From superhydrophobicity and water repellency to superhydrophilicity: smart polymer-functionalized surfaces†
Emmanuel
Stratakis
abc,
Anca
Mateescu
ad,
Marios
Barberoglou
ae,
Maria
Vamvakaki
ab,
Costas
Fotakis
ae and
Spiros H.
Anastasiadis
*ad
aInstitute of Electronic Structure and Laser, Foundation for Research & Technology-Hellas, 711 10 Heraklion, Crete, Greece. E-mail: spiros@iesl.forth.gr
bDepartment of Materials Science and Technology, University of Crete, Heraklion, Crete, Greece
cTechnological Educational Institute of Crete, Heraklion, Crete, Greece
dDepartment of Chemistry, University of Crete, Heraklion, Crete, Greece
eDepartment of Physics, University of Crete, Heraklion, Crete, Greece
First published on 13th May 2010
Abstract
pH-responsive surfaces, reversibly switching between superhydrophilicity and superhydrophobicity/water repellency, are developed by “grafting from” a pH-sensitive polymer onto a hierarchically micro/nano-structured substrate. We quantify the water repellency by investigating the restitution coefficient of water droplets bouncing off the surfaces. The water repellent state requires appropriate hydrophobicity of the functionalizing polymer as well as very low values of contact angle hysteresis.
The understanding and fabrication of functional and responsive surfaces with wetting properties that can be controllably altered on demand have attracted the interest of the scientific community due to their wide variety of potential applications,1 including microfluidic and “lab-on-chip” devices,2,3 drug delivery,4 enzyme immobilization,5 bioseparation,6 cell adhesion,7 chemical and biochemical gates,8 sensor development,9 and self-cleaning surfaces.10 These surfaces are able to reversibly switch their hydrophobicity/hydrophilicity in response to external stimuli.11
The development of water repellent surfaces12,13 has been the focus of current research. The strategy employed consists in mimicking superhydrophobic biosurfaces,14 like those of Nelumbo nucifera leaves (the sacred Lotus),14 by designing rough substrates out of hydrophobic materials.15 It is generally understood that the microstructured rough surface enhances the effect of hydrophobic surface chemistry into superhydrophobicity and water repellency.
Attempts to develop substrates with dual-scale roughness have created surfaces with high contact angles (CA) (∼150–160°) and low contact angle hysteresis (CAH, defined as the difference between the advancing and receding CA) (2.5–5°),16,17 both of which are particularly important with respect to water repellency.18 Functionalization of an artificially roughened substrate with a responsive coating has resulted in surfaces that can, in principle, switch between superhydrophobicity and superhydrophilicity in response to appropriate external stimuli;19 the possibility of multi-responsive surfaces has been explored as well.20 Polymer brushes created by end-grafting stimuli-sensitive polymers onto surfaces with hierarchical micro/nano roughness have been frequently utilized for the development of such smart surfaces. For responsive surfaces, only the static CA has been used to describe the superhydrophobic state whereas achieving low values of CAH was not demonstrated.18 The dynamic behavior of droplets impinging on such smart surfaces remains unexplored as well.
We have recently prepared17,21 artificial surfaces, possessing hierarchical micro- and nano-structures (Fig. S1a†) able to quantitatively mimic both the structure and the water repellence of Lotus leaf. In the present work, we utilize these surfaces to develop polymer functionalized pH-responsive substrates that can reversibly switch between superhydrophilicity at low pH and superhydrophobicity/water repellency at high pH. To the best of our knowledge, this is the first time that such a surface has been realized. To date polymers that become superhydrophobic at low pH and anionic and superhydrophilic at high pH have been reported;20 however, that imposes certain limitations for many applications, e.g., the anionic polymer surfaces cannot interact with DNA, enzymes, and polyanionic drugs by attractive electrostatic interactions. The responsive surfaces were prepared by “grafting from” poly(2-(diisopropylamino)ethyl methacrylate), PDPAEMA, brushes, at anchoring density σ = 0.78 chains nm−2, onto the dual-length-scale roughened substrates using surface-initiated atom transfer radical polymerization (ATRP) (Fig. S2†). Both the micro-scale conical features and the nano-scale protrusions are evident following the functionalization process (Fig. S1b†) verifying that the polymerization reaction did not perturb the hierarchical surface roughness. PDPAEMA homopolymer is a pH-responsive weak polybase, which undergoes reversible protonation/deprotonation upon changing the solution pH. The switching behavior of the surface is driven by the combined effects of the polymer chemical response and the dual-scale roughness. Herein, we quantify the water repellency of the surfaces in their superhydrophobic state by drop elasticity measurements. Moreover, we demonstrate that the water repellent state of the surfaces requires appropriate hydrophobicity of the functionalizing polymer, whereas for the first time we characterize the CAH along with the static CA in the superhydrophobic state of the responsive surfaces.
