 s, 623 m, 534 w.
Single-crystal X-ray diffraction data collections of 1–7 were performed on a Bruker Apex II CCD diffractometer operating at 50 kV and 30 mA using Mo-Kα radiation (λ = 0.71073 Å). Data collection and reduction were performed using the APEX II software. Multi-scan absorption corrections were applied for all the data sets using the APEX II program. All seven structures were solved by direct methods and refined by full-matrix least squares on F2 using the SHELXTL program package.14 All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms attached to carbon were placed in geometrically idealized positions and refined using a riding model. Hydrogen atoms on water molecules were located from difference Fourier maps and were also refined using a riding model. The crystallographic data and refinement parameters of 1–7 are listed in Table 1, selected bonds distances and angles are listed in Table 2.
Table 1
Crystal data and structure refinement parameters of the complexes 1–7a,b
| Complex |
1
|
2
|
3
|
4
|
5
|
6
|
7
|
|
R
1 = ∑‖Fo| − |Fc‖/∑|F0|.
wR2 = {∑[W(Fo2 − Fc2)2]/∑(Fo2)2}1/2.
|
| Empirical formula |
C22H16CuEuN2O11S2 |
C22H16CuCeN2O11S2 |
C22H16CuErN2O11S2 |
C22H16CuGdN2O11S2 |
C22H16CuSmN2O11S2 |
C22H16CuTbN2O11S2 |
C22H16CuDyN2O11S2 |
| Formula weight |
764.03 |
752.18 |
779.32 |
769.31 |
762.42 |
770.99 |
774.56 |
|
T/K |
298(2) |
298(2) |
298(2) |
298(2) |
298(2) |
298(2) |
298(2) |
| Crystal system |
Triclinic |
Triclinic |
Triclinic |
Triclinic |
Triclinic |
Triclinic |
Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
|
a/Å |
9.5144(6) |
9.5770(9) |
9.528(2) |
9.4878(10) |
9.5121(5) |
9.4951(6) |
9.4926(14) |
|
b/Å |
11.4162(7) |
11.5117(11) |
11.338(3) |
11.3999(12) |
11.4333(6) |
11.3913(7) |
11.3728(17) |
|
c/Å |
12.3690(12) |
12.3980(19) |
12.282(5) |
12.373(2) |
12.3811(10) |
12.3599(13) |
12.345(3) |
|
α/° |
99.4150(10) |
99.6810(10) |
99.109(3) |
99.3880(10) |
99.4600(10) |
99.2940(10) |
99.253(2) |
|
β/° |
100.2000(10) |
100.1280(10) |
100.275(3) |
100.1550(10) |
100.1680(10) |
100.2110(10) |
100.231(2) |
|
γ/° |
114.0100(10) |
114.1460(10) |
114.053(2) |
113.9630(10) |
114.0120(10) |
113.9990(10) |
114.0640(10) |
|
V/Å3 |
1165.63(15) |
1183.2(2) |
1152.1(6) |
1162.1(3) |
1168.14(13) |
1160.68(16) |
1156.5(4) |
|
Z
|
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
D
c/g cm3 |
2.177 |
2.111 |
2.247 |
2.199 |
2.168 |
2.206 |
2.224 |
|
μ/mm−1 |
3.827 |
3.043 |
4.792 |
3.993 |
3.647 |
4.188 |
4.376 |
|
F(000) |
748 |
738 |
758 |
750 |
746 |
752 |
754 |
| GOF |
1.039 |
1.027 |
1.029 |
1.029 |
1.047 |
1.057 |
1.073 |
|
R indices [I > 2σ(I)] |
R
1 = 0.0248 |
R
1 = 0.0240 |
R
1 = 0.0201 |
R
1 = 0.0215 |
R
1 = 0.0240 |
R
1 = 0.0256 |
R
1 = 0.0196 |
|
wR2 = 0.0562 |
wR2 = 0.0551 |
wR2 = 0.0479 |
wR2 = 0.0481 |
wR2 = 0.0536 |
wR2 = 0.0649 |
wR2 = 0.0476 |
|
R indices (all data) |
R
1 = 0.0271 |
R
1 = 0.0270 |
R
1 = 0.0217 |
R
1 = 0.0240 |
R
1 = 0.0266 |
R
1 = 0.0275 |
R
1 = 0.0209 |
|
wR2 = 0.0577 |
wR2 = 0.0571 |
wR2 = 0.0487 |
wR2 = 0.0492 |
wR2 = 0.0552 |
wR2 = 0.0659 |
wR2 = 0.0482 |
Table 2 Selected bond distances (Å) and angles (°) for 1–7a
|
Symmetry codes follow. For 1, #1 −x + 1, −y + 1, −z; #2 −x + 1, −y, −z + 1; #3 −x, −y + 1, −z + 1; #4 −x + 1, −y, −z.
