Selective enrichment of N-linked glycopeptides by using a highly hydrophilic matrix synthesized via click chemistry†
Long
Yu
,
Xiuling
Li
,
Jun
Dong
,
Xiuli
Zhang
,
Zhimou
Guo
* and
Xinmiao
Liang
*
Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, China. E-mail: liangxm@dicp.ac.cn; guozhimou@dicp.ac.cn; Fax: +86-411-8437 9539; Tel: +86-411-8437-9519
First published on 15th October 2010
Abstract
A highly hydrophilic matrix named Click CA was developed via click chemistry for the selective enrichment of N-linked glycopeptides. This matrix exhibited superior selectivity and coverage for N-linked glycopeptides.
Glycosylation is one of the most common and complex co-/post-translational modifications of proteins, which plays important roles in many cellular activities, such as cell growth, migration, differentiation, signaling and trafficking.1 Glycosylation analysis of proteins is crucial for decoding these biofunctions of glycosylation, where structural determination of glycopeptides by mass spectrometry (MS) is required.2 Due to the co-existence of non-glycosylated peptides in the protein digests, MS analysis of glycopeptides suffered a lot from the ion suppression effect caused by the non-glycosylated peptides that are more abundant and more strongly ionized than glycopeptides.3 To minimize the ion suppression effect, different strategies for selective enrichment of glycopeptides have been employed prior to MS analysis.4 Amongst these strategies, the hydrophilic interaction chromatography (HILIC)-based method has become a promising approach due to its global glycan affinity, high glycopeptide coverage, good reproducibility as well as the compatibility with MS.5 The further application of this method, however, is limited by the insufficient selectivity of the hydrophilic matrices, on which glycopeptides are captured.6 To this end, it is of great importance to develop hydrophilic matrices with high glycopeptide selectivity.
Under HILIC mode, peptide mixture dissolved in the relatively hydrophobic solvent was first loaded onto the hydrophilic matrix, followed successively by rinsing to remove non-glycosylated peptides and elution of glycopeptides using the solvent with higher water content.7 According to the partition model of HILIC, solute retention is determined by its partitioning capability between the solvent and the water-rich layer that is stagnant on the hydrophilic matrix.8 In principle, non-glycosylated peptides are prone to associate with the hydrophobic solvent and eluted earlier, while glycopeptides with polar glycans are prone to associate with the water-rich layer and eluted later. Therefore, the formation of the stable water-rich layer on the matrix determines the discrimination of glycopeptides from non-glycosylated peptides. In other words, the selectivity of the matrix for glycopeptides mainly relies on its hydrophilicity. Herein, we proposed a simple and effective click chemistry-based approach for the synthesis of a matrix with high hydrophilicity and evaluated its selectivity and coverage for N-linked glycopeptides.
