ssPNA templated assembly of oligo(p-phenylenevinylene)s†
Pim G. A.
Janssen
a,
Nico
Meeuwenoord
b,
Gijs
van der Marel
b,
Sara
Jabbari-Farouji
c,
Paul
van der Schoot
c,
Mathieu
Surin
d,
Željko
Tomović
a,
E. W.
Meijer
a and
Albertus P. H. J.
Schenning
*a
aLaboratory for Macromolecular and Organic Chemistry, Eindhoven University of Technology, P. O. Box 513, 5600 MB Eindhoven, The Netherlands. E-mail: a.p.h.j.schenning@tue.nl; Fax: +31 40 245 1036; Tel: +31 40 247 2655
bLaboratory of Bio-organic Synthesis, Leiden Institute of Chemistry, Gorlaeus Laboratories, Leiden University, 2300 RA Leiden, The Netherlands
cGroup Theory of Polymers and Soft Matter, P. O. Box 513, 5600 MB Eindhoven, The Netherlands
dLaboratory for Chemistry of Novel Materials, University of Mons-Hainaut, B-7000, Mons, Belgium
First published on 11th November 2009
Abstract
A single-stranded oligothymine peptide nucleic acid (PNA) was used as a template for the assembly of a chiral oligo(p-phenylenevinylene) diaminotriazine derivative (OPV) in methylcyclohexane (MCH) revealing nanostructures in which the size is controlled by the template.
In nature, templates with specific binding sites are used to efficiently form assemblies and polymers with definite size or sequence.1 This behavior has inspired many researchers to exploit templated polymerization2,3 as a tool to control the size and sequence of synthetic polymersvia a ‘bottom-up’ approach.4–8 Especially oligonucleotides are interesting building-blocks, since they can be obtained monodisperse, functionalized and used to create predefined nanosized structures via sticky-end cohesion.9
In a previous study, we showed that the single-stranded desoxyribonucleic acid (ssDNA) oligothymine can act as a template for the assembly of complementary diaminotriazine equipped guest molecules in water.7 In this construct, the single DNA strand templates a supramolecular strand of chromophores held together by π–π, hydrophobic and hydrogen bond interactions. The efficiency of this templated assembly depends on the host–guest and guest–guest interaction and can be described by a templated assembly model based on a one-dimensional Ising model.7a The use of DNA as template requires water as solvent10 and therefore the variety of guest molecules is limited. In order to broaden the scope of this templated approach to organic solvents, we now report on the use of a single-stranded peptide nucleic acid (ssPNA), consisting of 10 thymine residues (pT10,‡Scheme 1), as a template for the assembly of a chiral π-conjugated oligo(p-phenylenevinylene) diaminotriazine derivative11 (OPV, Scheme 1) in MCH. PNA12 is an achiral and uncharged analogue of DNA in which the phosphate backbone is replaced by an N-(2-aminoethyl)glycine backbone, making it soluble in a range of organic solvents. We have previously shown that OPV forms hydrogen bonded hexamers that subsequently self-assemble into helical fibers in heptane.11a Here, we describe the non-templated self-assembly and pT10 templated assembly process of OPV studied by means of temperature-dependent UV-vis absorption and CD spectroscopy. The assemblies were visualized with atomic force microscopy (AFM).
