The use of Zincon for preconcentration and determination of Zinc by flame atomic absorption spectrometry
Mariana Gonçalves
De Martino
,
Greice Trevisan
Macarovscha
and
Solange
Cadore
*
Institute of Chemistry - University of Campinas, P.O. Box 6154, 13083-970, Campinas, SP, Brasil. E-mail: cadore@iqm.unicamp.br
First published on 10th June 2010
Abstract
A method for the determination of zinc using zincon as an enrichment agent and flame atomic absorption spectrometry was developed. The system consists of a glass column containing 200 mg of Dowex 1X8 resin modified with 1% (w/w) of zincon. The recovery of the metal was quantitative at pH 7.0 to 8.0 with the use of a potassium dihydrogen-phosphate/sodium hydroxide buffer solution. The colored complex was eluted from the column with 10.0 mL of a 0.1 mol L−1 HNO3 solution. Using these conditions an enrichment factor of 25 and at least 15 re-utilizations of the column were possible. The limit of determination was 1.7 μg L−1 and a relative standard deviation of 1.2% was obtained, calculated from 10 measurements. The foreign ions effect was studied and the system showed no significant interferences. The accuracy was ascertained by using certified reference material and the results obtained were in agreement with the certified values. The proposed method was successfully used for the determination of zinc in fruit juices.
Introduction
Zinc is an essential trace element to the human organism as it is a constituent of several enzymes, such as carboxypeptidase A, which catalyzes ester and peptide hydrolysis. This element has an important role in the metabolic process of carbohydrates, lipids and proteins. It is also essential in the synthesis of proteins, RNA and DNA.1,2 Deficiency of zinc in humans may affect the cells and can cause problems such as stunted growth, loss of appetite, mental lethargy, dermatitis, abnormalities of the skull and in the immunologic system, and diarrhea.3The dietary recommendations for zinc are 13 mg day−1.4 The main sources of zinc are water and food and, in this case, its concentration depends on the kind of final product and also the type of industrial process to which it was submitted.
Several papers describe the determination of zinc in food and beverages5–8 where the concentration varied from ng g−1 to mg g−1, depending on the kind of food and of brand of the product.
The determination of zinc may be carried out by several analytical techniques. Flame atomic absorption spectrometry (FAAS) is a fast and versatile technique, of relatively simple operation but sometimes it has a low sensitivity for trace elements in beverages. In order to overcome this limitation a concentration step is necessary. For the concentration of metallic ions, liquid–solid extraction has been one of the most frequently used techniques and consists of the adsorption of the analyte by organic reagents fixed on solid materials.9–13 Some of the reagents used for the determination of zinc are α-naphthylthiocarbazone, 1-(2-pyridylazo)-2-naphthol, 1-(2-thiazolylazo)-2-naphthol, dithizone, diethylcarbamate, and zincon.14
Yoe and Rush15 reported that zincon forms a blue precipitate with zinc. The reaction is fast in the presence of an alkaline solution of this reagent. Its reactivity depends on the pH and the zinc–zincon complex is stable in the pH range 8.5 to 9.5. The compound zincon [1–2(hydroxy-5-sulfophenyl)-5-(2-carboxifenil formazane) (Fig. 1) is usually found as the monosodium salt, which has three acid hydrogens: a carboxylic (–COOH), a phenolic (–OH) and one on the secondary amine group (=NH). Ionization steps of this reagent are:15,16
| H3In− ↔ H2In2− ↔ HIn3− ↔ In4− |
| pKa1 = 4 pKa2 = 8 pKa3 = 15 |
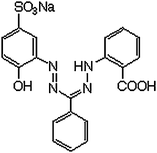 | ||
| Fig. 1 Zincon. | ||
For many years this reagent had been used for spectrophotometric determination of zinc and also for copper. Indeed, zincon also reacts with many other metals,16–22 but by working at appropriated pH interference problems may be controlled.
All these papers have used zincon as a colorimetric reagent but none of them explored the possibility of using this reagent for concentration procedures. Kocjan23 used a mixture of zincon and Aliquat 336 to modify the surface of silica gel and studied the sorption for several metallic ions including zinc. The results obtained suggest that this reagent could be used for the concentration of zinc.
