Double-codified nanogold particles based automated flow-through CLEIA for 2,4-dinitrotoluene
Zhen
Lin
a,
J. C.
Sauceda-Friebe
b,
Jin-Ming
Lin
*a,
Reinhard
Niessner
b and
Dietmar
Knopp
*b
aAnalysis Center and Department of Chemistry, Tsinghua University, Beijing, 100084, China. E-mail: jmlin@mail.tsinghua.edu.cn; Fax: +86-10-62792343; Tel: +86-10-62792343
bInstitute of Hydrochemistry and Chemical Balneology and Chair of Analytical Chemistry, Technische Universität München, Marchioninistrasse 17, D-81377, München, Germany. E-mail: dietmar.knopp@ch.tum.de; Fax: +49-89-218078252; Tel: +49-89-218078252
First published on 19th May 2010
Abstract
A sensitive, automated micro flow-through chemiluminescence enzyme immunoassay (CLEIA) was developed for the determination of 2,4-dinitrotoluene (DNT) which employed a DNT analog covalently immobilized on a glass microchip, and nanogold particles (AuNPs) modified with HRP-labeled anti-mouse antibodies. The HRP-luminol-H2O2 chemiluminescent system (because of its high sensitivity) was chosen as the detection system. The obtained chemiluminescence (CL) intensity was inversely proportional to the DNT concentration in the sample. The working range for DNT quantification was 0.01–1 μg mL−1. Both the intra- and inter-assay coefficients of variation were less than 10%. The proposed assay had a LOD of 0.009 μg mL−1, which was 3-fold lower than a traditional HRP-labeled anti-mouse antibody-based CLEIA. The overall assay time was 13 min for each sample, enabling relatively rapid analysis.
Introduction
The immunoassay is a very important bioanalytical technique which is currently used in a wide range of applications, such as (for example), clinical diagnosis and environmental monitoring. The most widely used immunoassays are enzyme-labeled immunoassays, which are usually performed on microtiter plates, and are based on the enzymatic catalysis of appropriate substrates to yield chromogenic or chemiluminescent (CL) products. However, time-consuming multiple incubation steps are generally necessary for such assays. Hence, the development of rapid, sensitive and automated immunoassays remains desirable.1Micro-immunoassays, with miniaturized analytical systems and special channel dimensions, can have greatly reduced reaction times.2 Furthermore, only small reaction volumes are needed. Flow-through techniques offer precise control over both reaction times and reagent volumes.3 By combining micro-immunoassays and flow-through techniques, superior platforms for routine immunoassays may be generated.4
Nanogold particles (AuNPs), a class of nanomaterial, have many unique properties and have been widely used for analytical and biomedical purposes for many years. With surface modification, AuNPs can bind with many types of biomolecule, such as peptides, lipids, enzymes, drugs, antibodies, and viruses.5 The coupling of surface plasmons on the AuNPs with plasmons on Au films can also make AuNPs suitable for signal amplification in surface plasmon resonance spectroscopy (SPR).6,7 In addition, special optical properties (of AuNPs) mean that they are widely used in dipstick immunoassays. AuNPs are also suitable substrates for surface-enhanced Raman scattering (SERS).8,9 Quartz crystal microbalance (QCM) immunosensors have also been developed which apply antibody-functionalized AuNPs as amplifying probes.10 Further, a CL-based method has been used to detect AuNPs tags in biological conjugates, using an immunoassay as a model.11 This technique was based on the CL reaction in a AuCl4−-luminol-H2O2 system, and used HNO3-HCl to dissolve the AuNPs. The dissolution time of several hours was however quite long, and a complex solution was necessary to adjust the pH to make it suitable for CL detection.