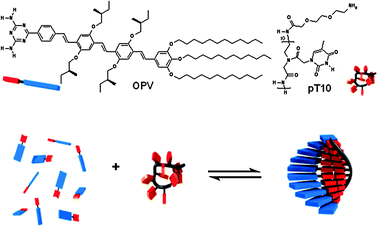 | ||
| Scheme 1 Molecular structures of the host template pT10 and the guest OPV and a schematic representation of ssPNA templated self-assembly (in blue and red OPV, and in black and red the PNA template). | ||
The synthesis of OPV11a and pT1013 were performed according to literature procedures. We have first investigated the non-templated self-assembly of OPV. In chloroform, OPV is molecularly dissolved and has an absorption maxima λmax at 430 nm. In MCH14 at 323 K, OPV (100 μM) is molecularly dissolved since a similar absorption maximum is found. Upon cooling to 263 K, hypochromicity, a red shift of the onset of the absorption, and an absorption maximum shifting from λmax = 430 to 440 nm (Fig. 1a) are observed.15 Simultaneously, at low temperatures, a positive Cotton effect is observed with a zero-crossing at λz−c = 434 nm,16 indicating that OPV self-assembles into right-handed helical aggregates, similar as earlier observed in heptane.11 The non-templated self-assembly process has been studied in more detail by monitoring the UV absorption at λ = 500 nm as a function of temperature at different concentrations. The self-assembly is fully reversible and the observed exponential transition is indicative of a cooperative non-templated self-assembly process.11,17 By fitting both the concentration- and the temperature-dependent self-assembly data to the cooperative self-assembly model,17 the enthalpy of binding (ΔHe ≈ −75 ± 8 kJ mol−1) was determined (Fig. 1e and f).17 To visualize the OPV assemblies, a MCH-solution has been drop-cast onto graphite (HOPG). AFM micrographs show the formation of fibers with a 4–6 nm height (Fig. 2a, c). This height corresponds well with the diameter of the fibers consisting of hexameric H bonded rosettes earlier reported for OPV in heptane.11a
![Absorption and CD spectra at temperatures between 323 and 263 K for OPV (a and c, respectively) and pT10–OPV (1 : 10) (b and d, respectively) mixtures in MCH. (e) The self-assembled fraction upon cooling of OPV and OPV–pT10 (10 : 1) mixtures and the fits to the cooperative self-assembly model17 and templated assembly model,7a respectively. [pT10] = 10 μM, [OPV] = 100 μM. (f) Te and Tet (inverted scale) as a function of [OPV] (logarithmic scale) for OPV and pT10–OPV (1 : 10) mixtures.](/image/article/2010/CC/b913307k/b913307k-f1.gif) | ||
| Fig. 1 Absorption and CD spectra at temperatures between 323 and 263 K for OPV (a and c, respectively) and pT10–OPV (1 : 10) (b and d, respectively) mixtures in MCH. (e) The self-assembled fraction upon cooling of OPV and OPV–pT10 (10 : 1) mixtures and the fits to the cooperative self-assembly model17 and templated assembly model,7a respectively. [pT10] = 10 μM, [OPV] = 100 μM. (f) Te and Tet (inverted scale) as a function of [OPV] (logarithmic scale) for OPV and pT10–OPV (1 : 10) mixtures. | ||
![Atomic force micrographs of (a) OPV (3 × 3 μm) and (b) pT10–OPV (1 × 1 μm) solutions drop-casted on HOPG at 273 K and the corresponding height cross-sections below. [pT10] = 10 μM, [OPV] = 100 μM.](/image/article/2010/CC/b913307k/b913307k-f2.gif) | ||
| Fig. 2 Atomic force micrographs of (a) OPV (3 × 3 μm) and (b) pT10–OPV (1 × 1 μm) solutions drop-casted on HOPG at 273 K and the corresponding height cross-sections below. [pT10] = 10 μM, [OPV] = 100 μM. | ||