Images of a water droplet lying on the PDPAEMA functionalized artificial surface are shown in Fig. 1 following successive immersions in solutions at pH 8.5 and pH 2.5. The complete wetting (superhydrophilicity) following the immersion at pH 2.5 and the superhydrophobic behavior (CA 154 ± 1°) following the immersion at pH 8.5 are due to the reversible protonation/deprotonation of DPAEMA (the pKα of PDPAEMA is 6.3). Fig. 1 also shows the respective behavior for a dual-scale roughened surface, functionalized with a poly(2-(diethylamino)ethyl methacrylate), PDEAEMA, brush (the pKα of PDEAEMA is 7.3). The responsive behavior is evident in this case as well; however, the behavior after immersion at pH 10 is far from superhydrophobic with the CA reaching only ∼126 ± 1°. It is noted that the water CA on a PDPAEMA brush on a flat Si surface is ∼88 ± 1° whereas that on a PDEAEMA brush on a flat Si surface is ∼83 ± 1° (both in the deprotonated state). It is, therefore, the combined effect of the dual-scale roughness and the sufficient hydrophobicity of the material22 that is necessary in order for a surface to become superhydrophobic; the incorporation of only two extra methyl groups per monomer unit (Fig. 1) can lead to a “hydrophobic enough” material.
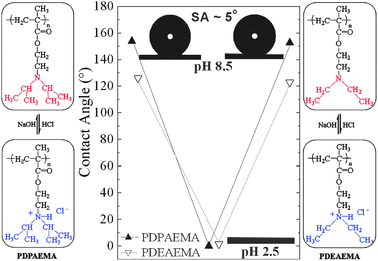 | ||
Fig. 1 Contact angles and images of water droplets residing on the PDPAEMA functionalized hierarchically structured surface following immersion at pH 8.5, pH 2.5 (complete wetting) and again at pH 8.5 ( ). The CA values for the PDEAEMA functionalized surface at pH 10 and pH 3 are shown for comparison ( ). The CA values for the PDEAEMA functionalized surface at pH 10 and pH 3 are shown for comparison ( ). The schemes on the two sides show the protonation/deprotonation process of PDPAEMA and PDEAEMA. ). The schemes on the two sides show the protonation/deprotonation process of PDPAEMA and PDEAEMA. | ||
The average CAs of water drops residing on the PDPAEMA functionalized artificially structured surface are shown in Fig. 2a following successive immersions at pH 8.5 and pH 2.5. It is evident that the responsiveness of the functionalized hierarchically-structured surface holds for at least ten cycles, with very stable values of the CAs of both the superhydrophobic and the superhydrophilic state. It is, thus, proven that the polymer brush synthesized onto the dually-roughened surface is resilient to pH variations, which is especially important since the brush is immersed in basic and strongly acidic solutions. The PDPAEMA brush synthesized onto a flat Si surface is responsive as well (Fig. 2b) with CA value variations, however, that are only within ∼30°.