Recently, several reports have shown that immunoassays which are based on HRP modified AuNPs have provided increased assay sensitivity. For example, Jia et al.12 immobilized a detector antibody and HRP on AuNPs, and these then acted as both carriers and amplifiers. However, the sensitivity needed to be improved. AuNPs are also widely used in electrochemical immunoassays.13 Tang and Ren14 for example, have used HRP-encapsulated nanogold hollow microspheres (HRP-GHS) conjugated to a secondary carcinoembryonic antigen antibody (HRP-GHS-anti-CEA), and developed an in situ amplified electrochemical immunoassay for CEA. Double-codified AuNPs (DC-AuNPs) have also been used to enhance immunoanalysis.15 The DC-AuNPs were modified with an anti-human IgG antibody-HRP conjugate for signal amplification, and both spectrophotometric and electrochemical detection methods were utilized for the human IgG measurement. Zhang et al.16,17 also developed a novel, ultrasensitive, enhanced CL immunoassay based on DC-AuNPs for the determination of α-fetoprotein (AFP). In this case, bromophenol blue (BPB) and 4-(4′-iodo) phenylphenol (IPP) were used, in turn, as the CL enhancers in a luminol-H2O2-HRP CL based system. The detection limit for the method was also significantly greater than for the conventional ELISA. However, the immunoreaction took place in an Eppendorf tube or microtiter plate plate, needed extended incubation times, and the separation steps used were relatively complex.
Until now, the combined application of AuNPs modified with HRP-labeled anti-mouse antibodies and an automated flow-through microchip-based CLEIA, have not been reported to the best of our knowledge. Here, a corresponding assay is developed and presented for use with the model compound 2,4-dinitrotoluene (DNT), a well known precursor in trinitrotoluene synthesis, which is used mainly in the polymer industry.
Briefly, a DNT analog was covalently immobilized on a glass microchip and free DNT (in sample or standard) then competed with the immobilized antigen for the limited number of DNT-antibody binding sites. After a separation step, AuNP-HRP-labeled anti-mouse antibodies in solution were passed over the microchip, and these bound to the primary antibodies on the microchip surface. Finally, a CL signal was generated by addition of a HRP substrate, and the signal was recorded using a CCD camera.
Experimental
Chemicals and instruments
All aqueous solutions were prepared using ultrapure water which was generated using reverse osmosis with UV treatment (Milli-RO 5 Plus, Milli-Q185 Plus, Millipore, Eschborn, Germany). The (3-glycidyloxypropyl)trimethoxysilane (GOPTS), poly(ethylene glycol) diglycidyl ether (PEGDG), gold(III) chloride trihydrate (HAuCl4·3H2O, 99.9% pure), sodium citrate tribasic dihydrate (Na3C6H5O7·2H2O), 2,4-dinitrotoluene (DNT), N1-(2,4-Dinitro-phenyl)-ethane-1,2-diamine, casein, and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO). The anti-DNT monoclonal antibody was obtained from Strategic Diagnostics Inc. (Newark, DE). Hellmanex II was obtained from Hellma GmbH (Müllheim, Germany). Diamino-poly(ethylene glycol) 2000 (DAPEG 2000 g mol−1) was from Huntsman Holland (Rozenburg, The Netherlands). HRP-labeled anti-mouse IgG (H + L) was purchased from Vectors Laboratories (Burlingame, CA). The CL substrate containing luminol and hydrogen peroxide was obtained from Thermo Scientific (Dreieich, Germany). The 0.1 M phosphate buffered saline (PBS) with a pH value of 7.4 was prepared by adding 12.2 g K2HPO4, 1.36 g KH2PO4, and 8.5 g NaCl to 1 L of ultrapure water. PBS with 0.5% casein (which was filtered after preparation) was used as the running buffer. The spotting buffer consisted of 30% DMSO, 2.5% glycerol and 67.5% 0.01 M carbonate buffer. All other chemicals mentioned were of analytical grade.Menzel glass slides (26 mm × 76 mm × 1 mm) were obtained from Carl Roth GmbH (Karlsruhe, Germany). Plastic containers (Carl Roth) were used during activation and cleaning of the glass slides. Single and multi-channel volume-adjustable pipettes and tips were purchased from Eppendorf (Hamburg, Germany). A Multilabel Counter (Victor2 Model 1420, Perkin Elmer (Waltham, USA)) was used for CL reading of the microtiter plates. The ultraviolet-visible (UV-vis) spectrophotometer (DU 650) was purchased from Beckman (Cambridge, UK). A BioOdyssey Calligrapher Miniarrayer (Bio-Rad, München, Germany) was utilized to spot the glass slides. For CL microarray readings and automated reagent supply, a Microarray Chip Reader (MCR 3, GWK Präzisionstechnik GmbH, München, Germany) was used.18 A JEM-1200EX electron microscope (JEOL Ltd., Tokyo, Japan) operating at 100 kV was used to characterise the AuNPs.
