The case for the use of unrefined natural reagents in analytical chemistry—A green chemical perspective
Kate
Grudpan
a,
Supaporn Kradtap
Hartwell
a,
Somchai
Lapanantnoppakhun
a and
Ian
McKelvie
b
aDepartment of Chemistry and Center for Innovation in Chemistry, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand
bWater Studies Centre, School of Chemistry, Monash University, Victoria, 3800, Australia
First published on 8th September 2010
Abstract
An important part of the Green Chemistry philosophy is the need to develop and adopt green analytical techniques and procedures. These include the reduction of reagent and solvent usage, the minimization of solid, liquid and gaseous materials produced by analytical processes, the replacement of reagents and solvents of high occupational or environmental toxicity with much more innocuous materials, and the reduction of energy use in analytical processes. One aspect that has received little attention in this context is the use of unrefined natural reagents derived from plant and animal tissues or microbial cells. Crude plant extracts may contain chemical compounds that enable their use as indicators in acid–base or redox titrations, or as chromogenic or fluorogenic agents. Enzymes extracted from plants may be used directly in soluble form, or incorporated in biosensors or solid phase reactors, for analytical measurements, or as a means of removing interferences or performing speciation studies. The use of natural reagents in conjunction with a flow injection system can confer a number of advantages. The enhanced kinetic control that flow analysis offers may assist in avoiding undesirable side reactions that would otherwise occur using unpurified reagents. The lifetime of natural reagents may be prolonged when used in a flow analysis system because their exposure to light or air can be controlled. Any changes in response that do occur may be readily corrected by regular standard checking and recalibration. This article reviews the use of natural reagents with an emphasis on flow-based analytical systems, and makes the case for further research in this latent area of green analytical chemistry.
Introduction
The late 1900's and early 2000's have seen the growth of interest in so-called Green Chemistry,1 which has been driven by concerns regarding the environmental impact and sustainability of chemistry, and to some extent by the poor public perceptions of the discipline of chemistry and the chemical industry in general. Implicit in the Green Chemistry paradigm is the need for the development and adoption of green analytical techniques and procedures.2,3 These include the reduction of reagent and solvent usage, the minimization of solid, liquid and gaseous materials produced by analytical processes, the replacement of reagents and solvents of high occupational or environmental toxicity with much more innocuous materials, and the reduction of energy use in analytical processes.4,5 As Armenta et al. have pointed out in their review on Green Analytical Chemistry,3 flow analytical techniques are inherently green, because they involve much smaller volumes of reagents and sample, produce much less waste, and offer the possibility of both efficient on-line sample pretreatment and even analytical waste treatment.However, a principle of Green Analytical Chemistry that apparently has not been widely annunciated is the potential and desirable use of natural or unrefined reagents as a replacement for highly refined or purified reagents, in analytical processes.3 This suggestion may at first seem to be counter to the best-held principles of analytical chemistry, in which reagents of the highest available purity are used in order to minimize reagent blanks and potential interferences. However, when a reagent is only required in excess, or a crude extract contains the necessary active reagent, then in some cases these may be substituted for more purified reagents without compromising the success of the determination. Clearly the use of such natural reagents is highly desirable because of the simplicity of their preparation and the inherent energy and chemical waste savings.
What is meant by the term “natural reagents”? These are reagents that may be obtained as extracts of plant or animal tissues with little purification or modification other than perhaps filtration or dialysis, and used in soluble form, immobilized on a solid substrate, or even packed as intact tissue in a flow-through reactor. In the genesis of analytical chemistry, use of natural reagents was the norm until advances in organic chemistry during the 19th Century enabled the synthesis of pure, synthetic reagents that were subsequently used for analytical purposes. For example, the alkaloid brucine, obtained from the seeds of the Strychnine tree (Strychnos nux-vomica L.), was described as early as 1863 for the determination of nitrate and nitric acid,6 and was in common usage for nitrate determination until the 1970's.7–10 However, it was not until the 1990's that the chemical basis of the brucine-based nitrate determination was thoroughly investigated and explained. Interestingly, the brucine method was largely superseded as a standard method11,12 by a supposedly better method involving Cd-reduction of nitrate followed by diazotization and coupling with N-1-naphthylethylenediamine dihydrochloride to form a diazo dye (the Griess reaction). This is used almost universally (it is an approved method of organisations such as the US EPA12), but is now recognized to have a number of deficiencies13 as well as being environmentally unfriendly because of the use of toxic cadmium. As a consequence, some laboratories (e.g. USGS) have recently adopted the use of nitrate reductase obtained from corn leaves (Zea mays) as an alternative means for reduction of nitrate.14
From a pedagogical perspective, considerable benefit may be derived from introducing either secondary or even tertiary students to introductory chemical techniques such as acid base titration or photometry through the use of natural product reagents. For example, students might extract an indicator from plant tissue, and then use this in an acid base titration.15 More advanced students could, e.g. extract acid phosphatase from potatoes and use this in the study of enzyme behaviour and kinetics. Apart from the obvious financial benefits (50 Units of acid phosphatase from a major US supplier costs about A$60 compared with ca. A$2 for a sack of potatoes), students studying such fundamental analytical and biochemical behaviour would also gain an appreciation for the important role of natural product chemistry. In a way, it is analogous to teaching city children that milk comes from cows, rather than from cartons in the supermarket!