The possibility of using this reagent for liquid–solid extraction was also suggested by Oehme et al.,24 who modified different solid materials with the ion pair zincon-tetraoctylammonium to obtain an optic membrane for the determination of copper.
In another paper, Kocjan et al.25 utilized silica (LiChroprep RP-8 and RP-18) modified with the ion pair Aliquat 336 and zincon to remove Zn and Cu from aqueous solutions. In this case, however, the authors studied the sorption capacity and the degradation of the modified silica. In a recent paper Li et al.26 used activated carbon modified with this reagent for the extraction and determination of lead and chromium in water samples.
The sequential determination of zinc and copper using zincon is possible due to the stability of both complexes considering the pH, that is, at pH 5.0 only Cu-zincon complex exists on the contrary of pH 9.0 where both Cu-zincon and Zn-zincon complexes are formed. Richter et al.27 described an injection system with two flows of pH (pH 5 and 9) that allowed the sequential formation of the colored complexes. Chemometric methods also allowed for the sequential determination of nickel, cobalt, zinc and copper using zincon as complexing reagent.28
The use of zincon is also appropriate for the determination of metals in metalloproteins, as described by Sabel et al.29 for Co, Cu and Zn.
As can be seen, zincon was largely used as concentration reagent in liquid–liquid extraction and as spectrophotometric reagent, mainly for the determination of zinc but also for other elements. However, its potential use as reagent for solid–liquid pre-concentration was not deeply investigated. Considering this point, in the present work a method for the determination of zinc, after its liquid–solid extraction using Dowex 1X8 resin modified with zincon, is described. The metal ion is eluted with a HNO3 solution and its concentration is determined by FAAS. In order to verify the possibility to use the proposed method in real samples it was applied for the determination of zinc in samples of fruit juices.
Experimental
Apparatus
The absorption measurements were made with a Perkin-Elmer 5100 Atomic Absorption Spectrometer (Norwalk, CT, USA) equipped with a hollow cathode lamp for zinc as well as a deuterium lamp for background correction, under the following conditions: wavelength 213.9 nm; slit width: 0,70; lamp current: 15 mA; flame: air/acetylene. The pH measurements were made by a pH-meter (Hanna Instruments, model pH200, São Paulo, SP, Brazil) equipped with a combined glass electrode (silver/silver chloride). The liquid–solid extraction system consisted of glass columns (4 mm internal diameter and 20 cm long) equipped with a Teflon® tap.30 The solution containing the analyte passed through the column, where the modified material was maintained between two portions of glass wool in order to avoid movement of the sorbent material. For sample treatment, a hot plate (Quimis, Piracicaba, SP, Brazil) was used.Reagents
All the reagents used were of analytical reagent grade. Ultra pure water (Milli-Q Water Purification System - Millipore, Bedford, USA) was used throughout. Laboratory glassware was kept overnight in a 10% v/v HNO3 solution and then rinsed with deionized water.The zinc solution was prepared from a standard solution (zinc standard per assorbimento atomico, Carlo Erba, 1000 mg L−1). Appropriate dilutions were made from this solution, whenever necessary, with deionized water.
Buffer solutions were prepared by mixing a 0.1 mol L−1 KH2PO4 solution and an adequate volume of 0.1 mol L−1 NaOH solution in order to adjust the pH.
Preparation of solid material modified with zincon
Before treatment with zincon, the chosen materials were washed with methanol for 1 h under constant stirring and left to dry in the oven (∼100 °C). The materials tried were silica gel (Merck, Darmstadt, Germany, 35–70 mesh ASTM), Dowex 1X8-200 resin (Aldrich Chemical Company, Milwaukee, USA, 100–200 mesh ASTM) and Amberlite XAD-7 HP (Acros Organics, Geel, Belgium).A 0.1% (w/v) zincon solution was prepared by weighing 0.100 g of zincon (Vetec, p.a., Rio de Janeiro, Brazil) and dissolving it in 3.0 mL of 1 mol L−1 NaOH. The volume was completed to 100.0 mL with deionized water. The solid material was then modified by mixing them (for 1 h) with the correct volume of the zincon solution to produce 1.0%, 0.4% and 0.2% (w/w) modified materials, that were filtered and then left to dry in the oven (100 °C). To avoid reagent decomposition the material was protected from the light during all procedures. The filtered solution was analyzed by UV-VIS spectrophotometry (spectrophotometer UV/VIS/Cary G5, Varian) and the concentration of zincon sorbed onto the solid material was calculated considering the initial and final concentration of the reagent.