Procedure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), then concentrated H2SO4 and then water, were used during a further cleaning stage; again the slides were dried under a stream of nitrogen. Water-free GOPTS (800 μL) was then spread over one activated side of each slide which was then covered with a second slide to form a sandwich configuration. After incubation for 1 h at room temperature (RT), slides were separated manually and cleaned with sonication in ethanol, then methanol, then finally in ethanol again for 15 min. After drying under a stream of nitrogen, molten DAPEG was poured over the glass slide and activation was carried out at ∼98 °C for 15 h. After rinsing, slides were dried with nitrogen again, and stored in a desiccator at RT for further use.
1 v/v), then concentrated H2SO4 and then water, were used during a further cleaning stage; again the slides were dried under a stream of nitrogen. Water-free GOPTS (800 μL) was then spread over one activated side of each slide which was then covered with a second slide to form a sandwich configuration. After incubation for 1 h at room temperature (RT), slides were separated manually and cleaned with sonication in ethanol, then methanol, then finally in ethanol again for 15 min. After drying under a stream of nitrogen, molten DAPEG was poured over the glass slide and activation was carried out at ∼98 °C for 15 h. After rinsing, slides were dried with nitrogen again, and stored in a desiccator at RT for further use.
The glass slide surfaces were activated with PEGDG before immobilization of the antigen began. PEGDG was poured over the slide which was then kept at ∼98 °C overnight. The slides were cleaned with water and dried under nitrogen. The BioOdyssey Calligrapher Miniarrayer and a solid pin were used to spot the DNT analog N1-(2,4-dinitro-phenyl)-ethane-1,2-diamine onto the glass chips. A grid of spots was printed; and humidity within the spotting chamber was set at 20%. Subsequently, slides were incubated at RT overnight. Slides were then blocked with 3 M tris buffer (overnight), rinsed with water, and then dried under nitrogen.
The glass slides with the antigen (DNT analog) microarray were then glued onto a black polycarbonate carrier using laser-cut double-sided adhesive foil. The foil provided an accurate space for the chip, and the black polycarbonate carrier (with inlet and outlet ports) provided a support for subsequent fluid flow. The black color also tends to reduce background signals. Before undertaking the immunoassay, the chip was inserted into a specific loading device which was then installed into the flow-through system.
 | ||
| Fig. 1 Schematic diagram of the fluidic set-up of the flow-through CLEIA. V1-8: valves used for fluid control; P1-6: pumps for delivering solutions, i.e., P1 – sample solution, P2 – anti-2,4-DNT antibody solution, P3 – AuNPs-HRP-labeled anti-mouse antibody solution, P4 – running buffer, P5 – substrate, P6 – regeneration buffer; CCD: charge couple device. | ||
The CL images were processed as described earlier.22 Spots were identified automatically using the SIP 0.4 software program (Karsunke Softwarebüro, Wolnzach, Germany) according to signal intensity and consistency with the spot grid. The CL intensities of each spot were calculated by integration of pixel intensities within a square of a given side length around each spot center. The mean intensity value (and standard deviation of the integrals) was determined for six spot replicates for the same analyte concentration. The overall time per run was 13 min per sample, enabling relatively rapid analysis.