|
| Compound 1 |
| Eu(1)–O(1) |
2.357(3) |
Eu(1)–O(6)#1 |
2.425(2) |
| Eu(1)–O(5) |
2.371(3) |
Eu(1)–O(1W) |
2.454(3) |
| Eu(1)–O(7) #1 |
2.382(3) |
Eu(1)–O(2) |
2.556(3) |
| Eu(1)–O(4) |
2.408(2) |
Cu(1)–N(2) #2 |
1.882(3) |
| Eu(1)–O(3) |
2.409(3) |
Cu(1)–N(1) |
1.888(3) |
| O(1)–Eu(1)–O(4) |
144.49(10) |
O(5)–Eu(1)–O(4) |
73.17(9) |
| O(3)–Eu(1)–O(2) |
52.58(9) |
C(17)–O(3)–Eu(1) |
95.8(2) |
|
O(6)#1–Eu(1)–O(2) |
154.06(9) |
C(17)–O(2)–Eu(1) |
88.8(2) |
|
N(2)#2–Cu(1)–N(1) |
172.76(15) |
S(1)–O(1)–Eu(1) |
164.1(2) |
| S(2)–O(4)–Eu(1) |
143.12(16) |
S(2)–O(7)–Eu(1)#1 |
150.32(16) |
| Compound 2 |
| Ce(1)–O(1) |
2.426(2) |
Ce(1)–O(6)#1 |
2.506(2) |
| Ce(1)–O(5) |
2.465(2) |
Ce(1)–O(1W) |
2.520(2) |
|
Ce(1)–O(7)#1 |
2.459(2) |
Ce(1)–O(2) |
2.620(2) |
| Ce(1)–O(4) |
2.480(2) |
Cu(1)–N(2)#2 |
1.881(3) |
| Ce(1)–O(3) |
2.484(2) |
Cu(1)–N(1) |
1.882(3) |
| O(1)–Ce(1)–O(4) |
145.80(8) |
O(5)–Ce(1)–O(4) |
72.86(8) |
| O(3)–Ce(1)–O(2) |
51.14(7) |
C(17)–O(3)–Ce(1) |
96.0(2) |
|
N(2)#2–Cu(1)–N(1) |
174.65(13) |
S(1)–O(1)–Ce(1) |
168.59(17) |
| Compound 3 |
| Er(1)–O(1) |
2.299(2) |
Er(1)–O(6)#1 |
2.334(2) |
| Er(1)–O(5) |
2.297(2) |
Er(1)–O(1W) |
2.393(2) |
|
Er(1)–O(7)#1 |
2.322(2) |
Er(1)–O(2) |
2.505(3) |
| Er(1)–O(4) |
2.361(2) |
Cu(1)–N(1) |
1.878(3) |
| Er(1)–O(3) |
2.348(2) |
Cu(1)–N(2)#2 |
1.878(3) |
| O(1)–Er(1)–O(4) |
144.00(8) |
O(5)–Er(1)–O(4) |
73.50(8) |
| O(3)–Er(1)–O(2) |
53.78(8) |
C(17)–O(3)–Er(1) |
95.3(2) |
|
N(2)#2–Cu(1)–N(1) |
171.28(13) |
S(1)–O(1)–Er(1) |
162.68(17) |
| Compound 4 |
| Gd(1)–O(1) |
2.340(2) |
Gd(1)–O(6)#1 |
2.412(2) |
| Gd(1)–O(5) |
2.356(2) |
Gd(1)–O(1W) |
2.439(2) |
|
Gd(1)–O(7)#1 |
2.368(2) |
Gd(1)–O(2) |
2.550(2) |
| Gd(1)–O(4) |
2.399(2) |
Cu(1)–N(1) |
1.882(3) |
| Gd(1)–O(3) |
2.402(2) |
Cu(1)–N(2)#2 |
1.877(3) |
| O(1)–Gd(1)–O(4) |
144.11(8) |
O(5)–Gd(1)–O(4) |
73.39(7) |
| O(3)–Gd(1)–O(2) |
52.83(7) |
C(17)–O(3)–Gd(1) |
95.3(2) |
|
N(2)#2–Cu(1)–N(1) |
172.37(12) |
S(1)–O(1)–Gd(1) |
163.28(16) |
| Compound 5 |
| Sm(1)–O(1) |
2.364(2) |
Sm(1)–O(6)#1 |
2.439(2) |
| Sm(1)–O(5) |
2.387(2) |
Sm(1)–O(1W) |
2.458(2) |
|
Sm(1)–O(7)#1 |
2.393(2) |
Sm(1)–O(2) |
2.567(2) |
| Sm(1)–O(4) |
2.422(2) |
Cu(1)–N(1) |
1.881(3) |
| Sm(1)–O(3) |
2.426(3) |
Cu(1)–N(2)#2 |
1.884(3) |
| O(1)–Sm(1)–O(4) |
144.53(9) |
O(5)–Sm(1)–O(4) |
73.11(8) |
| O(3)–Sm(1)–O(2) |
52.41(8) |
C(17)–O(3)–Sm(1) |
95.6(2) |
|
N(2)#2–Cu(1)–N(1) |
172.79(13) |
S(1)–O(1)–Sm(1) |
164.43(18) |
| Compound 6 |
| Tb(1)–O(1) |
2.337(3) |
Tb(1)–O(6)#1 |
2.386(3) |
| Tb(1)–O(5) |