Click chemistry, coined by Sharpless and co-workers,9 has been proved to be a practicable approach for the development of hydrophilic matrices in our laboratory.10 A common strategy for the construction of these matrices involves the synthesis of azide-silica and the alkyne derivatization of the target molecule (maltose, for example), followed by the click reaction. For Click CA,‡ however, the synthesis was much simpler, since the derivatization step was not necessary. Click CA was constructed by linking propiolic acid to the azide-silica through click chemistry (Scheme 1). The carbon and nitrogen content of the resulting matrix is 7.53% and 3.71%, while the surface coverage is about 2.98 mol m−2 calculated from the increased carbon content after the click reaction. The surface chemistry of Click CA is confirmed by the solid state 13C NMR spectrum (Fig. S2).†
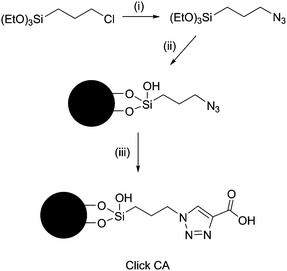 | ||
Scheme 1 Reagents and conditions: (i) NaN3, DMF, KI, 90–100 °C; (ii) silica gel, DMF, 100–110 °C; (iii) propiolic acid, CH3OH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), 75 °C. 1, v/v), 75 °C. | ||
To evaluate the selectivity of Click CA, three model N-linked glycoproteins with different types of glycans were selected, including human serum immunoglobulin G (IgG), bovine ribonuclease B (RNase B) and chicken ovalbumin (re-purified by reversed-phase liquid chromatography). Fig. 1 shows the spectrum of the IgG glycopeptide fraction enriched with Click CA (Fig. 1B), in comparison with that of the glycopeptide fraction enriched with Sepharose (Fig. 1C) and that of the desalted digest as control (Fig. 1A). In the desalted digest, the signals of the glycopeptides were severely suppressed by the co-eluted non-glycosylated peptides, resulting in the fact that only 10 glycopeptides were detected. After enrichment with Click CA, the amount and signal intensity of glycopeptides were remarkably enhanced, since the non-glycosylated peptides were effectively removed from the glycopeptide fraction, where up to 26 glycopeptides with complex-type glycans were detected. While in the glycopeptide fraction enriched with Sepharose, considerable amount of non-glycosylated peptides were still co-existing with the glycopeptides, due to the limited selectivity of the matrix. Only 12 glycopeptides were identified after enrichment with Sepharose. For RNase B, glycopeptides with glycans of increasing number of mannose from one to nine were clearly identified after enrichment with Click CA, whereas only five glycopeptides could be observed in the fraction enriched with Sepharose, as shown in Fig. 2. For ovalbumin, there were 17 glycopeptides with high-mannose or hybrid-type glycans that have been detected in the enriched fraction, compared to seven in the desalted digest (Fig. S3).†
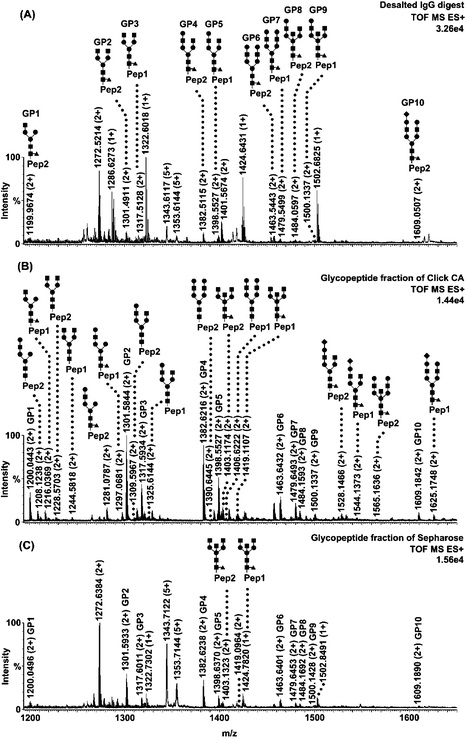 | ||
| Fig. 1 Nano-ESI-MS characterization of human IgG tryptic digest treated with different approaches. (A) Mass spectrum of IgG digest desalted with C18 (control). (B) Mass spectrum of IgG glycopeptide fraction enriched with Click CA. (C) Mass spectrum of IgG glycopeptide fraction enriched with Sepharose. Glycopeptides are labelled with their structures. The sequences of Pep1 and Pep2 are EEQYNSTYR and EEQFNSTFR, respectively. ■ N-acetylglucosamine; ● Mannose or Galactose; ◆ Sialic acid; ▲ Fucose. Note that the structures of 10 glycopeptides that appeared in the three fractions are only labelled in Fig. 1A. In Fig. 1B and 1C, they are marked with the symbols GP1-10. Other peaks are non-glycosylated peptides. | ||
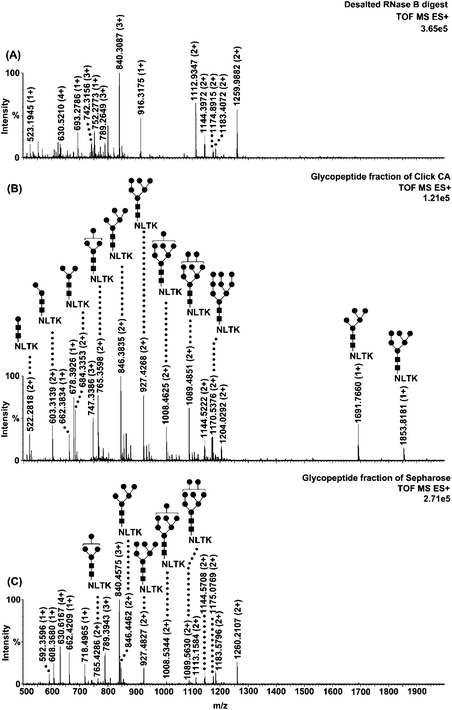 | ||
| Fig. 2 Nano-ESI-MS characterization of RNase B tryptic digest treated with different approaches. (A) Mass spectrum of RNase B digest desalted with C18 (control). (B) Mass spectrum of RNase B glycopeptide fraction enriched with Click CA. (C) Mass spectrum of RNase B glycopeptide fraction enriched with Sepharose. Glycopeptides are labelled with their structures. ■ N-acetylglucosamine; ● Mannose. Other peaks are non-glycosylated peptides. | ||