What should the “boundary conditions” be for the use of unrefined natural reagents, i.e. those requirements that must be met if the integrity of a particular analytical determination is not to be compromised? Reagent blanks must be measured to account for impurities in natural extracts used, and there should be strict adherence to normal quality assurance protocols, i.e. proper validation of methods including spike recoveries and evaluation of interferences, and traceability to an appropriate primary standard. Furthermore, natural reagents should not be treated like some sort of chemical “black box”, but their use should be based on informed knowledge of the active constituents and chemical properties of the extract of plant material or tissue in question, which in many cases may be derived from the very extensive natural product chemistry literature.16–18
This review briefly considers some applications of unrefined natural reagents in analysis (cf.Fig. 1). which may involve natural reagents as pH or redox indicators, or as chromo, fluoro and lumino-genic reagents. Tissues from plants and animals and microbes, or their extracts, will also contain some enzymes that can be used unmodified in enzymatic determinations and as a means of extraction or modification of interfering sample matrices. Similarly, extracts, tissues or cells may be incorporated into biosensors as a means of achieving bioselectivity
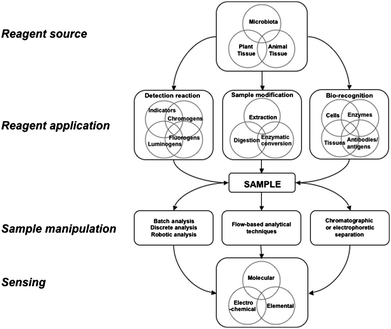 | ||
| Fig. 1 A schematic diagram showing generic strategies for the application of natural reagents in chemical analysis. | ||
A range of analytical applications of unrefined natural reagents have been tabulated in Tables 1–3 While the data in these tables are exemplary, rather than comprehensive, it is clear that there has been relatively little use of unrefined natural reagents in the last half century. However, what is evident, is that the more recent use of flow-based analytical techniques in the application of natural reagents is advantageous, and this approach is frequently used as the means of applying unrefined reagents, tissue reactors or bio-recognition devices. The enhanced kinetic control that FIA offers can assist in avoiding undesirable side reactions that would otherwise occur using unpurified reagents. Use of natural reagents in a flow analysis system may also prolong their active life by limiting their exposure to light or air; any changes in response that occur may be readily corrected by regular standard checking, and by recalibration if necessary. The rapid signal response and accompanying high sample throughput of flow analytical techniques even makes the use of relatively unstable reagents feasible. In many procedures, replacement of all reagents with natural reagents may not be possible, or even desirable, and thus flow analysis provides a practical modus operandi for the Green Chemistry objective of minimizing both reagent consumption and waste generation.3
| Natural pH indicators | |||||
|---|---|---|---|---|---|
| Analyte | Reagent | Source | Sample type | Method | Reference |
| pH | Anthocyanine dyes extracted from flowers and leaves | Cockscomb (Celosia cristate Linn), cotton tree (Bombax ceiba L.), flame tree (Poinciana regia) and Roselle (Hibiscus subdariffa) | — | Acid–base titration | 25 |
| pH | Roselle (Hibiscus subdariffa) | — | Acid–base titration | 15 | |
| pH | Crude extracts of flowers | Azalea (Rhododendron simsii) and Glory tree quaresmeira (Tibouchina granulosa) | — | Acid–base titration | 23 |
| Natural chromogenic and fluorogenic reagents | |||||
| B | Curcumin | Tumeric (Curcuma longa L) | Sea water | Spectrophotometry | 26 |
| Fe | Guava leaf (Psidium guajava L.) extract | — | FIA-spectrophotometry | 27 | |
| Mo(VI) | Slippery elm (Ulmus rubra) leaf extract | Waters sample | Spectrophotometry | 28 | |
| Hg(II) | Chlorophyll | Pea plant | — | Fluorescence inhibition | 29 |
| Zr | Crude plant extract | Oriental plane tree (Platanus orientalis) | Tap, wastewater, well water samples | Spectrophotometry | 30 |
| Analyte | Reagent | Source | Sample type | Method | Calibration range (detection limit) [RSD] | Ref |
|---|---|---|---|---|---|---|
| a Linear range. | ||||||
| Glutamine | Glutaminase (EC 3.2.1.5) | Porcine kidney tissue or immobilised Sarcina flava bacterial cells | — | Segmented continuous flow analysis system with ammonia gas-sensing electrode | 10−5–10−2 M | 33 |
| (—) | ||||||
| [—] | ||||||
| Lactic acid | α-hydroxy acid oxidase | Porcine kidney tissue | Blood, milk | FIA-chemiluminescence | 1 × 10−6–1 × 10−3 M | 34 |
| (2 × 10−7 M) | ||||||
| [1.2% at 1 × 10−4 M] | ||||||
| L(+)lactate | Lactate oxidase, Peroxidase (EC 1.1.3.2) | Zucchini (Cucurbita pepo) | Silage material | Multi-commutated FIA-spectrophotometry | 10–100 mgL−1 | 35 |
| (—) | ||||||
| [0.2% at 75 mgL−1] | ||||||
| L-ascorbate | L-ascorbate oxidase | Cucumber (Cucumis sativus L.) | — | FIA-amperometry | 5 × 10−4–7 × 10−3 Ma | 36 |
| Malic acid, dopa | Malate dehydrogenase (EC 1.1.1.37) and polyphenol oxidase (EC 1.10.3.1) | Apple (Malus domestica) tissue reactor | — | FIA-spectrophotometry | 5 × 10−4–2 × 10−2 Ma malic acid, 5 × 10−6–1 × 10−4 Ma Dopa | 37 |
| (—) | ||||||
| [—] | ||||||
| Hydrogen peroxide | Peroxidase (EC 1.11.1.7) | Zucchini (Cucurbita Pepo) | Waters | FIA-spectrophotometry | 1.6 × 10−5–6.6 × 10−4 Ma | 38 |
| (2.1 × 10−6 M) | ||||||
| [<0.2% at 2 × 10−4 M, n = 10] | ||||||
| Catechol | Polyphenol oxidase (EC 1.10.3.1) | Coconut (Cocos nucifera) based biosensor that converts catechol to quinone | River, waster waters | FIA-amperometry | 5 × 10−6–8 × 10−4 Ma | 39 |
| (2 × 10−6 M) | ||||||
| [1.8% at 1 × 10−4 M] | ||||||