To investigate the PNA templated assembly of OPV, a base-equivalent of pT10 was added to a 200 μM solution of OPV in chloroform at 263 K. No Cotton effect and spectral changes were observed in the OPV absorption region indicating that there is no interaction between OPV and pT10. To increase the host–guest and guest–guest interaction in the templated PNA assembly, MCH was used as a solvent. In this solventOPV itself already forms self-assembled fibers (vide supra) which have to be less stable than the proposed OPV–pT10 constructs. When OPV is mixed with a base-equivalent of pT10 in MCH at 323 K and cooled down to 263 K, hypochromicity is accompanied by a blue shift of λmax to 425 nm and a red shift of the onset (Fig. 1b). Compared to the non-templated OPVself-assembly, the OPV–pT10 mixture has a lower intensity of the Cotton effect (Fig. 1d). Furthermore, the zero-crossing of the Cotton effect λz−c is 410 nm16 for the pT10–OPV mixture, while for the OPV, λz−c is 434 nm (Fig. 1b, c). This indicates that OPV is differently organized when the template pT10 is present.18
The templated assembly process has been studied in more detail by monitoring the UV-vis absorption at λ = 500 nm as a function of temperature at different concentrations and compared to the non-templated self-assembly. The transition temperatures, below which the two types of self-assembly set in, are defined as the elongation temperature Te17 for non-templated self-assembly and as the apparent elongation temperature Tet for templated assembly.7a For a similar concentration, the Tet of the pT10–OPV mixtures is higher than the Te of OPV (Fig. 1e and f), showing that the pT10–OPV assemblies are more stable than the non-templated self-assemblies of OPV. When fitting the temperature-dependent data to the templated self-assembly model as described previously,7a an enthalphy of ΔHet ≈ −90 ± 10 kJ mol−1, a guest–guest interaction energy of ε = −6.2 ± 0.5 kTp was obtained.19
The enthalpy values extracted for the templated and non-templated assembly processes suggest that the higher stability of the templated assembly as indicated by the higher melting temperature is due to its larger enthalpy gain. A necessary condition for the predominance of templated assembly over self-assembly is that the free-energy change resulting from the combined effects of host–guest and templated guest–guest interaction molecules is larger than the free-energy gain from the stacking of guest molecules in self-assembly. As a consequence, the presence of the PNA template effectively suppresses the self-assembly of OPV unless a large excess of OPV is present in the solution and only then when the pT10 templates are filled.
To visualize the pT10–OPV assemblies, an MCH-solution was drop-casted on graphite (HOPG). In contrast to the sample containing only OPV (Fig. 2a), the AFM micrographs of the pT10–OPV mixture show uniform small particles with a height of 3–4 nm and a deconvoluted width20 of 5–10 nm (Fig. 2b, d).7a The expected dimensions of the pT10–OPV complexes are ∼4 × 4 × 4 nm (the length of pT10 and OPV are 3.6 and ∼4 nm, respectively) and correspond to the size of the objects observed, revealing that the PNA-template controls the size of the OPV assemblies.
In conclusion, PNA-templated assemblies have been constructed in MCH of which the size is controlled by the PNA template. This PNA-templated approach can in principle be applied to any functional molecule and makes it possible to construct size-controlled functional nanostructures in organic media.
The authors acknowledge K. Pieterse for the artwork and the EURYI scheme for the financial support.
Notes and references
- (a) Special Issue: Tobacco Mosaic Virus: Pioneering Research for a Century, Philos. Trans. R. Soc. London, Ser. B, 1999, 354, 517; (b) Biochemistry, ed. L. Stryer, 4th Revised edn, W. H. Freeman and Company, New York, United States of America, 1996 Search PubMed.
- Examples and reviews of templated polymerizations: (a) J. Gons, E. J. Vorenkamp and G. Challa, J. Polym. Sci., Part A: Polym. Chem., 1975, 13, 1699 Search PubMed; (b) T. Inoue and L. E. Orgel, Science, 1983, 219, 859 CrossRef CAS; (c) R. E. Kleiner, Y. Brudno, M. E. Birnbaum and D. R. Liu, J. Am. Chem. Soc., 2008, 130, 4646 CrossRef CAS; (d) R. Saito, Polymer, 2008, 49, 2625 CrossRef CAS; (e) J. C. M. van Hest, Nat. Chem. Biol., 2008, 4, 272 CrossRef CAS.