2.345(3) |
Tb(1)–O(1W) |
2.428(3) |
|
Tb(1)–O(7)#1 |
2.354(3) |
Tb(1)–O(2) |
2.541(3) |
| Tb(1)–O(4) |
2.391(3) |
Cu(1)–N(1) |
1.885(3) |
| Tb(1)–O(3) |
2.386(3) |
Cu(1)–N(2)#2 |
1.883(3) |
| O(1)–Tb(1)–O(4) |
144.01(11) |
O(5)–Tb(1)–O(4) |
73.44(10) |
| O(3)–Tb(1)–O(2) |
53.01(9) |
C(17)–O(3)–Tb(1) |
95.4(2) |
|
N(2)#2–Cu(1)–N(1) |
172.02(16) |
S(1)–O(1)–Tb(1) |
162.8(2) |
| Compound 7 |
| Dy(1)–O(1) |
2.319(2) |
Dy(1)–O(6)#1 |
2.373(2) |
| Dy(1)–O(5) |
2.326(2) |
Dy(1)–O(1W) |
2.412(2) |
|
Dy(1)–O(7)#1 |
2.342(2) |
Dy(1)–O(2) |
2.528(2) |
| Dy(1)–O(4) |
2.380(2) |
Cu(1)–N(1) |
1.884(3) |
| Dy(1)–O(3) |
2.369(2) |
Cu(1)–N(2)#2 |
1.882(3) |
| O(1)–Dy(1)–O(4) |
143.98(8) |
O(5)–Dy(1)–O(4) |
73.44(7) |
| O(3)–Dy(1)–O(2) |
53.29(7) |
C(17)–O(3)–Dy(1) |
95.71(19) |
|
N(2)#2–Cu(1)–N(1) |
171.86(12) |
S(1)–O(1)–Dy(1) |
162.45(16) |
Results and discussion
Single-crystal X-ray analysis reveals that 1–7 crystallize in the triclinic space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and posses 3D coordination frameworks based on the linkages of 2D [Ln(nds)(H2O)]+ sheets and [Cu(ina)2]− chains. Because these compounds are isostructural, only the structure of 1 is described in detail. An ORTEP view of 1 is shown in Fig. 1. The asymmetric unit consists of one Eu(III) ion, one Cu(I) ions, one nds ligand, two independent full deprotonated ina ligands, and one coordinated water molecule. The Eu(III) atom is eight-cooordinated in a bicapped trigonal prismatic coordination geometry by four oxygen atoms from the ina ligands, three oxygen atoms from the nds ligands and one oxygen atom from one coordinated water molecule, respectively. The Eu–O bond distances range from 2.357(3) to 2.556(3) Å. The bond angles of O–Eu–O are in the range of 52.58(9) to 144.49(10)°. It is not appropriate to use the terms syn and anti to describe the coordination mode of metal arenedisulfonate, the torsion angles of C–S–O–Eu are used.2,15The torsion angles of C(18)–S(1)–O(1)–Eu(1), C(4)–S(2)–O(7)–Eu(1) and C(4)–S(2)–O(4)–Eu(1) are 113.443(753)°, 84.155(338)° and 76.143(282)°, respectively. The crystallographically Cu(I) center has a nearly linear coordination environment made up of two N atoms from two bridging ina ligands with a Cu–N distance which varies from 1.882(3) to 1.888(3) Å, and N–Cu–N angles of 172.76(15)°. In 2, 5, 1, 4, 6, 7 and 3, the average bond distance of Ln–O is 2.495, 2.432, 2.420, 2.408, 2.396, 2.381 and 2.357 Å, respectively, which exhibits the characteristic of lanthanide contraction.