| Epinephrine | Polyphenol oxidase (EC 1.10.3.1) | Palm tree fruit (Livistona chinensis) bioreactor that converts epinephine to epinephrinequinone | Pharmaceutical formulations | FIA-amperometry | 5 × 10−5–3.5 × 10−4 Ma | 40 |
| (1.5×10−5 M) | ||||||
| [3.1% at 3×10−4 M, n = 10] | ||||||
| Isoproterenol | Polyphenol oxidase (EC 1.10.3.1) | Avocado (Persea americana) extract | Pharmaceutical formulations | FIA-spectrophotometry, Polyphenol oxidase immobilized on controlled pore silica reactor | 1.23 × 10−4–7.38 × 10−4 Ma | 41 |
| (6.25 × 10−5 M) | ||||||
| [<1% at 2 × 10−4 M, n = 10] | ||||||
| L-cysteine | Polyphenol oxidase (EC 1.10.3.1) | Sweet potato root (Ipomoea batatas L. Lam.) | Pharmaceutical formulations | FIA-spectrophotometry (inhibition of catechol oxidation) | 6 × 10−5–8 × 10−4 Ma | 42 |
| (4.4 × 10−6 M) | ||||||
| [<1.2% at 2 × 10−4 M, n = 10] | ||||||
| L-dopa and carbidopa | Polyphenol oxidase (EC 1.10.3.1) | Crude extract of sweet potato root (Ipomoea batatas (L.) Lam.) | Pharmaceutical formulations | FIA-spectrophotometry | 4 × 10−4–1 × 10−2 M | 43 |
| (1.5 × 10−5 M, L-dopa, 2 × 10−5 M, carbidopa) | ||||||
| [<1%, n = 6] | ||||||
| Methyldopa and dopamine | Polyphenol oxidase (EC 1.10.3.1) | Crude extract of sweet potato root (Ipomoea batatas (L.) Lam.) | Pharmaceutical formulations | Spectrophotometry | 2 × 10−4–6 × 10−3 Ma | 44 |
| (3.4 × 10−5 M methyldopa, 3 × 10−5 M dopamine) | ||||||
| [<1.27% and 1.68% at 5 × 10−3 M, n = 10] | ||||||
| Phenols | Polyphenol oxidase (EC 1.10.3.1) | Tissue slices from potato (Solanum tuberosum) and mushroom (Agaricus bisporus) | Industrial waste waters | FIA-amperometry | 1 × 10−3–1 × 10−2 M | 45 |
| (—) | ||||||
| [—] | ||||||
| Phenols, poly | Polyphenol oxidase (EC 1.10.3.1), catechol oxidase (dimerizing) (EC 1.1.3.14) | Banana (gen. Musa), spinach | — | FIA-amperometry | 1 × 10−4–6 × 10−3 M dopamine | 46 |
| 2 × 10−4–8 × 10−3 M catechol | ||||||
| 2 × 10−4–6 × 10−3 M DL-epinephrine | ||||||
| Phenols, total | Polyphenol oxidase (EC 1.14.18.1) | Crude extract of sweet potato root (Ipomoea batatas (L.) Lam.) | Industrial wastewaters | FIA-spectrophotometry | 2 × 10−4–2 × 10−3 Ma | 47 |
| (1 × 10−5 M) | ||||||
| [1.17% at 6 × 10−4 M, n = 12] | ||||||
| Sulfite | Catechonol oxidase (EC 1.14.18.1) | Crude extract of sweet potato root (Ipomoea batatas (L.) Lam.) | Juice, white wine, vinegar | FIA-spectrophotometry | 4 × 10−5–6 × 10−4 M | 48 |
| (2.2 × 10−6 M) | ||||||
| [<1% at 1 × 10−4 M, n = 12] | ||||||
| Urea | Urease (EC 3.5.1.5) | Pigeonpea (Cajanus cajan) tissue packed reactor | Animal blood | FIA-spectrophotometry | — | 49 |
| (—) | ||||||
| [1.4%, n = 12] | ||||||
| Urea | Urease (EC 3.5.1.5) | Crude extract of Jack bean meal (Canavalia ensiformis DC) | Milk, fertilizer samples | Potentiometric ammonia detection by SIA with gas diffusion | 1 × 10−3–1 × 10−2 M | 50 |
| (6 × 10−4 M) | ||||||
| [1.9, n = 10] | ||||||
| Urea | Urease (EC 3.5.1.5) | Soybean (Glycine max (L.) Merr) tissue packed in a reactor | Urine samples | FIA-chemiluminescence with either luminol or permanganate | 4 × 10−6–4 × 10−4 Ma | 51 |
| (2 × 10−6 M) | ||||||
| [6% at 1 × 10−5 M, n = 7] | ||||||
| Glycerol | Gluconobacter oxydans | Pilot process liquor | FIA-Thermometric biosensor | 5 × 10−3–1 × 10−1 M | 52 | |
| (—) | ||||||
| [—] | ||||||
| Hg and MeHg speciation | SPE on Dowex Optipore® SD-2 containing bacteria | Streptococcus pyogenes | Natural water, environmental samples | Cold vapour AAS | — | 53 |
| (2.1 ng g−1 Hg, 1.5 ng g−1 MeHg) | ||||||
| [<1.7%] | ||||||
| Cr(VI) and Cr(III) speciation | SPE on loaded Diaion® SP-850 resin containing bacteria | Bacillus sphaericus | Natural waters | — | 54 | |
| (0.5 μg L−1 Cr(III)) | ||||||
| [<1.5%] |
| Analyte | Reagent | Source | Sample type | Method | Calibration range (detection limit) [RSD] | Ref |
|---|---|---|---|---|---|---|
| Phosphate, fluoride | Acid phosphatase (EC 3.1.3.2). Immobilized glucose oxidase (EC 1.1.3.4). | Potato (Solanum tuberosum) tissue slice | Fertilizer, urine | Amperometric oxygen (Clark) electrode. Inhibition of glucose 6′ phosphate hydrolysis. | — | 64 |
| (2.5 × 10−5 M phosphate, 1×10−4 M fluoride) | ||||||
| [1.7% phosphate, 6.5% fluoride] | ||||||
| Catechol | Catechol oxidase (dimerizing) (EC 1.1.3.14) | Spinach (Spinacea oleracea) | — | Amperometric oxygen (Clark) electrode | 2 × 10−5–8 × 10−4 M | 65 |
| (0.5–1.0 × 10−5 M) | ||||||
| [RSD 3%, n = 10] | ||||||
| Glycolic acid | Glycolate oxidase (EC 1.1.3.1) and peroxidase (EC 1.11.1.7) | Sunflower (Helianthus annuus L.) leaf tissue | Urine | FIA-amperometric biosensor | 1 × 10−6–2 × 10−3 M | 66 |
| (1 × 10−6 M) | ||||||
| [1.67%, n = 15] | ||||||
| Dopamine | Peroxidase (EC 1.11.1.7) | Zucchini (Cucurbita pepo) | Pharmaceutical formulations | Amperometry | 5.×10−4–3×10−3 M | 67 |
| (2.6×10−5 M) | ||||||
| [<1.2% at 7.9×10−4 M, n = 10] | ||||||
| Epinephrine | Polyphenol oxidase (EC 1.10.3.1) | Palm tree fruit (Livistona chinensis) biosensor that converts epinephine to epinephrinequinone | Pharmaceutical formulations | FIA-amperometry | 5.0 × 10−5–3.5 × 10−4 M | 40 |
| (1.5 × 10–5 M) | ||||||
| [3.1% at 3 × 10−4 M, n = 10] | ||||||
| Hydrazine | Peroxidase (EC 1.11.1.7) | Sweet potato tissue (Ipomoea batatas L. Lam.) | Boiler feed waters | Amperometry, paraffin/graphite electrode, modified with sweet potato. Inhibition oxidation of hydroquinone | 7.0 × 10−6–1.2 × 10−4 M | 68 |
| (5.1 × 10−7 M) | ||||||
| [—] | ||||||
| Hydrogen peroxide | Peroxidase (EC 1.11.1.7) | Ground asparagus tissue (Asparagus officinalis) | — | Amperometry, with ferrocene mediated electrode | 5 × 10−6–7 × 10−5 M | 69 |
| (4.0 × 10−7 M) | ||||||
| [1.95% at 1 × 10−5M, n = 15] | ||||||
