Enzyme logic gate associated with a single responsive microparticle: scaling biocomputing to microsize systems†
Valeriya
Bychkova
b,
Alexey
Shvarev
*b,
Jian
Zhou
a,
Marcos
Pita
a and
Evgeny
Katz
*a
aDepartment of Chemistry and Biomolecular Science, and NanoBio Laboratory (NABLAB), Clarkson University, Potsdam, NY 13699-5810, USA. E-mail: ekatz@clarkson.edu; Fax: +1-315-2686610; Tel: +1-315-2684421
bDepartment of Chemistry, Oregon State University, Corvallis, OR 97331, USA. E-mail: Alexey.Shvarev@oregonstate.edu; Fax: +1-541-7372062; Tel: +1-541-7376704
First published on 3rd November 2009
Abstract
A microsize biocomputing system based on enzyme logic processing biochemical signals was developed. Optical transduction of pH signals generated in situ by the enzyme OR logic gate was achieved with the use of a single optode microparticle.
Unconventional chemical1 and biochemical2 computing systems meet several challenging problems: scaling up their complexity to mimic computing networks, scaling down their size to micro- and nanosystems and improving information processing for low noise performance. Different biomolecular tools, including proteins/enzymes,3DNA,4RNA,5 and whole cells6 were used to assemble computing systems processing biochemical information. Recently emerged enzyme logic systems7 have already achieved a complexity level to process several biochemical signals, mimicking logic networks composed of 3–4 logic gates,8 while their optimization predicts low noise performance up to 10 concatenated gates.9 Enzyme logic gates and their networks have been coupled with various signal-responsive materials, providing bulk material property changes in response to biochemical signals processed by the enzymes.10 Logic operations performed at the nanoscale11 or even at the single molecule level12 have been reported for non-biochemical systems. The present communication is the first report on an enzyme logic system coupled with a single responsive microparticle aiming towards the miniaturization of biocomputing systems. Functional coupling of enzyme logic systems with signal-responsive materials has been realized via bulk10 or local13 pH changes generated by enzyme reactions in situ, resulting in the transition of switchable materials between two distinct states (e.g. shrunken and swollen polymers). It should be noted that pH-controlled chemical transformations have also been used in various chemical logic systems without enzymes.14 In the present work we used a single pH-sensitive microparticle to report on the pH changes generated by an enzyme logic system.
Our OR logic gate system consisted of glucose oxidase (GOx, from Aspergillus niger, type X-S, E.C. 1.1.3.4; 3.1 unit mL−1) and esterase (Est, from porcine liver, E.C. 3.1.1.1; 3.1 unit mL−1) in a non-buffered 0.1 M Na2SO4 solution adjusted to an initial pH of 6.5 ± 0.3. The chemical inputs were 10 mM ethyl butyrate (input A) and 10 mM glucose (input B). Logic “0” and “1” in the input combinations refer to the absence and presence of the certain substance, respectively. The chemical input signals were applied to the system in four different combinations: 0,0; 0,1; 1,0 and 1,1, where the first notation corresponds to signal A and the second to signal B. The enzyme system responded to the signals by oxidation of glucose biocatalyzed by GOx resulting in gluconic acid (note that O2 was always present in the solution) and by hydrolysis of ethyl butyrate biocatalyzed by Est to yield butyric acid. Either (upon input signals 0,1; 1,0) or both (upon input signals 1,1) produced acids resulted in the pH decrease finally reaching pH 4.6 ± 0.6. Only in the absence of both the input signals (0,0) did the system preserve its initial pH value, thus resembling the Boolean OR logic function. After the pH was decreased (inputs 0,1; 1,0; 1,1), urease (from jack beans, E.C. 3.5.1.5; 10 unit mL−1) was added to the solution, performing the Reset function activated by the addition of 4 mM urea, thus resulting in the biocatalytic formation of ammonia and increasing the pH to the initial level, Scheme 1.
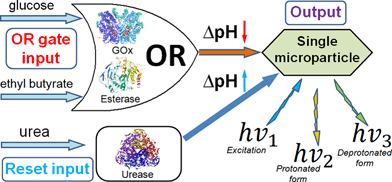 | ||
| Scheme 1 The OR–Reset enzyme system based on GOx–Est–Urease concerted operation producing in situ pH changes as the output signal when activated by glucose, ethyl butyrate and urea. | ||
The pH changes generated in situ were detected by a single pH-responsive optode microparticle, 6.5 ± 0.5 μm in diameter. A typical ion-selective optode15 contains an ionophoreL, which selectively forms a complex with a primary metal ionIz+ (Na+ in the present study) and a chromoionophore Ind, a lipophilic dye, that interacts with a reference ion (H+ in the present study) and changes optical properties upon protonation. The third additive is a lipophilic cation-exchanger. Because the concentration of ion-exchanger in the matrix is limited, the competition between two ions (Na+ and H+) for the ion-exchange sites affects the fraction of protonated chromoionophore IndH+ and determines the microsensor response. With the assumption that nI is a stoichiometry of the ion-ionophore complex and zI is the charge of the primary ion, the theoretical optode response function obeys eqn (1),15 where Kexch is the ion-exchange constant.