and posses 3D coordination frameworks based on the linkages of 2D [Ln(nds)(H2O)]+ sheets and [Cu(ina)2]− chains. Because these compounds are isostructural, only the structure of 1 is described in detail. An ORTEP view of 1 is shown in Fig. 1. The asymmetric unit consists of one Eu(III) ion, one Cu(I) ions, one nds ligand, two independent full deprotonated ina ligands, and one coordinated water molecule. The Eu(III) atom is eight-cooordinated in a bicapped trigonal prismatic coordination geometry by four oxygen atoms from the ina ligands, three oxygen atoms from the nds ligands and one oxygen atom from one coordinated water molecule, respectively. The Eu–O bond distances range from 2.357(3) to 2.556(3) Å. The bond angles of O–Eu–O are in the range of 52.58(9) to 144.49(10)°. It is not appropriate to use the terms syn and anti to describe the coordination mode of metal arenedisulfonate, the torsion angles of C–S–O–Eu are used.2,15The torsion angles of C(18)–S(1)–O(1)–Eu(1), C(4)–S(2)–O(7)–Eu(1) and C(4)–S(2)–O(4)–Eu(1) are 113.443(753)°, 84.155(338)° and 76.143(282)°, respectively. The crystallographically Cu(I) center has a nearly linear coordination environment made up of two N atoms from two bridging ina ligands with a Cu–N distance which varies from 1.882(3) to 1.888(3) Å, and N–Cu–N angles of 172.76(15)°. In 2, 5, 1, 4, 6, 7 and 3, the average bond distance of Ln–O is 2.495, 2.432, 2.420, 2.408, 2.396, 2.381 and 2.357 Å, respectively, which exhibits the characteristic of lanthanide contraction.
 |
| | Fig. 1 ORTEP drawing for compound 1 (50% thermal ellipsoids). All H atoms are omitted for clarity. Symmetry code: A (−x + 1, −y, −z); B (−x + 1, −y + 1, −z); C (−x, −y + 1, −z + 1). | |
The ina ligand adopts two kinds of bridging coordination modes: (i) the carboxylate coordinates to two europium centers with a bis-monodendate mode, while the N atom coordinates to one copper centre (Scheme 1a); (ii) the carboxylate coordinates adopt a bidendate mode while the N atom also coordinates to one copper center (Scheme 1b). The nds also acts two kinds of coordination modes: (i) each nds ligand is bonded to only two europium atoms (η1μ1-η1μ1), and leaves four free oxygen atoms (Scheme 1c). (ii) the nds-sulfonate linker adopts the η2μ2-η2μ2 coordination mode, leaving two uncoordinated O atoms (Scheme 1d). Both coordination modes are proved to be crucial in constructing [Ln(nds)(H2O)]+ layers and Cu(ina)2− chains. All carboxyl groups are deprotonated, in agreement with the IR data in which no strong absorption peaks around 1700 cm−1 for –COOH are observed. Both vas and vs of the SO3−groups found at 1240–1180(vas) and 1100–1040(vs) cm−1.
 |
| | Scheme 1 Coordination modes of ina and nds ligands | |
Two Eu(III) centres were linked, through a pair of ina ligands and nds ligands, respectively, and resulted in a dinuclear unit with a Eu⋯Eu distance of 4.5531(4) Å. The connection of adjacent dinuclear units through nds entity adopting a symmetric η1μ1-η1μ1 mode generate infinite 1D zigzag strings as shown in Fig. 2a. The nds ligand also bridging Eu2O16 dimers from one neighbouring chain in the η2μ2-η2μ2 coordination mode (Fig. 2b). In doing that, the structure could be extended in terms of two-dimensional rectangle grid structure of [Ln(nds)(H2O)]+ with a dimension of 11.4 × 10.9 Å, each sharing the Eu2O16 dimers with two differently coordinated nds connectors, as illustrated in Fig. 2d. Undoubtedly, the geometrical features imposed by the position of the sulfonate groups in the nds connector, to our knowledge, is the first example of 2D Ln(nds)(H2O)+ sheets by directional covalent bond. It should be noticed that two different ina ligands give rise to a nearly perpendicular arrangement (dihedral angle 74.954°), which leads to the linear N–Cu–N mode in Cu(ina)2− coordination. The 2D layers are packed into a 3D framework by the bridging Cu(ina)2− chains (Fig. 2c). And the grids are packed without dislocation. From the topological point of view, each Eu/nds layer acts as a six/three-connected node, respectively, and a network is formed with the Schläfli symbol {4·82}{47·5·64·7·82}{42·6} (Fig. 3), providing a rare example of 3d–4f open framework.