Results and discussion
Principle of the AuNPs-HRP-labeled anti-mouse antibody-based CLEIA
The principle of the AuNPs-HRP-labeled anti-mouse antibody-based CLEIA is presented in Fig. 2. Firstly, the antigen analog was covalently bound to the glass chip, then, the anti-DNT monoclonal antibody and DNT analyte were preincubated within the tubing loop, and then pumped over the glass chip. The immobilized analyte then competed with the free analyte in the sample/standard for a limited number of free antibody binding sites. Following a washing step, the secondary HRP-labeled anti-mouse antibody, or, the AuNPs-HRP-labeled anti-mouse antibody conjugate were added, which then bound to the primary antibody on the chip. After further washing, the enzyme substrate was added, and the CL signal generated was read by the CCD camera. The principle behind the HRP-labeled anti-mouse antibody based CLEIA was exactly the same as for the AuNPs-HRP-labeled anti-mouse antibody-based CLEIA, except, the immunocomplex was formed among the primary antibody and secondary HRP-labeled anti-mouse antibody (Fig. 2.4a). Critically, because a high number of HRP-labeled anti-mouse antibody molecules can actually be immobilized on the surface of AuNPs, a concurrent signal amplification, and therefore a significant sensitivity improvement can be obtained (Fig. 2.4b). | ||
| Fig. 2 Schematic illustration of a traditional HRP-labeled anti-mouse antibody based CLEIA procedure (upper part) and the AuNPs-HRP-labeled anti-mouse antibody based CLEIA (lower part). The following steps were performed: (1) Immobilization of analyte analog on the chip; (2) Incubation with the primary antibody and target sample analyte; (3) Washing and separation; (4a) Incubation with the HRP-labeled anti-mouse antibody conjugate, or (4b) Incubation with the AuNPs-HRP-labeled anti-mouse antibody conjugate. | ||
Characterization of the synthesized AuNPs and AuNPs-HRP-labeled anti-mouse antibody conjugate
Fig. 3a shows the UV-vis absorption spectrum for the AuNPs. The absorption peak (at about 520 nm) indicates the formation of AuNPs with an average diameter of around 16 nm,23 which was confirmed by TEM analysis (Fig. 3c). With the HRP-labeled anti-mouse antibody coating mounted on the AuNPs, a second absorption peak became apparent (at 280 nm) which was characteristic of proteins (Fig. 3b). As such, the enzyme-labeled antibody was apparently successfully bound to the AuNPs.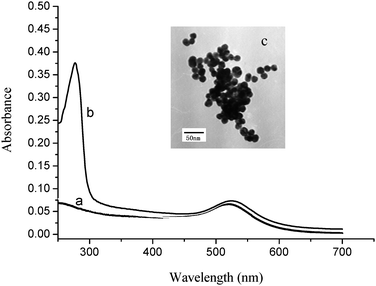 | ||
| Fig. 3 (a) UV-vis spectrum of AuNPs with a characteristic absorption peak at about 520 nm; (b) UV-vis spectrum of HRP-labeled anti-mouse antibody; (c) TEM of AuNPs (size 16 nm). | ||
Unless the ratio of HRP-labeled anti-mouse antibody to AuNPs is correct, nonspecific antibody binding may occur, which may then reduce the sensitivity of the assay. The gold flocculation test, previously reported by Ambrosi et al.15 and Yang et al.16 was utilized to determine the optimal ratio for the HRP-labeled anti-mouse antibodies to the AuNPs. In detail, the pH value of AuNPs solution (250 μL) was adjusted with Na2CO3 to 9.0. Then, 30 μL of HRP-labeled anti-mouse antibody at different concentrations was added to the AuNPs solution. After a 15 min incubation period, 166 μL of 10% NaCl was added. Finally, the AuNPs aggregation was analyzed. If the AuNPs are inadequately covered by protein, the addition of a certain amount of NaCl will induce a redshift in the maximum absorption wavelength. In this investigation, a protein concentration ≤3.33 × 10−2 mg mL−1 induced an obvious redshift, i.e., a very small difference was observed between the absorbance at 520 nm and at 580 nm (Fig. 4A). At increasing protein concentrations from 0.16 to 1 mg mL−1, the spectrum for the treated AuNPs was similar to that of the pure AuNPs (without the addition of NaCl), which indicated that at 0.16 mg mL−1 the antibody concentration was sufficient for particle labeling.
 | ||
| Fig. 4 (A) (a) AuNPs without the addition of NaCl; (b–e) 0.033, 0.16, 0.50, and 1.0 mg mL−1 HRP-labeled anti-mouse antibody conjugate concentrations added to 250 μL of AuNPs solution before the addition of NaCl; (f) Addition of NaCl to 250 μL AuNPs solution without protein stabilization. (B) CL intensity obtained using the microtiter plate based CLEIA in terms of the concentration of HRP-labeled anti-mouse antibody conjugate used to coat the AuNPs. | ||
In addition, a microtiter plate-based CLEIA was used to confirm that the estimated optimal AuNPs coating concentration of HRP-labeled anti-mouse antibody was correct. Briefly, the monoclonal antibody against DNT was immobilized on a microtiter plate. After incubation with 1% casein in PBS (to block unoccupied sites), the coated AuNPs (with different amounts of bound HRP-labeled secondary antibody) were added to the microtiter plate. After incubation for 20 min at RT, the enzyme substrate was added. The CL intensity reached a plateau at the point where the nanoparticles were coated with an antibody concentration of ≥0.16 mg mL−1 (Fig. 4B), confirming the initial findings.