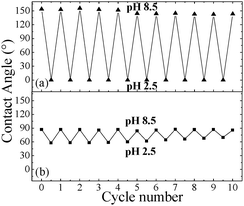 | ||
| Fig. 2 (a) Average CA values of water drops residing on the PDPAEMA functionalized hierarchically structured (a) and flat (b) surfaces following successive immersions at pH 8.5 and pH 2.5. | ||
The kinetics of the hydrophilic/hydrophobic transition determines the speed of the surface responsiveness. Fig. 3 shows the average CAs of water droplets on the PDPAEMA functionalized artificial surface following immersions in a solution at pH 8.5 together with the respective sliding angles (SA23) for 10 μL drops as a function of immersion time. It is clearly seen that, although the equilibrium value of the CA of ∼154° is reached after only about 10 s, the equilibrium value of the SA of ∼5°, which results in water repellency, is only reached after much longer times of ∼40–50 s. Most probably, although the incomplete deprotonation of the polymer brush can be sufficient to result in an apparent hydrophobicity of the surface (CA >150°), the presence of even a few charged monomer units can lead to chemical heterogeneities24 that cause contact line pinning and, thus, high CAH.18
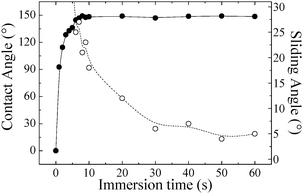 | ||
| Fig. 3 Average CA (●) and SA (○) values for a PDPAEMA functionalized hierarchically structured surface as a function of immersion time in a solution at pH 8.5. | ||
The inset of Fig. 4 presents selected snapshots of a free-falling water droplet (radius 1.35 mm) impinging on the PDPAEMA functionalized artificial surface following its immersion in a pH 8.5 solution. The drop impacts the surface with a velocity that corresponds to a dimensionless Weber number of We = 3.5.25 The surface is so water-repellent that the drop bounces back numerous times and selected maxima of its trajectory are shown as a function of time. The drop finally comes to rest on the surface after ∼250 ms. The full video can be viewed in Movie S1† (the video of a droplet completely wetting the surface in the superhydrophilic state can be viewed in Movie S2†). The elasticity of the collisions on the PDPAEMA brush-functionalized artificially-structured surface indicates a high degree of repellency. A direct measure of this elasticity is the restitution coefficient, ε = V′/V, defined as the ratio of the center of mass velocity of a droplet just after impact, V′, to that just before impact, V. This coefficient is deduced from the recorded video images and is shown in Fig. 4 as a function of the impact velocity V in comparison with that of the natural Lotus leaf.17 The highest elasticity is observed at intermediate velocities, from ∼0.18 m s−1 to ∼0.30 m s−1, where the restitution coefficient exceeds 0.90. For small velocities, ε decreases abruptly with decreasing V and reaches zero at a certain velocity (0.17 m s−1). This is the threshold that quantifies the water repellency of the surface; the smaller this velocity, the more water repellent the surface is. The similarity of the restitution coefficient (to our knowledge, this is among the highest ever reported18) as well as the threshold velocity value (necessary to avoid sticking of the drops) between the functionalized artificial surface and the Lotus leaf surface is evident. It is considered that the bouncing to non-bouncing transition arises from the presence of surface defects; the contact line pins on defects resulting in a difference between advancing and receding CA, θa and θr, i.e., in hysteresis. The pinning force per unit length is F = γLV(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θr − cos
θr − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θa) = γLVΔ(cos
θa) = γLVΔ(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ);10 the energy dissipated will, thus, scale as γLVR2Δ(cos
θ);10 the energy dissipated will, thus, scale as γLVR2Δ(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ). The drop will bounce provided that its kinetic energy scaling as ρR3V2 can overcome this dissipation. An estimate of the threshold velocity for water repellency can be obtained by equating the two energies. The relative CAs were measured as θa = 154 ± 1° and θr = 149 ± 1° for the PDPAEMA superhydrophobic surface. For a drop with radius R ∼ 1.35 mm, the estimated threshold velocity is calculated as ∼0.07 m s−1, a value close to that observed experimentally.
θ). The drop will bounce provided that its kinetic energy scaling as ρR3V2 can overcome this dissipation. An estimate of the threshold velocity for water repellency can be obtained by equating the two energies. The relative CAs were measured as θa = 154 ± 1° and θr = 149 ± 1° for the PDPAEMA superhydrophobic surface. For a drop with radius R ∼ 1.35 mm, the estimated threshold velocity is calculated as ∼0.07 m s−1, a value close to that observed experimentally.