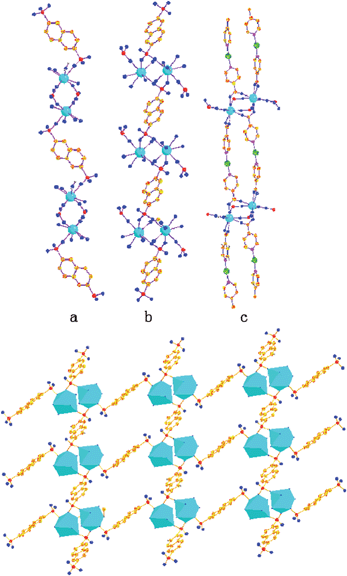 |
| | Fig. 2 (a) Chain 1: infinite 1D zigzag, with a η1μ1-η1μ1 mode between dinuclear unit and nds. (b) Chain 2: view of (Eu2O16)–nds–(Eu2O16) chains, nds bridging dinuclear unit exhibiting the η2μ2-η2μ2 coordination mode. (c) The bridging Cu(ina)2− chain 1D with N–Cu–N mode. (d) Ln(nds)+ layers formed in compound 1. All hydrogen atoms are omitted for clarity. | |
 |
| | Fig. 3 Diagram for the (3,6)-connected net of 1. Green: Eu; yellow: S; dark: C. | |
Luminescent properties
Due to the excellent photoluminescence properties of the lanthanide compounds,16 the luminescence of 1, 5 and 6 containing Eu(III), Sm(III) and Tb(III) ions, respectively, in the solid state were investigated. As shown in Fig. 4, the emission spectrum of 1 upon excitation at 395 nm exhibits the characteristic transition of Eu(III) ion. They are attributed to 5D0 → 7FJ (J = 0 → 4) transitions, i.e. 579 nm (5D0 → 7F0), 592 nm (5D0 → 7F1), 613 and 619 nm (5D0 → 7F2), 654 nm (5D0 → 7F3), 704 nm (5D0 → 7F4). The 5D0 → 7F2 transition is stronger than that of the 5D0 → 7F1 transition, indicating that Eu(III) ions have a low symmetric coordination environment and without an inversion center. This is in agreement with the results of the single-crystal X-ray analysis.17 Compound 5 emits an orange light when excited at 362 nm (Fig. 5), it gives a typical Sm(III) emission spectrum. The emission at 561, 599, and 645 nm correspond to the characteristic emission of 4G5/2 → 6HJ (J =5/2, 7/2, 9/2) transitions of the Sm(III) ion, where the peak at 599 nm is the strongest one. Compound 6 yields green light and exhibits the characteristic emission of 5D4 → 7FJ (J = 3–6) of the Tb(III) ion (Fig. 6). Two intense emission bands at 489 and 544 nm is attributed to 5D4 → 7F6 and 5D4 → 7F5, while the weaker emission bands at 590 nm and 619 nm originate from 5D4 → 7F4 and 5D4 → 7F3.
Thermal stability and PXRD analysis
Owing to the similarity of the structures for 1–7, compound 1 was selected for the TGA to examine the thermal stability of both compounds. The TG curve was performed in air atmosphere from 50 to 750 °C at a heating rate of 10 °C min−1(Fig. S1).† It can be seen from the TG curve that there is no weight loss between 50 and 225 °C. Such a thermal stability of 1 may be attributed to the formation of the 2D layer of [Ln(nds)(H2O)]+ and the 1D chain of Cu(ina)2−. A Weight loss occurs between 225 and 335 °C, accompanied by an endothermic effect corresponding to the loss of the coordinated water molecule per formula unit (found, 2.8%; calcd, 2.4%). Above 335 °C, all of the organic components are removed and the framework is collapsed.
The as-isolated samples of the complexes were also characterized by powder X-ray diffraction at room temperature (Fig. 7 and S1).† When compared to the simulated patterns based on the single crystal data samples, the experimental patterns are in agreement with the calculated diffractograms, thus basically pointing toward the formation of only a single phase product under the reaction conditions employed for these complexes.
 |
| | Fig. 7 The experimental PXRD pattern of complex 4 at room temperature (top). The simulated PXRD pattern from single crystal X-ray data of complex 4 (bottom). | |
Magnetic property
Solid-state dc magnetic susceptibility measurement for compound 4 was performed in the range of 2–300 K under a field of 1000 Oe, and the magnetic behaviour is shown in Fig. 8 as plots of χMTvs.T and χM−1vs.T, where χM is the magnetic susceptibility per [Gd2] unit. The room-temperature χMT value is 15.7 cm3 K mol−1, closed to the spin-only value 15.75 cm3 K mol−1 for two Gd(III) (S = 7/2). As the temperature lowered from 300 to 15 K, the χMT value remained the unchanged value 15.7 cm3 K mol−1, and then decreased steadily to 14.5 cm3 K mol−1 at 2 K, which reveals an overall antiferromagnetic behaviour in compound 4. The temperature dependent χM−1 value obey the Curie–Weiss law with Curie constant C = 15.74 cm3 K mol−1 and Weiss constant θ = −0.15 K. Because of the complicated three-dimensional framework of 4, no exact theoretical expression could be used to simulate the magnetic data. Since the organosulfonic ligands separated the Gd2 units well, the magnetic bahaviour of 4 can be roughly represented by Gd2 interaction expression, and the best fitting parameters can be given as g = 2.01, J = 0.008 cm−1. Because the small positive J value can not explain the decrease of the χMT value at low temperature, the magnetic interaction expression of 4 should be further developed.
 |
| | Fig. 8
χ
M
T vs.