- Examples of templated self-assembly: (a) J. S. Lindsey, New J. Chem., 1991, 15, 153 CAS; (b) T. Sugimoto, T. Suzuki, S. Shinkai and K. Sada, J. Am. Chem. Soc., 2007, 129, 270 CrossRef CAS; (c) S. R. Bull, L. C. Palmer, N. J. Fry, M. A. Greenfield, B. W. Messmore, T. J. Meade and S. I. Stupp, J. Am. Chem. Soc., 2008, 130, 2742 CrossRef CAS; (d) Y. Xu, J. Ye, H. Liu, E. Cheng, Y. Yang, W. Wang, M. Zhao, D. Zhou, D. Liu and R. Fang, Chem. Commun., 2008, 49 RSC; (e) A. L. Benvin, Y. Creeger, G. W. Fisher, B. Ballou, A. S. Waggoner and B. A. Armitage, J. Am. Chem. Soc., 2007, 129, 2025 CrossRef CAS.
- F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491 CrossRef CAS.
- Examples of DNA as structural scaffold for particles: (a) Y. Pinto, J. D. Le, N. C. Seeman, K. Musier-Forsyth, T. A. Taton and R. A. Kiehl, Nano Lett., 2005, 5, 2399 CrossRef CAS; (b) K. V. Gothelf and T. H. LaBean, Org. Biomol. Chem., 2005, 3, 4023 RSC.
- (a) R. Iwaura, F. J. M. Hoeben, M. Masuda, A. P. H. J. Schenning, E. W. Meijer and T. Shimizu, J. Am. Chem. Soc., 2006, 128, 13298 CrossRef CAS; (b) R. Iwaura, K. Yoshida, M. Masuda, M. Ohnishi-Kameyama, M. Yoshida and T. Shimizu, Angew. Chem., Int. Ed., 2003, 42, 1009 CrossRef CAS.
- (a) P. G. A. Janssen, S. Jabbari-Farouji, M. Surin, X. Vila, J. C. Gielen, T. F. A. de Greef, M. R. J. Vos, P. H. H. Bomans, N. A. J. M. Sommerdijk, P. C. M. Christianen, P. Leclère, R. Lazzaroni, P. van der Schoot, E. W. Meijer and A. P. H. J. Schenning, J. Am. Chem. Soc., 2009, 131, 1222 CrossRef CAS; (b) P. G. A. Janssen, J. Vandenbergh, J. L. J. van Dongen, E. W. Meijer and A. P. H. J. Schenning, J. Am. Chem. Soc., 2007, 129, 6078 CrossRef CAS; (c) P. G. A. Janssen, J. L. J. van Dongen, E. W. Meijer and A. P. H. J. Schenning, Chem.–Eur. J., 2009, 15, 352 CrossRef CAS; (d) M. Surin, P. G. A. Janssen, R. Lazzaroni, E. W. Meijer and A. P. H. J. Schenning, Adv. Mater., 2009, 21, 1126 CrossRef CAS.
- (a) P. K. Lo and H. F. Sleiman, J. Am. Chem. Soc., 2009, 131, 4182 CrossRef CAS; (b) P. K. Lo and H. F. Sleiman, Macromolecules, 2008, 41, 5590 CrossRef CAS.
- Examples of 2D and 3D DNA structures: (a) N. C. Seeman, Methods Mol. Biol., 2005, 303, 143 CAS; (b) F. A. Aldaye, A. L. Palmer and H. F. Sleiman, Science, 2008, 321, 1795 CrossRef CAS.
- It should be noted that DNA has been used as a template in aqueous solvent mixtures, see for example: (a) A. Furstenberg, M. D. Julliard, T. G. Deligeorgiev, N. I. Gadjev, A. A. Vasilev and E. Vauthey, J. Am. Chem. Soc., 2006, 128, 7661 CrossRef; (b) B. A. Armitage, Top. Curr. Chem., 2005, 253, 55 CAS.
- (a) P. Jonkheijm, A. Miura, M. Zdanowska, F. J. M. Hoeben, S. de Feyter, A. P. H. J. Schenning, F. C. de Schryver and E. W. Meijer, Angew. Chem., Int. Ed., 2004, 43, 74 CrossRef; (b) F. Würthner, Z. Chen, F. J. M. Hoeben, P. Osswald, C.-C. You, P. Jonkheijm, J. van Herrikhuyzen, A. P. H. J. Schenning, P. P. A. M. van der Schoot, E. W. Meijer, E. H. A. Beckers, S. C. J. Meskers and R. A. J. Janssen, J. Am. Chem. Soc., 2004, 126, 10611 CrossRef.