The effect of the AuNPs on the CL of the luminol-H2O2 system
Often, AuNPs show an enhancing effect in many CL systems, i.e., in KIO4-NaOH-Na2CO3,24 peroxymonocarbonate-eosin Y,25 bis(2,4,6-trichlorophenyl)oxalate-H2O226 and luminol-ferricyanide27 based techniques. Therefore, the effect of AuNPs on the luminol-H2O2 system used here was investigated before they were used in the associated CLEIA. Firstly, the prepared AuNPs solution was centrifuged, and particles were then dispersed in ultrapure H2O. The AuNPs were then added to a mixed solution of luminol and hydrogen peroxide. The CL intensities of the luminol-H2O2 systems with and without AuNPs were then compared. Again, a AuNPs enhancing effect on the CL of the luminol-H2O2 system was observed (Fig. 5). This was in accordance with a previously published report.28 However, the magnitude of CL intensity enhancement (caused by the AuNPs) was quite negligible when compared to the strong catalytic oxidation effect excerted by the HRP on the luminol. | ||
| Fig. 5 The effect of AuNPs on the CL signal intensity of the luminol-H2O2 system in relation to incubation time. | ||
Effect of differing concentrations of immunoreagents
Generally speaking, the amount of immobilized antigen and antibody in a competitive immunoassay should be optimized, to obtain a method with both good sensitivity and reproducibility. A concentration of anti-DNT monoclonal antibody ranging from 5.45 × 10−5 mg mL−1 to 2.18 × 10−4 mg mL−1 was therefore investigated. As shown in Fig. 6A, the CL intensity increased in line with the concentration of primary antibody. The logit-log plots showed that sensitivity was at its highest when a concentration of 1.09 × 10−4 mg mL−1 of antibody was used (Fig. 6B). This concentration was therefore used in subsequent experiments. | ||
| Fig. 6 (A) CL intensity versus target analyte concentration, whilst using different concentrations of AuNPs; (B) The linear logit-log plots established for different concentrations of AuNPs; (C) CLmax, CLmax/IC50versus the immobilized target analyte (analog) concentration; (D) Brightness of spots obtained with variable amounts of target analyte immobilized on the glass chip. | ||
In a second experiment, the influence of the immobilized antigen concentration on the assay sensitivity was investigated. Briefly, the antigen was immobilized on a microchip at different concentrations, and the assay ran using a blank sample (i.e., without the analyte). IC50 values were then calculated, i.e., to determine the DNT concentration that caused a 50% reduction in the maximal chemiluminescence intensity (the CLmax). The IC50 values obtained at different immobilized antigen concentrations, and the corresponding CLmax results were then used to evaluate the microchip. The CLmax increased with increasing concentration of immobilized antigen, reaching a plateau at a concentration of 0.5 mg mL−1. The quotient of CLmax/IC50 reached a maximum at ≈ 0.2 mg mL−1 (Fig. 6C). When taking into account the spot shapes obtained by the CCD camera (Fig. 6D), it was determined that an antigen concentration of 0.5 mg mL−1 should be utilized in subsequent experiments.