T and χM−1vs.T plots for compound 4. The dots represent the experimental data, and the solid lines represent the best fitting data which were calculated with the method as shown in the text. | |
Conclusions
In summary, seven novel 3D Ln–TM coordination compounds were successfully synthesized by applying hydrothermal strategy. All of the seven compounds were constructed by the linkages of 2D [Ln(nds)(H2O)]+ layers and Cu(ina)2− chains. The 2D layers are the consequence of the naphthalenedisulfonic acid used as linkers with two different coordination mode. It is of interest that the novel (3,6)-connected net exhibited by the seven frameworks are the first examples, in the family of Ln–TM heterometallic coordination polymers with organodisulfonate ligand. The luminescence and magnetic properties have also been discussed. Moreover, the successful syntheses of the title compounds provide a good method for constructing high dimensional coordination polymers by organodisulfonate ligands.
Acknowledgements
This work was financially supported by Guangdong Provincial Science and Technology Bureau (grant 2008B010600009), and NSFC (grant no. 20971047 and U0734005).
References
-
(a) G. A. Lawrance, Chem. Rev., 1986, 86, 17 CrossRef CAS;
(b) A. P. Côté and G. K. H. Shimizu, Coord. Chem. Rev., 2003, 245, 49 CrossRef CAS;
(c) G. K. H. Shimizu, R. Vaidhyanathan and J. M. Taylor, Chem. Soc. Rev., 2009, 38, 1430 RSC.
-
(a) J. W. Cai, C. H. Chen, C. Z. Liao, J. H. Yao, X. P. Hu and X. M. Chen, J. Chem. Soc., Dalton Trans., 2001, 1137 RSC;
(b) J. W. Cai, C. H. Chen, C. Z. Liao, X. L. Feng and X. M. Chen, J. Chem. Soc., Dalton Trans., 2001, 2370 RSC.
-
(a) P. Kanoo, K. L. Gurunatha and T. K. Maji, Cryst. Growth Des., 2009, 9, 4147 CrossRef CAS;
(b) B. D. Chandler, G. D. Enright, S. Pawsey, J. A. Ripmeester, D. T. Cramb and G. K. H. Shimizu, Nat. Mater., 2008, 7, 229 CrossRef CAS;
(c) S. A. Dalrymple and G. K. H. Shimizu, J. Am. Chem. Soc., 2007, 129, 12114 CrossRef CAS;
(d) B. D. Chandler, D. T. Cramb and G. K. H. Shimizu, J. Am. Chem. Soc., 2006, 128, 10403 CrossRef CAS;
(e) B. D. Chandler, J. O. Yu, D. T. Cramb and G. K. H. Shimizu, Chem. Mater., 2007, 19, 4467 CrossRef CAS.
-
(a) F. Gándara, A. García-Cortés, C. Cascales, B. Gómez-Lor, E. Gutiérrez-Puebla, M. Iglesias, A. Monge and N. Snejko, Inorg. Chem., 2007, 46, 3475 CrossRef CAS;
(b) F. Gándara, C. Fortes-Revilla, N. Snejko, E. Gutiérrez-Puebla, M. Iglesias and A. Monge, Inorg. Chem., 2006, 45, 9680 CrossRef CAS.
- X. Ribas, D. Maspoch, K. Wurst, J. Veciana and C. Rovira, Inorg. Chem., 2006, 45, 5383 CrossRef CAS.
-
(a) S. R. Miller, G. M. Pearce, P. A. Wright, F. Bonino, S. Chavan, S. Bordiga, I. Margiolaki, N. Guillou, G. Ferey, S. Bourrelly and P. L. Llewellyn, J. Am. Chem. Soc., 2008, 130, 15967 CrossRef CAS;
(b) S. K. Mäkinen, N. J. Melcer, M. Parvez and G. K. H. Shimizu, Chem.–Eur. J., 2001, 7, 5176 CrossRef CAS;
(c) J. O. Yu, A. P. Côté, G. D. Enright and G. K. H. Shimizu, Inorg. Chem., 2001, 40, 582 CrossRef CAS;
(d) A. P. Côté and G. K. H. Shimizu, Chem. Commun., 2001, 251 RSC;
(e) P. Römbde, A. Schier and H. Schmidbaur, J. Chem. Soc., Dalton Trans., 2001, 2482 RSC;
(f) G. K. H. Shimizu, G. D. Enright, C. I. Ratcliffe and J. A. Ripmeester, Chem. Commun., 1999, 461 RSC;
(g) J. H. Teles, S. Brodes and M. Chabanas, Angew. Chem., Int. Ed., 1998, 37, 1415 CrossRef CAS.