| Hydroquinone | Peroxidase (EC 1.11.1.7) | Sweet potato root (Ipomoea batatas L. Lam.) | Cosmetic creams | Amperometry-stearic acid-graphite powder electrode modified with sweet potato, for use in organic solvents | 6.2 × 10−5–1.5 × 10−3 M | 63 |
| (8.5 × 10−6M) | ||||||
| [<1% at 7.3 × 10−4 M, n = 10] | ||||||
| Hydroquinone | Peroxidase (EC 1.11.1.7) | Ginger (Zingiber officinales Rosc.) | Photographic waste waters | Square wave voltammetry, carbon paste electrode modified with peroxidase immobilized on chitosan | 2.5 × 10−4–2.4 × 10−3 M | 70 |
| (2.5 × 10−5 M) | ||||||
| [<1.2% at 4.9×10−4 M, n = 10] | ||||||
| Hydroquinone | Peroxidase (EC 1.11.1.7) | Gilo (Solanum gilo) | Photographic waste waters | Square wave voltammetry, carbon paste electrode modified with peroxidase immobilized on chitosan | 2.5 × 10−4–5.5 × 10−3 M | 71 |
| (2 × 10−6 M) | ||||||
| [<1% at 3 × 10−4 M, n = 8] | ||||||
| Hydrazine | Polyphenol oxidase (EC 1.10.3.1) | Sweet potato root (Ipomoea batatas L. Lam.) | Photographic waste waters | Cyclic voltammetry-graphite-epoxy resin electrode modified with sweet potato tissue | — | 72 |
| (—) | ||||||
| [0.7%, n = 10] | ||||||
| Mono- and di-phenols | Polyphenol oxidase (EC 1.10.3.1) | Sweet potato (Ipomoea batatas (L.) Lam.) | Industrial waste waters | Amperometric oxygen (Clark) electrode | 2 × 10−5–4 × 10−4 M | 73 |
| (1.2 × 10−5 M) | ||||||
| [1.02%] | ||||||
| Paracetamol | Polyphenol oxidase (EC 1.10.3.1) | Avocado (Persea americana) | Pharmaceutical formulations | Chronoamperometry-vaseline-graphite electrode modified with avocado tissue | 1.2 × 10−4–5.8 × 10−3 M | 74 |
| (8.8 × 10−5 M) | ||||||
| [<0.5% at 5 × 10−3 M, n = 10] | ||||||
| Phenols, total | Polyphenol oxidase (EC 1.10.3.1) | Jack fruit (Artocarpus integrifolia L.) | Waste waters | Amperometry-capric acid-graphite powder electrode modified with crude extract of jack fruit | 5.25 × 10−6–7.8 × 10−4 M | 75 |
| (2 × 10−7 M) | ||||||
| [3% at 2.5 × 10−3 M, n = 10] |
Detection reagents: Indicators, chromo-, fluoro-, and lumnino-gens
There is a wealth of high quality chemical information on potential natural analytical reagents in the compendia of natural product and food chemistry. For example the indicator and fluorescent properties of anthocyanins in crude extracts from plants such as red cabbage (Brassica oleracea),19,20 red grapes21 and Morning Glory flowers22 are well documented, but they are not widely used in analysis despite ready availablity and low cost. It has been demonstrated that crude extracts of the flowers of azalea (Rhododendron simsii) and quaresmeira (Tibouchina granulosa) used as pH indicators gave endpoints within 2% of those obtained by potentiometry.23 There are also reports of the use of extracts of Chinese hibiscus (Hibiscus rosa sinensis L.) and Indian shot (Canna indica L.) being used as simple visual γ-ray dosimetry indicators.24Other natural reagents were historically used either for spot tests, e.g. cinchonine from Calisaya (Cinchona calisaya, Wedd) was used for the detection of bismuth, while salicylate, derived from willow bark (Salix spp), was known as an effective reagent for iron.6
Table 1 lists some natural indicators, as well as examples of some plant-based materials that have been used for spectrophotometric and fluorimetric determinations. For example, curcumin, which is obtained by extraction from the rhizomes of turmeric (Curcuma longa L.) has long been known as a suitable reagent for the determination of boron.26 Similarly, aqueous extracts of guava leaves have been shown to be useful in the FIA spectrophotometric determination of Fe.27 Three flow injection configurations were tested, viz; a normal FIA mode in which sample was injected into a flowing stream of guava extract, a reactor containing guava leaves for on-line leaching of the extract, and a reverse FIA configuration in which extract was injected into a stream of sample. The latter mode was preferred because it minimized reagent use and gave greater sensitivity. However, all modes required the incorporation of a washing step to remove an unidentified precipitate that formed (Fig. 2). If performed in batch mode, precipitate formation would be problematic, and may mean that the analytical procedure is unviable, whereas when performed in flow analysis mode, washing to remove precipitate can be performed in a controlled and highly reproducible manner.
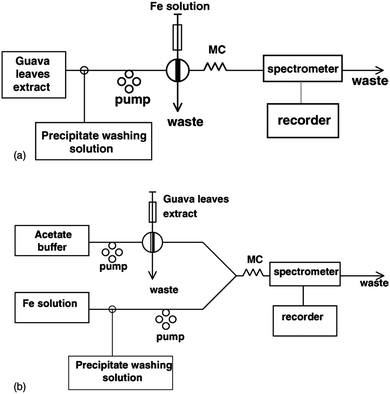 | ||
| Fig. 2 Two FIA manifold configurations tested for the determination of iron using guava leaf extract (a) normal FIA, (b) reverse FIA. (Reproduced from Ref. 27 with permission of Elsevier BV). | ||
Some plants contain fluorescent constituents, e.g quinine is naturally derived from Cinchona bark, and ripe banana skins are known to fluoresce under UV light, due to the break down products of chlorophyll.31 The determination of Hg2+ by fluorescence quenching of chlorophyll29 is a particularly good example of a natural reagent-based determination, in that it is simple, selective and very sensitive, with a limit of detection of ca. 1 μg L−1. In a method for the speciation of Fe(II)/Fe(III) that has very good green chemistry credentials, Pulido-Tofiño et al. described a flow injection method based on a fluorescent siderophore, pyoverdin, that is biosynthesised by Pseudomonas fluorescens. Pyoverdin was immobilized on controlled pore glass and used repeatedly, while peroxydisulfate for Fe(II) oxidation was immobilized on Dowex resin and used with a switching valve to enable determination of either Fe(III) or total Fe.32 Methods based on fluorescence measurement such as these certainly warrant further investigation and validation.