In addition to selectivity, glycopeptide coverage is another important factor that reflects the ability of the matrix for providing global glycosylation profile. For glycopeptide coverage assessment, the glycopeptides from human serum α1-acid glycoprotein (AGP) were enriched and characterized. About 220 glycopeptides covering all the five sites of the two AGP variants (AGP-1 and 2) were identified. Fig. 3 shows the glycosylation profile on site III (Asn54), which is contained in the peptide backbone SVQEIQATFFYFTPN54K. Most glycopeptides of this site are sialylated and the glycans varied from mono- to tetra-antennary structures. To confirm the structures of the identified glycopeptides on this site, MS/MS experiments on ion 1594.7 (3+) and 1917.5 (2+) were performed, as shown in Fig. S4 and S5.†
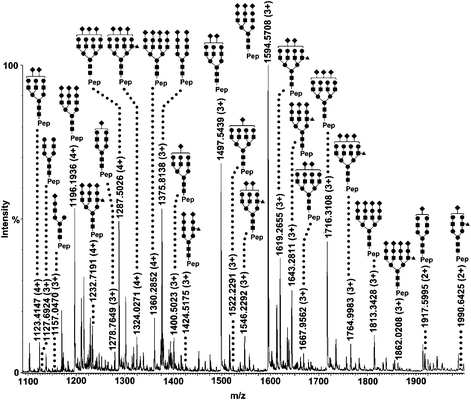 | ||
| Fig. 3 Nano-RPLC-MS characterization of human AGP glycopeptides from glycosylation site III. Glycopeptide peaks with hydrogen adduction are labelled with their structures. The peptide sequence is SVQEIQATFFYFTPNK. ■ N-acetylglucosamine; ● Mannose or Galactose; ◆ Sialic acid; ▲ Fucose. | ||
The enrichment effectiveness of Click CA, especially the glycopeptide coverage, is superior to that of other HILIC matrices employed for glycopeptide enrichment, such as Click Maltose,11 Sepharose5b or ZIC-HILIC.12 This can be attributed to the high hydrophilicity of Click CA, which was indicated by the loading and rinsing conditions. During loading and rinsing, solvents with stringently-controlled water content were used in order to maximally remove non-glycosylated peptides from the matrix, on which glycopeptides should be retained still simultaneously. For most hydrophilic matrices, the water content of loading and rinsing solvents was usually 20% when no salt was added.5,11–13 Whereas, for Click CA, the samples can be loaded and rinsed by using solvents containing 30% of water. The 10% difference in water content reflects the fact that the retention of glycopeptides on Click CA is remarkably stronger than that on other hydrophilic matrices, since solute retention is very sensitive to the variation of water content under HILIC mode.8b By performing the RNase B glycopeptide elution window experiments, we further compared the hydrophilicity of Click CA with that of a weak cationic exchanger (WCX), which contains an aliphatic carboxylic acid group bonded to silica (Fig. 4). RNase B glycopeptides were eluted from the WCX column when the rinsing solvent with 20% water content was used, while the same glycopeptides could be retained still on the Click CA column when using the rinsing solvent containing 30% of water (Fig. S6 and S7).† Apparently, the hydrophilicity of Click CA is higher than that of the WCX matrix. Since the structure difference of Click CA from the WCX matrix is the existence of the triazole ring, it can be deduced that the triazole ring with strong dipole property enhances the hydrophilicity of the matrix. However, this is not the only reason for the high hydrophilicity of Click CA, since other matrices synthesized via click chemistry, such as Click Maltose, did not exhibit such strong hydrophilicity.11 Therefore, we speculates that the combination structure of the triazole ring and the carboxylic acid group might have distinct charge distribution that has dominant impact on the hydrophilicity of Click CA.