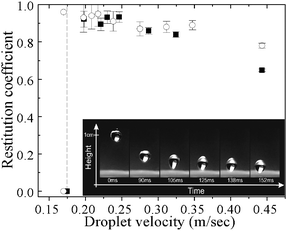 | ||
| Fig. 4 Restitution coefficient as a function of the impact velocity for the PDPAEMA functionalized surface, after immersion at pH 8.5 (■), and a natural Lotus leaf surface17 (○). The dashed line signifies the threshold velocity. Inset: Selected snapshots of a water drop impinging on the surface for We = 3.5.24 Maxima of the drop trajectory are shown as a function of time. | ||
In summary, pH-responsive surfaces were developed, which can reversibly switch between superhydrophilic at low pH and superhydrophobic and water repellent at high pH. The surfaces were developed by “grafting from” a pH-sensitive polymer brush onto controlled hierarchically micro- and nano-structured substrates, which mimic the hierarchical morphology of natural water repellent surfaces. The responsive behavior is due to the combined effect of the hierarchical micro- and nano-roughness and the appropriate hydrophobicity of the functionalizing polymer brush. We show the kinetic behavior for achieving low values of the CAH, which, together with high values of the static CA, are required for superhydrophobicity and water repellency.
This work was supported by the Integrated European Laser Laboratories LASERLAB-EUROPE (Contract No. RII3-CT-2003-506350), the Greek General Secretariat of Research and Technology (ΠENEΔ Programme 03ΕΔ581), and NATO's Scientific Affairs Division (Science for Peace Programme).
Notes and references
- M. A. Cohen Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101 CrossRef.
- B. Zhao, J. S. Moore and D. J. Beebe, Science, 2001, 291, 1023 CrossRef CAS.
- D. J. Beebe, J. S. Moore, Q. Yu, R. H. Liu, M. L. Kraft, B.-H. Jo and C. Devadoss, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 13488 CrossRef CAS.
- D. A. LaVan, T. McGuire and R. Langer, Nat. Biotechnol., 2003, 21, 1184–1191 CrossRef.
- K. Christian, H. Andreas and S. Wolfgang, Anal. Chem., 2002, 74, 355 CrossRef CAS.
- I. Roy, M. V. S. Rao and M. N. Gupta, Biotechnol. Appl. Biochem., 2003, 37, 9 CrossRef CAS.
- J.-I. Edahiro, K. Sumaru, Y. Tada, K. Ohi, T. Takagi, M. Kameda, T. Shinbo, T. Kanamori and Y. Yoshimi, Biomacromolecules, 2005, 6, 970 CrossRef CAS.
- I. Tokarev and S. Minko, Adv. Mater., 2009, 21, 241 CrossRef CAS.
- C. M. Ruan, K. G. Ong, C. Mungle, M. Paulose, N. J. Nickl and C. A. Grimes, Sens. Actuators, B, 2003, 96, 61 CrossRef.
- R. Blossey, Nat. Mater., 2003, 2, 301 CrossRef CAS.
- T. P. Russell, Science, 2002, 297, 964 CrossRef CAS; S. H. Anastasiadis, H. Retsos, S. Pispas, N. Hadjichristidis and S. Neophytides, Macromolecules, 2003, 36, 1994 CrossRef CAS; W. Jiang, G. Wang, Y. He, X. Wang, Y. An, Y. Song and L. Jiang, Chem. Commun., 2005, 3550 RSC; A. Athanassiou, M. I. Lygeraki, D. Pisignano, K. Lakiotaki, M. Varda, C. Fotakis, R. Cingolani and S. H. Anastasiadis, Langmuir, 2006, 22, 2329 CrossRef CAS; T. N. Krupenkin, J. A. Taylor, E. N. Wang, P. Kolodner, M. Hodes and T. R. Salamon, Langmuir, 2007, 23, 9128 CrossRef CAS; P. F. Xia, Y. Zhu, L. Feng and L. Jiang, Soft Matter, 2009, 5, 275 RSC.
- P. Roach, N. J. Shirtcliffe and M. I. Newton, Soft Matter, 2008, 4, 224 RSC.