Sample modification: Enzyme extracts and immobilized enzyme reactors
Many crude plant extracts contain a range of enzymes that can be utilized in an analytical schema. Table 2 lists examples of some of these, and it is evident that plant derived enzymes such as polyphenol oxidase and peroxidase dominate the analytical literature.Analytical applications of enzymes extracted from plants or animal tissues may involve direct enzymatic interaction with the analyte, e.g in the determination of hydrogen peroxide using zucchini extract, peroxidase catalyses the oxidation of guaiacol in the presence of H2O2 to tetraguaiacol, which absorbs strongly at 470 nm.38 The peroxidase activity in the zucchini extract was shown to be extremely stable both in daily use, and when stored at ≤ 4 °C over five months.
Alternately, the enzymatic method may rely on kinetic inhibition by the analyte, e.g.L-cysteine can be determined based on its inhibition of the activity of polyphenol oxidase on the oxidation of catechol to o-quinone (λmax = 410nm).42
A third application of enzymes invokes the use of enzymes in crude extracts of plants, plant juices or tissue bioreactors as a means of minimizing sample matrix effects. This approach is particularly suitable in flow-based analytical procedures where the enzyme can be added continuously to the sample, or be used in an immobilised form. For example, surface active proteins and oxidisable species such as ascorbic acid that might otherwise interfere in electrochemical determinations, have been eliminated by the use of tissue bioreactors comprised of plants such as papaya, that are rich sources of ascorbic acid oxidase.55 In addition to these reactors being cheap, there is the added advantage that the stability and activity of the enzymes in the natural, immobilized form is often superior to that in extracted, purified forms.
Some authors have used immobilized live bacteria as the basis of pretreatment or analysis in some assays. Navratil et al. immobilized Gluconobacter oxydans cells on calcium pectate gel in a reactor to convert glycerol to dihydroxyacetone. Both reactant and product were detected by flow injection using glycerokinase (EC 2.7.1.30) and galactose oxidase (EC 1.1.3.9) immobilized on controlled pore glass with microcalorimetric measurement.52
Table 2 also lists two examples of the use of bacteria immobilized on solid matrices for solid phase extraction (SPE) for use in the speciation of metals. The use of immobilized cells or bacteria is a biomimetic approach that has been used for measurement of different bio-available or adsorbed species which are detected e.g by atomic spectrometry after elution.
One practical aspect that should be considered is the most appropriate means of isolation, and preservation or storage of natural reagents that may be more susceptible to oxidation, microbial attack and enzyme denaturation than purified substances. Techniques such as air drying of solids such as plant material, refrigeration, freezing or even freeze drying of liquid extracts, should all be investigated. Preservation with agents such as sodium azide or mercuric chloride would not be compatible with the general philosophy of green chemistry.
Bio-recognition based on plant and animal tissues and microbes
The term “bio-recognition” refers to the ability of a biological entity to recognise a target analyte and to elicit a signal proportional to that recognition reaction,56 and encompasses techniques such as biosensing, affinity chromatography, immunoassay, the avidin–biotin system and molecular imprinting.Biosensors are devices that couple bio-recognition via enzymes, tissues, antibody–antigen systems, entire cells or parts thereof, with various electrical, optical or thermal transducers.57 Electrical transduction may involve either potentiometric or amperometric measurements.58 In first generation biosensors, the signal measured may result from direct interaction of the reaction products as they diffuse to the transducer, e.g. a Pt electrode, in second generation biosensors a mediator such as ferrocene is used to improve electron transfer between the biochemical reaction and the transducer, while in the third generation devices, the bio-recognition process is an integral part of the transducer, e.g. though the use of conductive polymers or nanoparticulate surfaces.59,60
In addition to the selectivity achieved through the use of e.g. an enzymatic reaction, the transducer may also afford an extra level of selectivity. In amperometric detection, selectivity may be aided by the careful selection of the applied potential, while in fluorescence transduction, the choice of excitation and emission wavelengths will be important. Biosensors utilizing crude extracts of plants or slices of animal or plant tissue have become relatively common,61–63 as shown in Table 3.
Of those reported, many utilise either peroxidase (EC 1.11.1.7) or polyphenol oxidase (EC 1.10.3.1) in soluble extracts of a range of plants for the preparation of carbon paste electrodes, or variants thereof. For example, glycolic acid was determined in urine using a flow injection-biosensor system that incorporated glycolate oxidase (EC 1.1.3.1) and peroxidase (EC 1.11.1.7) from sunflower (Helianthus annuus L.) leaf tissue. Sensor response was observed to diminish by approx. 30% during continuous measurement of 90 samples over a period of ca. 6 h.66 This highlights one of the important advantages of the use of biosensors in conjunction with a flow analysis system. Where a natural extract reagent undergoes relatively quick degradation, e.g. over the space of one day, flow analysis is advantageous because the rate at which sensitivity changes can be readily monitored by periodic standard checking, and appropriate corrections made.
Use of biosensors with flow-based analytical techniques is advantageous because sensor life can be prolonged by minimizing its exposure to the sample matrix. The sensing element may also be reconditioned in buffer or electrolyte after every measurement. Furthermore, stop-flow techniques for enhancement of sensitivity, and switching of sample and standard streams for the purpose of regular recalibration are readily facilitated through simple flow programming.