Effect of pH
The pH effect on the extraction of Zn was investigated in the pH range of 6.0 to 10.0 with and without the use of a buffer solution. For pH lower than 8.0, a potassium dihydrogen-phosphate/sodium hydroxide buffer solution was used, and for pH higher than 8.0 a borate/sodium hydroxide buffer solution was used. These kind of buffer solution were used considering the previous work with zincon.15,16Column capacity
The capacity of the column was evaluated by passing a solution with constant mass of Zn and varying the volume considering that sometimes it is desirable or necessary to have a large volume of sample in order to reach the detection limit of the technique.Effect of foreign ions
Considering that zincon may react with many elements, it is important to carry out an interference study. The determination of zinc was evaluated in the presence of Ca, Co, Cu, Fe, K, Mg, Mn, Na and Ni, individually, in different proportions Zn: metallic ion, from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Two mixtures of all of the interferent ions were also studied: a) 75 μg of Cu, Fe, Co and Ni, 150 μg of Mn, 1500 μg of Mg, 1875 μg of Ca, 3000 μg of Na and 18750 μg of K were added to 7,5 μg of Zn. b) 7,5 μg Zn, 75 μg Cu and Fe, 150 μg Mn and Co, 375 μg Ni, 3000 μg Mg, 11250 μg Ca, 15000 μg Na and 75000 μg K.
10. Two mixtures of all of the interferent ions were also studied: a) 75 μg of Cu, Fe, Co and Ni, 150 μg of Mn, 1500 μg of Mg, 1875 μg of Ca, 3000 μg of Na and 18750 μg of K were added to 7,5 μg of Zn. b) 7,5 μg Zn, 75 μg Cu and Fe, 150 μg Mn and Co, 375 μg Ni, 3000 μg Mg, 11250 μg Ca, 15000 μg Na and 75000 μg K.
General procedure
The columns were packed with 200 mg of the modified material, washed with approximately 3.0 mL of a 0.1 mol L−1 nitric acid solution and conditioned with a pH 8.0 potassium dihydrogen-phosphate buffer solution.To optimize the conditions involved in the liquid–solid system, a known aliquot of a standard zinc solution was taken in a 100 mL beaker and the pH was adjusted to 8.0 with the buffer (dihydrogen-phosphate) solution. It was then passed through the column, and eluted with adequate volumes (5.0 to 25.0 mL) of 0.1 mol L−1 HNO3, using a flow rate of 2.0 mL min−1. The samples and certified material were prepared in similar manner.
The final solution was aspirated into an air-acetylene flame and the absorbance was measured against standard solutions of Zn. In order to verify the mass balance of the system the solution that passed through the column and was not retained by the sorbent material was also analyzed by FAAS after treating with nitric acid and heating.