- X. Zhang, F. Shi, J. Niu, Y. G. Jiang and Z. Q. Wang, J. Mater. Chem., 2008, 18, 621 RSC; F. Xia and L. Jiang, Adv. Mater., 2008, 20, 2842 CrossRef CAS.
- W. Barthlott and C. Neinhuis, Planta, 1997, 202, 1 CrossRef CAS.
- T. L. Sun, L. Feng, C. F. Gao and L. Jiang, Acc. Chem. Res., 2005, 38, 644 CrossRef CAS; X.-M. Li, D. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 1350 RSC; D. Quéré, Rep. Prog. Phys., 2005, 68, 2495 CrossRef.
- M. Miwa, A. Nakajima, A. Fujishima, K. Hashimoto and T. Watanabe, Langmuir, 2000, 16, 5754 CrossRef CAS; N. J. Shirtcliffe, G. McHale, M. I. Newton, G. Chabrol and C. C. Perry, Adv. Mater., 2004, 16, 1929 CrossRef CAS.
- V. Zorba, E. Stratakis, M. Barberoglou, E. Spanakis, P. Tzanetakis, S. H. Anastasiadis and C. Fotakis, Adv. Mater., 2008, 20, 4049 CrossRef CAS.
- M. Callies and D. Quéré, Soft Matter, 2005, 1, 55 RSC; D. Quéré, Annu. Rev. Mater. Res., 2008, 38, 71 CrossRef CAS; C. W. Extrand, Langmuir, 2002, 18, 7991 CrossRef CAS.
- S. Minko, M. Müller, M. Motornov and M. Nitschke, J. Am. Chem. Soc., 2003, 125, 3896 CrossRef CAS; H. S. Lim, D. Kwak, D. Y. Lee, S. G. Lee and K. Cho, J. Am. Chem. Soc., 2007, 129, 4128 CrossRef CAS.
- F. Xia, H. Ge, Y. Hou, T. Sun, L. Chen, G. Z. Zhang and L. Jiang, Adv. Mater., 2007, 19, 2520 CrossRef CAS; F. Xia, L. Feng, S. Wang, T. Sun, W. Song, W. Jiang and L. Jiang, Adv. Mater., 2006, 18, 432 CrossRef CAS.
- V. Zorba, L. Persano, D. Pisignano, A. Athanassiou, E. Stratakis, R. Cingolani, P. Tzanetakis and C. Fotakis, Nanotechnology, 2006, 17, 3234 CrossRef CAS.
- Q. Zhang, F. Xia, T. Sun, W. Song, T. Zhao, M. Liu and L. Jiang, Chem. Commun., 2008, 1199 RSC.
- The SA, α, is defined as the critical angle above which a droplet begins to slide down the inclined surface; it is used as a criterion for assessing the dynamic hydrophobicity of a surface. It is related to the CAH by the equation mgsin
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (α)/2R = γLV(cos
(α)/2R = γLV(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θr − cos
θr − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θa) = γLVΔ(cos
θa) = γLVΔ(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), where m is the mass of the drop and R its radius, g the gravitational constant, θa and θr the advancing and receding CA, and γLV the surface tension. For a drop of radius R = 1.35 mm (10 μL volume) and for θa = 154°, a sliding angle of 5° corresponds to a CAH, θa − θr, of ∼5°.
θ), where m is the mass of the drop and R its radius, g the gravitational constant, θa and θr the advancing and receding CA, and γLV the surface tension. For a drop of radius R = 1.35 mm (10 μL volume) and for θa = 154°, a sliding angle of 5° corresponds to a CAH, θa − θr, of ∼5°. - R. E. Johnson Jr. and R. H. Dettre, Adv. Chem., 1964, 43, 112 Search PubMed; R. E. Johnson Jr. and R. H. Dettre, J. Phys. Chem., 1964, 68, 1744 CrossRef.
- The Weber number is defined as We = ρV2R/γLV, where ρ is the liquid density and V the drop impact velocity.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, SEM images of the surfaces, schematic representation of the functionalization procedure and movies of water droplets on superhydrophobic and superhydrophilic surfaces. See DOI: 10.1039/c003294h |
| This journal is © The Royal Society of Chemistry 2010 |