Biosensors for determination of biochemical oxygen demand (BOD) provide a good example of the use of whole cells as the sensing element in biosensors. Liu and Mattiasson76 have reviewed the use of immobilized microbial cells in biofilm biosensors used to measure short-term BOD (see Fig. 3(a)). Either single strain cultures, complex cultures or heat killed microbes can be immobilised on a porous matrix adjacent to an oxygen sensing transducer (e.g. Clark electrode, O2 sensitive optode), and the short term oxygen consumption is monitored. Single strain cultures tend to be more substrate specific in their response to organic matter but give more stable long term responses than do those biosensors based on a microbial consortium which will nevertheless be more suitable for a wide range of wastes and concentrations.77 A flow system suitable for the application of a micrbial sensor for the determination of short-term BOD (BODst) is shown in Fig. 3(b).78
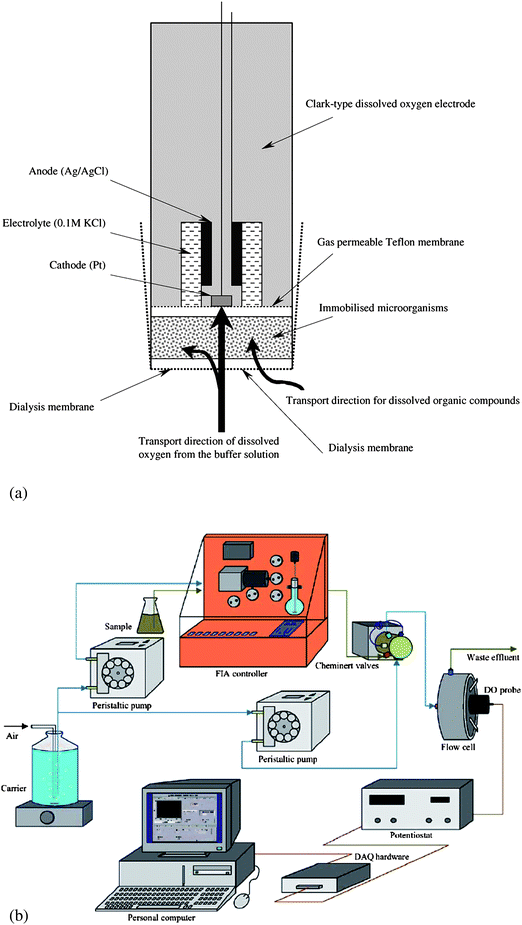 | ||
| Fig. 3 Diagrammatic view of (a) a BOD biosensor showing the immobilised microbial population in juxtaposed with a Clark-type dissolved oxygen electrode, and (b) a flow injection system for automated application of this microbial sensor. (Reproduced from Ref. 76,78 with permission of Elsevier Science B.V.). | ||
Another important area of bio-recognition is that of immunoassays. These are widely used for identification and quantification of analytes in clinical and biological chemistry. They also have an important role in environmental, agriculture and process analysis, in the detection of organic molecules, such as pesticides, where the emphasis is usually on the detection and quantification of trace concentrations in complex matrices. Enzyme-linked immunosorbent assays are widely used (ELISAs) and most typically involve colorimetric, fluorometric and chemiluminometric methods of detection. The common format for these assays is the 96-well plate system, but some workers have shown that flow-based automated systems, which exhibit high precision, rapid throughput, economical reagent use, and ready fluidic automation, are eminently suitable for immunassays, especially where on-line or process analysis is required. The flow analysis format lends itself well to heterogeneous immunoassays where the separation step can be carried out on-line,79,80 as illustrated in Fig. 4.
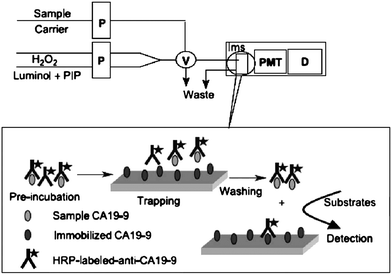 | ||
| Fig. 4 Schematic diagram of a flow injection chemiluminescent non-competitive immunoassay system for carbohydrate antigen 19-9. P, Peristaltic pump; V, Eight-way valve; Ims, Immunosensor; PMT, Photo-multiplier; D, Detector (Reproduced from Ref. 80 with permission of Elsevier Science B.V.). | ||
There are almost innumerable descriptions of immunoassay applications for natural and biological materials in the literature, including many describing the use of flow-based analytical techniques for this purpose.81–86 By their nature, immunoassays involve the use of “natural reagents” (enzymes, antibodies, etc) albeit in purified forms. However, the question remains whether this technique can be successfully applied using less purified, crude extracts, without significant compromise to analytical quality? There is also considerable scope for the use of flow-based techniques as the means of on-line cleanup of natural reagents in immunoassays.
Concluding remarks
The sources of natural reagents and their applications are numerous, and as yet they are a largely untapped resource. Use of unrefined natural reagents as a source of greener and cheaper reagents for a range of analytical tasks should not be seen as an option just for the developing world, but as something that is equally applicable worldwide. Where a natural reagent can be legitimately substituted for a purified or synthetic material without compromising the viability or quality of the analytical process, there is a strong case for doing so. Although not explored here, there is also great potential to utilize food grade or domestic chemicals in a range of analytical procedures, again without prejudice to analytical quality. As part of the Green Chemical Revolution the whole issue of analytical fitness for purpose needs to be reexamined, and this will necessarily involve consideration of the source, purity and cost of production and transport of chemicals, and the most appropriate means of employing these reagents in analysis. The flow analysis approach, whether in conventional or micro/nanofluidic mode, would appear to offer a feasible means of applying unrefined natural reagents for green chemical analysis.Most of the examples cited in this paper involve the use of photometric or electrochemical detection with natural reagents. The detection limits and linear ranges are probably constrained by higher blank values originating from the reagents themselves, and the limitation of the detection systems used. However, of the applications reported in Tables 2 and 3, the most sensitive were for determinations involving tissue reactors in concert with FIA and chemiluminescence detection. While most of these examples focus on agricultural, clinical or wastewater samples, which have higher analyte concentrations, the use of unrefined natural reagents for trace analytical purposes, e.g. in environmental waters, should be possible if the enhanced analytical control of FIA or SIA is utilized in combination with intrinsically sensitive detection methods such as chemiluminescence.
Thus, when applied with recognition of their limitations, unrefined natural reagents, especially when used in combination with flow based systems, can offer an alternative approach to sustainable and greener analytical measurement.
Acknowledgements
The authors are grateful for support by the Commission on Higher Education (CHE) through the Research Group (RG) grant (which supported IDMcK's visit to Chiang Mai), the Center for Innovation in Chemistry (PERCH-ICI), and the Thailand Research Fund (TRF).References
- P. T. Anastas and J. C. Warner, in Green Chemistry: Theory and Practice, ed. P. T. Anastas and J. C. Warner, Oxford University Press, New York, 1998, pp. 29–56 Search PubMed.
- P. T. Anastas, Crit. Rev. Anal. Chem., 1999, 29, 167–175 CrossRef CAS.