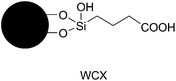 | ||
| Fig. 4 The structure of the WCX matrix. | ||
In summary, we introduced a highly hydrophilic matrix named Click CA for the selective enrichment of glycopeptides. In our assessment, Click CA exhibited high selectivity and coverage for N-linked glycopeptides with various glycan structures. The high hydrophilicity of Click CA results from its unique structure constructed via simple click chemistry.
This work was supported by the National Natural Science Funds for Distinguished Young Scholar (20825518), Project of National Science Foundation of China (20805046) and Project of Knowledge Innovation Program of Chinese Academy of Sciences (KSCX2-YW-R-170).
Notes and references
- (a) Y. Y. Zhao, M. Takahashi, J. G. Gu, E. Miyoshi, A. Matsumoto, S. Kitazume and N. Taniguchi, Cancer Sci., 2008, 99, 1304–1310 CrossRef CAS; (b) K. Ohtsubo and J. D. Marth, Cell, 2006, 126, 855–867 CrossRef CAS.
- (a) D. S. Dalpathado and H. Desaire, Analyst, 2008, 133, 731–738 RSC; (b) M. Wuhrer, M. I. Catalina, A. M. Deelder and C. H. Hokke, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 849, 115–128 CrossRef CAS; (c) W. Morelle, K. Canis, F. Chirat, V. Faid and J. C. Michalski, Proteomics, 2006, 6, 3993–4015 CrossRef CAS.
- B. A. Budnik, R. S. Lee and J. A. J. Steen, Biochim. Biophys. Acta, Proteins Proteomics, 2006, 1764, 1870–1880 CrossRef CAS.
- H. Geyer and R. Geyer, Biochim. Biophys. Acta, Proteins Proteomics, 2006, 1764, 1853–1869 CrossRef CAS.
- (a) M. Thaysen-Andersen, S. Mysling and P. Hojrup, Anal. Chem., 2009, 81, 3933–3943 CrossRef CAS; (b) Y. Zhang, E. P. Go and H. Desaire, Anal. Chem., 2008, 80, 3144–3158 CrossRef CAS.
- (a) W. Ding, J. J. Hill and J. Kelly, Anal. Chem., 2007, 79, 8891–8899 CrossRef CAS; (b) J. Cao, C. P. Shen, H. Wang, H. L. Shen, Y. C. Chen, A. Y. Nie, G. Q. Yan, H. J. Lu, Y. K. Liu and P. Y. Yang, J. Proteome Res., 2009, 8, 662–672 CrossRef CAS.
- Y. Wada, M. Tajiri and S. Yoshida, Anal. Chem., 2004, 76, 6560–6565 CrossRef CAS.
- (a) A. J. Alpert, J. Chromatogr., A, 1990, 499, 177–196 CrossRef CAS; (b) P. Hemstrom and K. Irgum, J. Sep. Sci., 2006, 29, 1784–1821 CrossRef.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- (a) Z. M. Guo, A. W. Lei, Y. P. Zhang, Q. Xu, X. Y. Xue, F. F. Zhang and X. M. Liang, Chem. Commun., 2007, 2491–2493 RSC; (b) H. X. Huang, Y. Jin, M. Y. Xue, L. Yu, Q. Fu, Y. X. Ke, C. H. Chu and X. M. Liang, Chem. Commun., 2009, 6973–6975 RSC; (c) Q. Fu, Z. M. Guo, T. Liang, X. L. Zhang, Q. Xu and X. M. Liang, Anal. Methods, 2010, 2, 217–224 RSC.
- L. Yu, X. L. Li, Z. M. Guo, X. L. Zhang and X. M. Liang, Chem.–Eur. J., 2009, 15, 12618–12626 CrossRef CAS.
- P. Hagglund, J. Bunkenborg, F. Elortza, O. N. Jensen and P. Roepstorff, J. Proteome Res., 2004, 3, 556–566 CrossRef.
- C. D. Calvano, C. G. Zambonin and O. N. Jensen, J. Proteomics, 2008, 71, 304–317 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, Fig. S1–S7 and Table S1–S13. See DOI: 10.1039/c0ay00569j |
| ‡ The naming of our matrices was based on the functional group that has been linked to the silica. The novel matrix was named as Click CA, where CA means Carboxylic Acid. |
| This journal is © The Royal Society of Chemistry 2010 |