-
(a) H. Wu, X. W. Dong, H. Y. Liu, J. F. Ma, S. L. Li, J. Yang, Y. Y. Liu and Z. M. Su, J. Chem. Soc., Dalton Trans., 2008, 5331 RSC;
(b) Z. X. Lian, J. W. Cai, C. H. Chen and H. B. Luo, CrystEngComm, 2007, 9, 319 RSC;
(c) C. H. Chen and J. W. Cai, Inorg. Chem., 2002, 41, 4967 CrossRef CAS;
(d) A. P. Côté, M. J. Ferguson, K. A. Khan, G. D. Enright, A. D. Kulynych, S. A. Dalrymple and G. K. H. Shimizu, Inorg. Chem., 2002, 41, 287 CrossRef CAS;
(e) J. W. Cai, C. H. Chen, C. Z. Liao, X. L. Feng and X. M. Chen, Acta Crystallogr., Sect. B: Struct. Sci., 2001, 57, 520 CrossRef CAS;
(f) N. S. Hu1sgen, G. A. Luinstra, F. Schaper and K. Schmidt, Inorg. Chem., 1998, 37, 3471 CrossRef.
-
(a) A. P. Côté and G. K. H. Shimizu, Coord. Chem. Rev., 2003, 86, 17;
(b) T. Z. Forbes and S. C. Sevov, Inorg. Chem., 2009, 48, 6873 CrossRef CAS;
(c) N. J. Burke, A. D. Burrows, M. F. Mahon and J. E. Warren, CrystEngComm, 2008, 10, 15 RSC.
- J. W. Cai, Coord. Chem. Rev., 2004, 248, 1061 CrossRef CAS.
-
(a) J. P. Zhao, B. W. Hu, F. C. Liu, X. Hu, Y. F. Zeng and X. H. Bu, CrystEngComm, 2007, 9, 902 RSC;
(b) F. Gándara, J. Perles, N. Snejko, M. Iglesias, B. Gómez-Lor, E. Gutiérrez-Puebla and M. A. Monge, Angew. Chem., Int. Ed., 2006, 45, 7998 CrossRef CAS;
(c) J.-L. Song, C. Lei and J. G. Mao, Inorg. Chem., 2004, 43, 5631;
(d) N. Snejko, C. Cascales, B. Gomez-Lor, E. Gutiérrez-Puebla, M. Iglesias, C. Ruiz-Valero and M. A. Monge, Chem. Commun., 2002, 1366 RSC;
(e) R. G. Xiong, J. Zhang, Z. F. Chen, X. Z. You, C. M. Che and H. K. Fun, J. Chem. Soc., Dalton Trans., 2001, 780 RSC;
(f) G. B. Deacon, A. Gitilits, G. Zelestny, D. Stellfeldt and G. Meyer, Z. Anorg. Allg. Chem., 1999, 625, 764 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533 CrossRef CAS.
-
(a) Z. Y. Li, N. Wang, J. W. Dai, S. T. Yue and Y. L. Liu, CrystEngComm, 2009, 11, 2003 RSC;
(b) N. Wang, S. T. Yue, Y. L. Liu, H. Y. Yang and H. Y. Wu, Cryst. Growth Des., 2009, 9, 368 CrossRef CAS;
(c) Y. F. Zhou, F. L. Jiang, D. Q. Yuan, B. L. Wu, R. H. Wang, Z. Z. Lin and M. C. Hong, Angew. Chem., Int. Ed., 2004, 43, 5665 CrossRef CAS;
(d) Y. F. Zhou, M. C. Hong and X. T. Wu, Chem. Commun., 2006, 135 RSC;
(e) C. M. Zaleski, E. C. Depperman, J. W. Kampf, M. L. Kirk and V. L. Pecoraro, Angew. Chem., Int. Ed., 2004, 43, 3912 CrossRef CAS;
(f) B. Zhao, X. Y. Chen, P. Cheng, D. Z. Liao, S. P. Yan and Z. H. Jiang, J. Am. Chem. Soc., 2004, 126, 15394 CrossRef CAS;
(g) A. D. Cutland-Van Noord, J. W. Kampf and V. L. Pecoraro, Angew. Chem., Int. Ed., 2002, 41, 4667 CrossRef CAS;
(h) F. C. Liu, Y. F. Zeng, J. Jiao, J. R. Li, X. H. Bu, J. Ribas and S. R. Batten, Inorg. Chem., 2006, 45, 6129 CrossRef CAS;
(i) X. Hu, Y. F. Zeng, Z. Chen, E. C. Sañudo, F. C. Liu, J. Ribas and X. H. Bu, Cryst. Growth Des., 2009, 9, 421 CrossRef CAS.
- V. V. Adrabinska, Coord. Chem. Rev., 2007, 251, 1987 CrossRef CAS.
-
(a)
G. M. Sheldrick, SHELXS–97, Program for Crystal Structure Solution, University of Göttingen: Göttingen, Germany, 1997 Search PubMed;
(b)
G. M. Sheldrick, SHELXL–97, Program for Crystal Structure Refinement, University of Göttingen: Göttingen, Germany, 1997 Search PubMed.