- S. Armenta, S. Garrigues and M. de la Guardia, TrAC, Trends Anal. Chem., 2008, 27, 497–511 CrossRef CAS.
- J. Namiesnik, J. Sep. Sci., 2001, 24, 151–153 CrossRef CAS.
- L. H. Keith, L. U. Gron and J. L. Young, Chem. Rev., 2007, 107, 2695–2708 CrossRef CAS.
- W. I. Stephen, Analyst, 1977, 102, 793–803 RSC.
- L. Kahn and F. T. Brezenski, Environ. Sci. Technol., 1967, 1, 492–494 CrossRef CAS.
- H. Fadrus and J. Maly, Fresenius' Z. Anal. Chem., 1964, 202, 164–176 CrossRef CAS.
- D. Jenkins and L. L. Medsker, Anal. Chem., 1964, 36, 610–612 CrossRef CAS.
- L. Kahn and F. T. Brezenski, Environ. Sci. Technol., 1967, 1, 488–491 CrossRef CAS.
- Anon, in Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Baltimore, 1998, pp. 4_121–124.122 Search PubMed.
- USEPA, ed. J. W. O'Dell, US Environment Protection Agency, Cincinati, OHIO 45268, 1993, p. 14.
- C. Gal, W. Frenzel and J. Möller, Microchim. Acta, 2004, 146, 155–164 CrossRef CAS.
- C. J. Patton, A. E. Fischer, W. H. Campbell and E. R. Campbell, Environ. Sci. Technol., 2002, 36, 729–735 CrossRef CAS.
- I. A. Yakasai, H. Musa, I. Hamisu, B. S. Sagagi and M. I. Usman, ChemClass Journal, 2005, 2, 62–64 Search PubMed.
- Atta-ur-Rahman, ed., Bioactive Natural Products-Studies in Natural Products Chemistry, Elsevier, 2000 onwards Search PubMed.
- A. Y. Leung, ed., Encyclopedia of common natural ingredients used in food, drugs, and cosmetics, Wiley, New York, 1996 Search PubMed.
- W. Steglich, B. Fugmann and S. Lang-Fugmann, ed., RÖMPP Encyclopedia Natural Products, Thieme Chemistry, Stuttgart, 2001 Search PubMed.
- G. M. Sapers, I. Taffer and L. R. Ross, J. Food Sci., 1981, 46, 105–109 CrossRef CAS.
- B. Pliszka, T. Olszewska and R. Drabent, in Proceedings of SPIE - The International Society for Optical Engineering, 2000, pp. 103–107 Search PubMed.
- F. J. Heredia, E. M. Francia-Aricha, J. C. Rivas-Gonzalo, I. M. Vicario and C. Santos-Buelga, Food Chem., 1998, 63, 491–498 CrossRef CAS.
- S. Asen, R. N. Stewart and K. H. Norris, Phytochemistry, 1977, 16, 1118–1119 CrossRef CAS.
- M. S. Cortes, L. A. Ramos and T. G. Cavalheiro, Quim. Nova, 2007, 30, 1014–1019 CAS.
- S. Saisomboon and C. Siri-Upathum, Rev. Sci. Instrum., 1987, 58, 1951–1952 CrossRef CAS.
- M. E. Soltan and S. M. Sirry, J. Chin. Chem. Soc., 2002, 49, 63–68 CAS.
- Y.-M. Liu and K. Lee, Mar. Chem., 2009, 115, 110–117 CrossRef CAS.
- T. Settheeworrarit, S. K. Hartwell, S. Lapanatnoppakhun, J. Jakmunee, G. D. Christian and K. Grudpan, Talanta, 2005, 68, 262–267 CrossRef.
- A. B. Monji, E. Zolfonoun and S. J. Ahmadi, Toxicol. Environ. Chem., 2009, 91, 1229–1235 CrossRef CAS.
- S. Gao, G. Tan, H. Yuan, D. Xiao and M. M. F. Choi, Microchim. Acta, 2006, 153, 159–162 CrossRef CAS.
- A. B. Monji, Green Chem. Lett. Rev., 2008, 1, 107–112 Search PubMed.
- S. Moser, T. Müller, M. O. Ebert, S. Jockusch, N. J. Turro and B. Kräutler, Angew. Chem., Int. Ed., 2008, 47, 8954–8957 CrossRef CAS.
- P. Pulido-Tofiño, J. M. Barrero-Moreno and M. C. Pérez-Conde, Talanta, 2000, 51, 537–545 CrossRef CAS.
- M. Mascini and G. A. Rechnitz, Anal. Chim. Acta, 1980, 116, 169–173 CrossRef CAS.
- F. Wu, Y. Huang and C. Huang, Biosens. Bioelectron., 2005, 21, 518–522 CrossRef CAS.
- C. A. Tumang, E. P. Borges and B. F. Reis, Anal. Chim. Acta, 2001, 438, 59–65 CrossRef CAS.
- S. Uchiyama, Y. Tofuku and S. Suzuki, Anal. Chim. Acta, 1988, 208, 291–294 CrossRef CAS.
- H. Horie and G. A. Rechnitz, J. Flow Injection Anal., 1995, 12, 91–97 Search PubMed.
- I. C. Vieira and O. Fatibello-Filho, Analyst, 1998, 123, 1809–1812 RSC.
- A. W. O. Lima, V. B. Nascimento, J. J. Pedrotti and L. Angnes, Anal. Chim. Acta, 1997, 354, 325–331 CrossRef CAS.
- F. S. Felix, M. Yamashita and L.c. Angnes, Biosens. Bioelectron., 2006, 21, 2283–2289 CrossRef CAS.
- K. O. Lupetti, I. C. Vieira and O. Fatibello-Filho, Talanta, 2002, 57, 135–143 CrossRef CAS.
- I. C. Vieira and O. Fatibello-Filho, Anal. Chim. Acta, 1999, 399, 287–293 CrossRef CAS.
- O. Fatibello-Filho and I. Da Cruz Vieira, Analyst, 1997, 122, 345–350 RSC.
- I. C. Vieira and O. Fatibello-Filho, Talanta, 1998, 46, 559–564 CrossRef.
- S. Canofeni, S. S. Di and R. Pilloton, Life Chem. Rep., 1994, 11, 321–331 CAS.
- S. Uchiyama and S. Suzuki, Anal. Chim. Acta, 1992, 261, 361–365 CrossRef CAS.
- I. C. Vieira and O. Fatibello, Anal. Chim. Acta, 1998, 366, 111–118 CrossRef.
- O. Fatibello-Filho and I. da Cruz Vieira, Anal. Chim. Acta, 1997, 354, 51–57 CrossRef CAS.