- (a) P. E. Nielsen and G. Haaima, Chem. Soc. Rev., 1997, 26, 73 RSC; (b) P. E. Nielsen, Lett. Pept. Sci., 2004, 10, 135; (c) M. C. de Koning, G. A. van der Marel and M. Overhand, Curr. Opin. Chem. Biol., 2003, 7, 734 CrossRef CAS.
- E. A. L. Biessen, K. Sliedregt-Bol, P. A. C. T. Hoen, P. Prince, E. van der Bilt, A. R. P. M. Valentijn, N. J. Meeuwenoord, H. Princen, M. K. Bijsterbosch, G. van der Marel, J. H. van Boom and T. J. C. van Berkel, Bioconjugate Chem., 2002, 13, 295 CrossRef CAS.
- MCH was used as apolar solvent to avoid artefacts in the CD measurements, see (a) M. Wolffs, S. J. George, Z. Tomovic, S. C. J. Meskers, A. P. H. J. Schenning and E. W. Meijer, Angew. Chem., Int. Ed., 2007, 46, 8203 CrossRef CAS; (b) S. J. George, Z. Tomovic, M. M. J. Smulders, T. F. A. de Greef, P. E. L. G. Leclere, E. W. Meijer and A. P. H. J. Schenning, Angew. Chem., Int. Ed., 2007, 46, 8206 CrossRef CAS.
- In heptane, the absorption of OPV shifts hypsochromically upon self-assembly, see ref. 11a.
- It should be noted that λz−c is not at λmax since the vibronic shoulders also give rise to a Cotton effect.
- (a) P. Jonkheijm, P. van der Schoot, A. P. H. J. Schenning and E. W. Meijer, Science, 2006, 313, 80 CrossRef CAS; (b) M. M. J. Smulders, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2008, 130, 606 CrossRef CAS.
- Remarkably, preliminary molecular modeling simulations reveal disordered pT10–OPV assemblies (see ESI†). Molecular dynamics hint that the assembly of OPV along pT10 is not only driven by H-bonds of the OPV diaminotriazine unit with the thymine bases of pT10 but also with the peptide backbone. Simulations of the CD spectra will be carried out to elucidate the supramolecular organization of the OPVs in these aggregates, see: F. C. Spano, S. C. J. Meskers, E. Hennebicq and D. Beljonne, J. Am. Chem. Soc., 2007, 129, 7044 Search PubMed.
- It should be noted that a steeper slope for the non-templated self-assembly curve is obtained near the melting temperature, even though the binding enthalpy is lower. This paradox originates from the fact that although this slope is proportional to the enthalpy change, it has different prefactors for each of the two processes. It is found that the ratio of these slopes curves is approximately given by ∼ΔHe/Te2/(η − 1/2)ΔHet/Tet2 where η is the stoichiometric ratio, equal to one in our experiments. Calculating the ratio of the two slopes according to this equation gives a value of 1.6 which is comparable to the value 1.67 coming directly from the graphs; S. Jabbari-Farouji and P. van der Schoot, manuscript in preparation.
- C. Bustamante, J. Vesenka, C. L. Tang, W. Rees, M. Guthold and R. Keller, Biochemistry, 1992, 31, 22 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Molecular modeling. See DOI: 10.1039/b913307k |
| ‡ pT10:13MALDI-TOF MS (M = 2823.5) m/z = 2824.6 [M + H+]. For experimental details and general methods see ref. 7a. Sample preparation: pT10 and OPV were dissolved in chloroform. After solvent removal and intensive drying, MCH was added to obtain the appropriate concentration and the solution was heated to 333 K to dissolve both components and slowly cooled. For atomic force microscopy, 2 μl of the solution at 273 K was drop-cast on freshly cleaved HOPG and allowed to dry in air. |
| This journal is © The Royal Society of Chemistry 2010 |