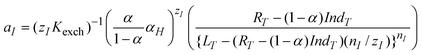 | (1) |
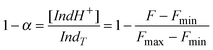 | (2) |
The pH-reporting microparticle was immobilized on a glass support and the optical signals were read by an inverted fluorescence microscope (see details in the ESI† ). The fluorescence spectrum of the microparticle was determined by the ion exchange controlled by the pH value, Fig. 1, thus allowing pH analysis in the solution adjacent to the microparticle. The chromoionophore protonated fraction (1 − α) was calculated from the fluorescence spectra, Fig. 1, inset, using eqn (2). The microsensor stability, good reversibility and short response time allowed us to use a single optode microparticle for the continuous measurements and to control the localized rapid pH changes.16
![The protonated fraction of the chromoionophore in the optode microparticle as a function of the pH value. Inset: The fluorescence spectra of the fully unprotonated, 10 mM NaOH, (a) and fully protonated, 10 mM HCl, (b) chromoionophore: 9-(diethylamino)-5-octadecanoylimino-5H-benzo[a]phenoxazine. (NFI = normalized fluorescence intensity).](/image/article/2010/CC/b917611j/b917611j-f1.gif) | ||
| Fig. 1 The protonated fraction of the chromoionophore in the optode microparticle as a function of the pH value. Inset: The fluorescence spectra of the fully unprotonated, 10 mM NaOH, (a) and fully protonated, 10 mM HCl, (b) chromoionophore: 9-(diethylamino)-5-octadecanoylimino-5H-benzo[a]phenoxazine. (NFI = normalized fluorescence intensity). | ||
The pH evolutions generated in situ by the enzyme OR logic gate, Fig. 2, followed by the Reset function were reported by a single microparticle in the form of fluorescence spectrum changes. A rough estimation, based on the amount of the pH-sensitive fluorescent dye in the microparticle (ca. 8.7 × 109 molecules), the concentration/activity of the enzymes and the time-period of the biocatalytic reactions, resulted in the conclusion that ca. 1 × 104 enzyme molecules localized in ca. 100–150 nm of the solution adjacent to the microparticle are able to produce the required pH changes. The diffusion layer thickness is relatively small compared to the sphere radius of the microparticle, meaning that the diffusion is fast (less than 10 μs) and the reaction occurs on the surface of the optode.
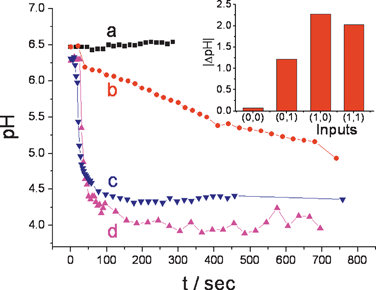 | ||
| Fig. 2 In situ pH changes generated by the enzyme OR logic gate and reported by the single optode microparticle upon application of the input signals: (a) 0,0; (b) 0,1; (c) 1,0 and (d) 1,1. Inset: Bar diagram featuring the pH changes generated by the different combinations of inputs. | ||
In a control experiment, the enzyme-generated pH changes were produced in the absence of the chromoionophore-functionalized microparticles. In this case no fluorescence was detected in the system.
It should be noted that in the present preliminary study the whole solution containing the reacting enzymes and their substrates produced the pH changes. However, only the adjacent thin-layer of the solution affected the fluorescent output signal generated by the microparticle. The obtained results provide the background for the next step, when the enzymes will be immobilized on the microparticle surface allowing not only their functional, but also spatial integration with the reporting microparticle. Also the complexity of the enzyme-signal processing system could be easily scaled up by applying multi-enzyme systems converting many biochemical input signals to the pH changes and mimicking computing networks.8b
The present work opens the way to enzyme logic networks associated with single responsive microparticles dramatically miniaturizing biocomputing systems. We anticipate that such miniaturized enzyme logic gates processing biochemical signals will find numerous analytical and biomedical applications, facilitate decision-making in connection to autonomous feedback-loop drug-delivery systems and will revolutionize monitoring and treatment of patients.
NSF grants DMR-0706209, CCF-0726698, ONR grant N00014-08-1-1202 and SRC award 2008-RJ-1839G are gratefully acknowledged by the Clarkson University team. The authors from OSU acknowledge ONR and ONAMI for financial support (N00014-07-1-0457).
Notes and references
- (a) A. P. De Silva and S. Uchiyama, Nat. Nanotechnol., 2007, 2, 399–410 CrossRef CAS; (b) K. Szacilowski, Chem. Rev., 2008, 108, 3481–3548 CrossRef CAS; (c) A. P. De Silva, S. Uchiyama, T. P. Vance and B. Wannalerse, Coord. Chem. Rev., 2007, 251, 1623–1632 CrossRef.