- G. C. Luca and B. F. Reis, Quim. Nova, 2001, 24, 191–194 CAS.
- F. V. Silva, A. R. A. Nogueira, G. B. Souza, B. F. Reis, A. N. Araujo, M. C. M. B. S. Montenegro and J. L. F. C. Lima, Talanta, 2000, 53, 331–336 CrossRef CAS.
- W. Qin, Z. Zhang and Y. Peng, Anal. Chim. Acta, 2000, 407, 81–86 CrossRef CAS.
- M. Navratil, J. Tkac, J. Svitel, B. Danielsson and E. Sturdik, Process Biochem., 2001, 36, 1045–1052 CrossRef CAS.
- M. Tuzen, O. D. Uluozlu, I. Karaman and M. Soylak, J. Hazard. Mater., 2009, 169, 345–350 CrossRef CAS.
- M. Tuzen, O. D. Uluozlu and M. Soylak, J. Hazard. Mater., 2007, 144, 549–555 CrossRef CAS.
- J. Wang and N. Naser, Anal. Chem., 1992, 64, 2469–2471 CrossRef CAS.
- Anon, in Encyclopedia of Microfluidics and Nanofluidics, Springer Verlag, Berlin, 2008, pp. 96–96 Search PubMed.
- M. Nic, J. Jirat, B. Kosata and A. Jenkins, IUPAC Compendium of Chemical Terminology (Gold Book), International Union of Pure and Applied Chemistry, 2006 Search PubMed.
- A. Ivaska, in Advances in Flow Injection Analysis and Related Techniques, ed. S. D. Kolev, and I. D. McKelvie, Elsevier, 2008, pp. 441–459 Search PubMed.
- Y. H. Lee, R. Mutharasan and S. W. Jon, in Sensor Technology Handbook, Newnes, Burlington, 2005, pp. 161–180 Search PubMed.
- Y. Shen, Y. Zhang, X. Qiu, H. Guo, L. Niu and A. Ivaska, Green Chem., 2007, 9, 746–753 RSC.
- O. Fatibello-Filho, K. O. Lupetti, O. D. Leite and I. C. Vieira, in Electrochemical Sensor Analysis, ed. S. Alegret and A. Merkoçi, Elsevier, Amsterdam, 2007, pp. e163–e168 Search PubMed.
- O. Fatibello-Filho, K. O. Lupetti, O. D. Leite and I. C. Vieira, in Electrochemical Sensor Analysis, Elsevier, Amsterdam, 2007, pp. 357–377 Search PubMed.
- O. Fatibello-Filho and I. C. Vieira, Fresenius J. Anal. Chem., 2000, 368, 338–343 CrossRef.
- F. Schubert, R. Renneberg, F. W. Scheller and L. Kirstein, Anal. Chem., 1984, 56, 1677–1682 CrossRef CAS.
- S. Uchiyama, M. Tamata, Y. Tofuku and S. Suzuki, Anal. Chim. Acta, 1988, 208, 287–290 CrossRef CAS.
- S. Liawrungrath, P. Purachat, W. Oungpipat and C. Dongduen, Talanta, 2008, 77, 500–506 CrossRef CAS.
- K. O. Lupetti, L. A. Ramos, I. C. Vieira and O. Fatibello-Filho, Farmaco, 2005, 60, 179–183 CrossRef CAS.
- I. C. Vieira, K. Omuro Lupetti and O. Fatibello-Filho, Anal. Lett., 2002, 35, 2221–2231 CrossRef CAS.
- W. Oungpipat, P. W. Alexander and P. Southwell-Keely, Anal. Chim. Acta, 1995, 309, 35–45 CrossRef CAS.
- I. R. W. Z. de Oliveira, I. C. Vieira, K. O. Lupetti, O. Fatibello-Filho, V. T. de Fevere and M. C. M. Laranjeira, Anal. Lett., 2004, 37, 3111–3127 CrossRef.
- I. R. W. Z. de Oliveira and I. C. Vieira, Enzyme Microb. Technol., 2006, 38, 449–456 CrossRef.
- K. O. Lupetti, G. Zanotto-Neto and O. Fatibello-Filho, J. Braz. Chem. Soc., 2006, 17, 1329–1333 CAS.
- I. C. Vieira, O. Fatibello-Filho, A. C. Granato and K. O. Lupetti, Eclet. Quim, 2004, 29, 7–14 Search PubMed.
- O. Fatibello-Filho, K. O. Lupetti and I. C. Vieira, Talanta, 2001, 55, 685–692 CrossRef.
- K. O. Lupetti, I. C. Vieira and O. Fatibello-Filho, Anal. Lett., 2004, 37, 1833–1846 CrossRef CAS.
- J. Liu and B. Mattiasson, Water Res., 2002, 36, 3786–3802 CrossRef CAS.
- S. Rastogi, P. Rathee, T. K. Saxena, N. K. Mehra and R. Kumar, Curr. Appl. Phys., 2003, 3, 191–194 CrossRef.
- J. Liu, G. Olsson and B. Mattiasson, Biosens. Bioelectron., 2004, 20, 571–578 CrossRef CAS.
- L. Zhao, L. Sun and X. Chu, TrAC, Trends Anal. Chem., 2009, 28, 404–415 CrossRef CAS.
- J. Lin, F. Yan, X. Hu and H. Ju, J. Immunol. Methods, 2004, 291, 165–174 CrossRef CAS.
- S. J. Hu, M. T. French, D. A. Palmer, M. Evans, S. M. Zhou, G. H. Sarpara and J. N. Miller, Anal. Chim. Acta, 2002, 454, 31–35 CrossRef CAS.
- A. D. Carroll, L. Scampavia, D. Luo, A. Lernmark and J. Ruzicka, Analyst, 2003, 128, 1157–1162 RSC.
- S. K. Hartwell, B. Srisawang, P. Kongtawelert, J. Jakmunee and K. Grudpan, Talanta, 2005, 66, 521–527 CrossRef CAS.
- J. Ruzicka, A. D. Carroll and I. Lahdesmaki, Analyst, 2006, 131, 799–808 RSC.
- S. K. Hartwell, K. Pathanon, D. Fongmoon, P. Kongtawelert and K. Grudpan, Anal. Bioanal. Chem., 2007, 388, 1839–1846 CrossRef CAS.
- I. Lahdesmaki, Y. K. Park, A. D. Carroll, M. Decuir and J. Ruzicka, Analyst, 2007, 132, 811–817 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