Re-usage capability of the microchip
The regeneration of the proposed chip was investigated using two kinds of regeneration buffer for washing. The ultimate purpose of the washing was to remove the anti-DNT antibody and AuNPs-HRP-labeled anti-mouse antibody immunocomplex from the covalently immobilized antigen on the chip surface. The solutions used were: 0.1 M glycine buffer (with a pH value of 3.0) containing either 0.1% Tween 20 and 1% DMSO, or, 0.1% SDS and 0.1 M NaCl. Experiments showed that the chip could be re-used at least 25 times whilst making consecutive measurements when the glycine buffer with SDS + sodium chloride was used (Fig. 7). Over the first 4 cycles, an about 10% increase in CLmax intensity was noticed which may indicate some surface activation caused by swelling in the specified liquid medium. Starting from regeneration cycle no. 30, a small but significant loss in response of the device was observed which would allow additional measurements of unknowns only after periodical recalibrations. The relative standard deviation (R.S.D.) of CLmax acquired for spot replicates (n = 6) and 25 cycles (cycle 5 to 29) of 4.3% was deemed acceptable. Regeneration with glycine buffer containing Tween 20 + DMSO caused a sharp decline in the CLmax signal intensity almost immediately, but the underlying reason for this loss in intensity was not clear. | ||
| Fig. 7 The relative CLmax intensity signal obtained with an increasing number of regeneration cycles of the spotted glass slides using as regeneration solvents 0.1 M glycine buffer (pH 3.0) with (a) 0.1% SDS and 0.1 M NaCl; (b) 0.1% Tween 20 and 1% DMSO. | ||
The calibration curve
A typical calibration curve obtained during the experiment is presented in Fig. 8A. The higher the DNT standard concentration, the lower the amount of primary antibody that was bound to the immobilized antigen on the chip, and thus, the lower the relative light intensity recorded. Under optimized conditions, a linear calibration curve was obtained within the 0.01–1 μg mL−1 range with a correlation coefficient R = 0.9957 (n = 30). | ||
| Fig. 8 Calibration curves showing CL intensity versus the concentration of DNT using: (A) AuNPs-HRP labeled anti-mouse antibody. (B) HRP labeled anti-mouse antibody. The inserted logit-log plots are linear over the 0.01 to 1.0 μg mL−1 range. | ||
Comparison between AuNPs-labeled and HRP-labeled anti-mouse antibody based CLEIAs
Because AuNPs have excellent physical and chemical characteristics, they have already been used widely as labels for several biomolecules. For example, on homogeneous AuNPs many HRP-labeled anti-mouse antibody conjugates can be immobilized in order to enhance CL signal intensity and thus improve assay performance. CL intensity in both the HRP-labeled and the AuNPs-HRP-labeled antibody based CLEIA was compared using a set of calibrators (Fig. 8). The limit of detection (LOD) was defined as the minimum dose that could be distinguished from blank. According to the IUPAC definition, this can be calculated at a signal-to-noise ratio of 3sB (where sB is the standard deviation of a blank solution). The LOD of the AuNPs-HRP-labeled CLEIA was 0.009 μg mL−1 (Fig. 8A), which was 3-fold lower than that recorded for the HRP-labeled CLEIA (0.030 μg mL−1; Fig. 8B). The precision of both types of CLEIA was assessed by estimating the intra- and inter-assay coefficients of variation (CVs). The intra-assay precision was evaluated when analyzing 3 concentration levels 6 times per run. The CVs were 2.8%, 3.7% and 1.5% for the 0.01, 0.1 and 1 μg mL−1 DNT standards, respectively. The inter-assay CVs were calculated using 3 consecutive runs, and were 3.5%, 4.5% and 1.6% at 0.01, 0.1 and 1 μg mL−1. Thus, the precision of the proposed immunoassay was acceptable. The functionalized particles could be stored at 4 °C for at least 2 weeks.Conclusions
The objective of the present work was to develop a sensitive, automated CLEIA for the determination of levels of the model analyte, DNT. Signal amplification was accomplished by the use of double-codified AuNPs. The estimated LOD for the technique was 0.009 ng mL−1, which was 3-fold lower when compared to a similar CLEIA which did not use AuNPs or particle labelling. The automated flow-through immunoassay greatly reduced analysis time to just 13 min per assay versus ∼60 min for the conventional procedure. With appropriate regeneration, the spotted glass slides were also re-usable (approximately 30 consecutive runs). Both the intra- and inter-assay coefficients of variation were between 1.5% and 4.5% for the AuNPs-based CLEIA. The new method could possibly be applied for the determination of DNT environmental samples such as surface and groundwaters.Acknowledgements
This work was supported by National Natural Science Foundation of China (Project No. 90813015), the National Basic Research Program of China (Project No. 2007CB714507), and the German Research Council (DFG, project Sino-German Cooperation Research Group for Separation and Analysis of Complex Samples). A critical pre-publication review was undertaken by Dr Mark Taggart (IREC, Spain).References
- I. Abdel-Hamid, P. Atanasov, A. L. Ghindilis and E. Wilkins, Sens. Actuators, B, 1998, 49, 202 CrossRef.