- (a) X. G. Shao, H. Y. Jiang and W. S. Cai, Huaxue Jinzhan, 2002, 14, 37–46 Search PubMed; (b) P. Fu, Biotechnol. J., 2007, 2, 91–101 Search PubMed; (c) Y. Benenson, T. Paz-Elizur, R. Adar, E. Keinan, Z. Livneh and E. Shapiro, Nature, 2001, 414, 430–434 CrossRef CAS.
- (a) S. Sivan and N. Lotan, Biotechnol. Prog., 1999, 15, 964–970 CrossRef CAS; (b) S. Sivan, S. Tuchman and N. Lotan, BioSystems, 2003, 70, 21–33 CrossRef CAS; (c) A. S. Deonarine, S. M. Clark and L. Konermann, Future Gener. Comput. Syst., 2003, 19, 87–97 CrossRef; (d) G. Ashkenazi, D. R. Ripoll, N. Lotan and H. A. Scheraga, Biosens. Bioelectron., 1997, 12, 85–95 CrossRef CAS; (e) R. Unger and J. Moult, Proteins: Struct., Funct., Bioinf., 2006, 63, 53–54 Search PubMed.
- (a) M. N. Stojanovic, D. Stefanovic, T. LaBeanH. Yan, in Bioelectronics: From Theory to Applications, ed. I. Willner and E. Katz, Wiley-VCH, Weinheim, 2005, ch. 14, pp. 427–455 Search PubMed; (b) A. Saghatelian, N. H. Volcker, K. M. Guckian, V. S. Y. Lin and M. R. Ghadiri, J. Am. Chem. Soc., 2003, 125, 346–347 CrossRef CAS; (c) G. Ashkenasy and M. R. Ghadiri, J. Am. Chem. Soc., 2004, 126, 11140–11141 CrossRef CAS.
- M. N. Win and C. D. Smolke, Science, 2008, 322, 456–460 CrossRef CAS.
- M. L. Simpson, G. S. Sayler, J. T. Fleming and B. Applegate, Trends Biotechnol., 2001, 19, 317–323 CrossRef CAS.
- (a) G. Strack, M. Pita, M. Ornatska and E. Katz, ChemBioChem, 2008, 9, 1260–1266 CrossRef CAS; (b) R. Baron, O. Lioubashevski, E. Katz, T. Niazov and I. Willner, J. Phys. Chem. A, 2006, 110, 8548–8553 CrossRef CAS.
- (a) G. Strack, M. Ornatska, M. Pita and E. Katz, J. Am. Chem. Soc., 2008, 130, 4234–4235 CrossRef CAS; (b) M. Privman, T. K. Tam, M. Pita and E. Katz, J. Am. Chem. Soc., 2009, 131, 1314–1321 CrossRef CAS.
- (a) V. Privman, G. Strack, D. Solenov, M. Pita and E. Katz, J. Phys. Chem. B, 2008, 112, 11777–11784 CrossRef CAS; (b) V. Privman, M. A. Arugula, J. Halámek, M. Pita and E. Katz, J. Phys. Chem. B, 2009, 113, 5301–5310 CrossRef CAS.
- (a) I. Tokarev, V. Gopishetty, J. Zhou, M. Pita, M. Motornov, E. Katz and S. Minko, ACS Appl. Mater. Interfaces, 2009, 1, 532–536 Search PubMed; (b) M. Motornov, J. Zhou, M. Pita, I. Tokarev, V. Gopishetty, E. Katz and S. Minko, Small, 2009, 5, 817–820 CrossRef CAS; (c) M. Motornov, J. Zhou, M. Pita, V. Gopishetty, I. Tokarev, E. Katz and S. Minko, Nano Lett., 2008, 8, 2993–2997 CrossRef CAS.
- (a) A. P. De Silva, Y. Leydet, C. Lincheneau and N. D. McClenaghan, J. Phys.: Condens. Matter, 2006, 18, S1847–S1872 CrossRef; (b) A. Ogawa and M. Maeda, Chem. Commun., 2009, 4666–4668 RSC.
- R. Stadler, S. Ami, C. Joachim and M. Forshaw, Nanotechnology, 2004, 15, S115–S121 CrossRef CAS.
- M. Pita, T. K. Tam, S. Minko and E. Katz, ACS Appl. Mater. Interfaces, 2009, 1, 1166–1168 Search PubMed.
- S. Silvi, E. C. Constable, C. E. Housecroft, J. E. Beves, E. L. Dunphy, M. Tomasulo, F. M. Raymo and A. Credi, Chem.–Eur. J., 2009, 15, 178–185 CrossRef CAS.
- E. Bakker, P. Buhlmann and E. Pretsch, Chem. Rev., 1997, 97, 3083–3132 CrossRef CAS.
- V. Bychkova and A. Shvarev, Anal. Chem., 2009, 81, 2325–2331 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of optode microparticles and single-particle measurements of the signals generated by the enzyme logic gate. See DOI: 10.1039/b917611j |
| This journal is © The Royal Society of Chemistry 2010 |