-
(a) A. J. Shubnell, E. J. Kosnic and P. J. Squattrito, Inorg. Chim. Acta, 1994, 216, 101 CrossRef CAS;
(b) B. J. Gunderman, I. D. Kabell, P. J. Squattrito and S. N. Dubey, Inorg. Chim. Acta, 1997, 258, 237 CrossRef CAS;
(c) E. J. Kosnic, E. L. McClymont, R. A. Hodder and P. J. Squattrito, Inorg. Chim. Acta, 1992, 201, 143 CrossRef CAS;
(d) C. H. Chen, J. W. Cai, X. L. Feng and X. M. Chen, J. Chem. Crystallogr., 2001, 31, 271 CrossRef CAS;
(e) J. W. Cai, C. H. Chen and J. S. Zhou, Chin. J. Inorg. Chem., 2003, 19, 81;
(f) J. W. Cai, J. S. Zhou and M. L. Lin, J. Mater. Chem., 2003, 13, 1806 RSC.
- Y. Li, F. Zheng, X. Liu, W. Zou, G. Guo, C. Lu and J. Huang, Inorg. Chem., 2006, 45, 6308 CrossRef CAS and references therein.
-
(a) A. de Bettencourt–Dias and S. Viswanathan, Chem. Commun., 2004, 1024 RSC;
(b) Y. Q. Sun, J. Zhang and G. Y. Yang, Chem. Commun., 2006, 4700 RSC.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and posses 3D coordination frameworks based on the linkages of 2D [Ln(nds)(H2O)]+ sheets and [Cu(ina)2]− chains. Because these compounds are isostructural, only the structure of 1 is described in detail. An ORTEP view of 1 is shown in Fig. 1. The asymmetric unit consists of one Eu(III) ion, one Cu(I) ions, one nds ligand, two independent full deprotonated ina ligands, and one coordinated water molecule. The Eu(III) atom is eight-cooordinated in a bicapped trigonal prismatic coordination geometry by four oxygen atoms from the ina ligands, three oxygen atoms from the nds ligands and one oxygen atom from one coordinated water molecule, respectively. The Eu–O bond distances range from 2.357(3) to 2.556(3) Å. The bond angles of O–Eu–O are in the range of 52.58(9) to 144.49(10)°. It is not appropriate to use the terms syn and anti to describe the coordination mode of metal arenedisulfonate, the torsion angles of C–S–O–Eu are used.2,15The torsion angles of C(18)–S(1)–O(1)–Eu(1), C(4)–S(2)–O(7)–Eu(1) and C(4)–S(2)–O(4)–Eu(1) are 113.443(753)°, 84.155(338)° and 76.143(282)°, respectively. The crystallographically Cu(I) center has a nearly linear coordination environment made up of two N atoms from two bridging ina ligands with a Cu–N distance which varies from 1.882(3) to 1.888(3) Å, and N–Cu–N angles of 172.76(15)°. In 2, 5, 1, 4, 6, 7 and 3, the average bond distance of Ln–O is 2.495, 2.432, 2.420, 2.408, 2.396, 2.381 and 2.357 Å, respectively, which exhibits the characteristic of lanthanide contraction.
and posses 3D coordination frameworks based on the linkages of 2D [Ln(nds)(H2O)]+ sheets and [Cu(ina)2]− chains. Because these compounds are isostructural, only the structure of 1 is described in detail. An ORTEP view of 1 is shown in Fig. 1. The asymmetric unit consists of one Eu(III) ion, one Cu(I) ions, one nds ligand, two independent full deprotonated ina ligands, and one coordinated water molecule. The Eu(III) atom is eight-cooordinated in a bicapped trigonal prismatic coordination geometry by four oxygen atoms from the ina ligands, three oxygen atoms from the nds ligands and one oxygen atom from one coordinated water molecule, respectively. The Eu–O bond distances range from 2.357(3) to 2.556(3) Å. The bond angles of O–Eu–O are in the range of 52.58(9) to 144.49(10)°. It is not appropriate to use the terms syn and anti to describe the coordination mode of metal arenedisulfonate, the torsion angles of C–S–O–Eu are used.2,15The torsion angles of C(18)–S(1)–O(1)–Eu(1), C(4)–S(2)–O(7)–Eu(1) and C(4)–S(2)–O(4)–Eu(1) are 113.443(753)°, 84.155(338)° and 76.143(282)°, respectively. The crystallographically Cu(I) center has a nearly linear coordination environment made up of two N atoms from two bridging ina ligands with a Cu–N distance which varies from 1.882(3) to 1.888(3) Å, and N–Cu–N angles of 172.76(15)°. In 2, 5, 1, 4, 6, 7 and 3, the average bond distance of Ln–O is 2.495, 2.432, 2.420, 2.408, 2.396, 2.381 and 2.357 Å, respectively, which exhibits the characteristic of lanthanide contraction.