- Y. Murakami, T. Endo, S. Yamamura, N. Nagatani, Y. Takamura and E. Tamiya, Anal. Biochem., 2004, 334, 111 CrossRef CAS.
- R. Puchades and A. Maquieira, Crit. Rev. Anal. Chem., 1996, 26, 195 CrossRef CAS.
- G. Q. Hu, Y. L. Gao and D. Q. Li, Biosens. Bioelectron., 2007, 22, 1403 CrossRef CAS.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS.
- F. A. Wang, J. L. Wang, X. Q. Liu and S. J. Dong, Talanta, 2008, 77, 628 CrossRef CAS.
- U. Pieper-Fürst, W. F. M. Stöcklein and A. Warsinke, Anal. Chim. Acta, 2005, 550, 69 CrossRef CAS.
- B. Bozzini, C. Mele and V. Romanello, J. Electroanal. Chem., 2006, 592, 25 CrossRef CAS.
- W. Q. Ma and Y. Fang, J. Colloid Interface Sci., 2006, 303, 1 CrossRef CAS.
- X. Chu, Z. L. Zhao and G. L. Shen, Sens. Actuators, B, 2006, 114, 696 CrossRef.
- Z. P. Li, Y. C. Wang, C. H. Liu and Y. K. Li, Anal. Chim. Acta, 2005, 551, 85 CrossRef CAS.
- C. P. Jia, X. Q. Zhong, B. Hua, M. Y. Liu, F. X. Jing, X. H. Lou, S. H. Yao, J. Q. Xiang, Q. H. Jin and J. L. Zhao, Biosens. Bioelectron., 2009, 24, 2836 CrossRef CAS.
- G. D. Liu and Y. H. Lin, Talanta, 2007, 74, 308 CrossRef CAS.
- D. P. Tang and J. J. Ren, Anal. Chem., 2008, 80, 8064 CrossRef CAS.
- A. Ambrosi, M. T. Castañeda, A. J. Killard, M. R. Smyth, S. Alegret and A. Merkoçi, Anal. Chem., 2007, 79, 5232 CrossRef CAS.
- X. Y. Yang, Y. S. Guo, S. Bi and S. S. Zhang, Biosens. Bioelectron., 2009, 24, 2707 CrossRef CAS.
- S. Bi, Y. M. Yan, X. Y. Yang and S. S. Zhang, Chem.–Eur. J., 2009, 15, 4704 CrossRef CAS.
- K. Kloth, R. Niessner and M. Seidel, Biosens. Bioelectron., 2009, 24, 2106 CrossRef CAS.
- A. Wolter, R. Niessner and M. Seidel, Anal. Chem., 2007, 79, 4529 CrossRef CAS.
- G. Frens, Nat. Phys. Sci., 1973, 241, 20 Search PubMed.
- P. Zhou, Y. T. Lu, J. Zhu, J. B. Hong, B. Li, J. Zhou, D. Gong and A. Montoya, J. Agric. Food Chem., 2004, 52, 4355 CrossRef CAS.
- M.. Rieger, C. Cervino, J. C. Sauceda, R. Niessner and D. Knopp, Anal. Chem., 2009, 81, 2373 CrossRef CAS.
- S. Basu, S. K. Ghosh, S. Kundu, S. Panigrahi, S. Praharaj, S. Pande, S. Jana and T. Pal, J. Colloid Interface Sci., 2007, 313, 724 CrossRef CAS.
- H. Cui, Z. F. Zhang and M. J. Shi, J. Phys. Chem. B, 2005, 109, 3099 CrossRef CAS.
- J.-M. Lin and M. L. Liu, J. Phys. Chem. B, 2008, 112, 7850 CrossRef CAS.
- H. Cui, Z. F. Zhang, M. J. Shi, Y. Xu and Y. L. Wu, Anal. Chem., 2005, 77, 6402 CrossRef CAS.
- C. F. Duan, H. Cui, Z. F. Zhang, B. Liu, J. Z. Guo and W. Wang, J. Phys. Chem. C, 2007, 111, 4561 CrossRef CAS.
- Z. F. Zhang, H. Cui, C. Z. Lai and L. J. Liu, Anal. Chem., 2005, 77, 3324 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