Determination of zinc in certified material
For evaluating the accuracy of the method, two certified samples were analyzed: “Mixed Food Diet” (High Purity Standards, CRM-MFD Lot123215) and “Natural Water” (NIST, SRM 1640 LotH-483). Appropriate aliquots of the samples, considering the concentration of zinc described in the certified concentration of zinc, were transferred to a beaker and after the pH was adjusted to 8.0 the final solution was transferred to the column.Determination of zinc in beverages
The zinc content was determined in samples of fruit juices purchased from local supermarkets. The chosen juices were peach, grape, pineapple, passion fruit, cashew, acerola, guava and mango. Before the concentration step, the samples were mineralized on a hot plate. Some of the samples were also enriched with 0.2 μg L−1 of Zn before sample treatment, in order to verify the analyte recovery. For treatment, a mass of 5.0 g of the samples was taken in a beaker and 30.0 mL of concentrated nitric acid and 3.0 mL of 30% (v/v) H2O2 were added. After almost complete dryness, the solution was transferred to a 50.0 mL volumetric flask and the volume was completed with deionized water. An aliquot of 25.0 mL of the final solution was transferred to a 100 mL beaker and after pH adjustment, it was introduced into the concentration column. After this, the concentration of zinc in these beverages was determined by FAAS. In those samples enriched with the analyte the recovery of zinc was evaluated.31Results and discussion
Modification of the solid material
The sorption of zincon on the different materials showed that the best material for this purpose is the Dowex 1X8 resin, which was then modified with 0.2%, 0.4%, 1.0%, 2.0% and 3.0% (w/w) of zincon. As the concentration of zincon in the sorbent materials increased, so did the extraction of zinc. But for ratios greater than 1.0% (w/w) there was no significant increase, therefore the use of the Dowex 1X8 resin with 1.0% of zincon was selected. The amount of material to be used was also investigated and it was found that for amounts smaller than 200 mg the extraction decreases while for quantities larger than this the system becomes inconveniently slow.Effect of pH
The pH of the aqueous phase is an important factor in solute distribution due to the acid-basic properties of the complexing reagent. The zinc–zincon complex is stable in the pH interval of 8.5 to 9.5. At these pH values the species H2In2− and HIn3− are predominant.14,15 The pH effect on the extraction of Zn was investigated in the pH range of 6.0 to 10.0 with and without the use of a buffer solution. For pH lower than 8.0, a potassium dihydrogen-phosphate/sodium hydroxide buffer solution was used, and for pH higher than 8.0 a borate/sodium hydroxide buffer solution was used. The results showed that the use of a buffer solution is necessary to obtain better results, but also that they are different from the expected due to effects from the different buffer solutions used.The literature14,15 describes the use of pH 8.5–9.5 for the zinc–zincon complex formation, but using liquid–solid extraction lower values of pH were required probably due to kinetics effects occurring for this complex in the sorption process. For this reason, a more detailed investigation concerning the type of buffer solution was carried out. Solutions of potassium dihydrogen-phosphate/sodium hydroxide, ammonium hydroxide/ammonium chloride borate/sodium hydroxide, and acetic acid/sodium acetate were tested. Fig. 2 shows the best results obtained with the buffer solutions that allowed recovery of zinc. The use of potassium dihydrogen-phosphate/sodium hydroxide buffer solution in the pH range of 7.0 to 8.0, shows extraction efficiencies of 97% and was selected as the optimized condition for this work.
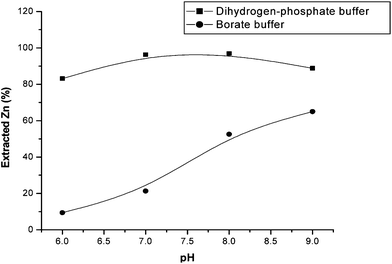 | ||
| Fig. 2 Effect of pH on zinc extraction using zincon. | ||
Eluent for the system
To optimize the system aiming quantitative recovery of zinc, two eluents were evaluated in concentrations that varied from 0.05 to 0.5 mol L−1. The volume of acid required to extract all the zinc from the column was also investigated. The performance of both eluents, HNO3 and HCl, was good considering concentration between 0.1 and 0.3 mol L−1, but HNO3 showed slightly better results. In this case a solution of 0.1 mol L−1 HNO3 was selected as the eluent for the system. Using 5.0 mL of 0.1 mol L−1 HNO3 it was possible to obtain a good recovery of zinc (90%) while a volume of 10.0 mL allowed recoveries of 96% of the analyte. For the next experiments a volume of 10.0 mL of 0.1 ml L−1 HNO3 was selected but it is important to know that, if necessary, small volumes may be used with quantitative recoveries. For both steps, preconcentration of zinc and its elution a flow rate of 2.0 mL min−1 was used. This flow rate showed to be the best when values from 0.5 to 3.0 mL min−1 were investigated.Column capacity
In this study it was possible to introduce into the column up to 300 mL of samples containing 7.5 μg of zinc without affecting the complete retention of the element.Column re-utilization
To reduce the cost and time spent in the analysis as well as the generation of residues, it is important to reutilize the sorbent material in the columns. In this case it was possible to use each column 15 times without affecting the recovery of zinc. Recoveries higher than 94% were obtained in all the tests.Effect of foreign ions
It is well known that the complexing reagent, zincon, reacts not only with zinc, but also with other elements such as Cu, Ni, Fe and Co.14,15 For this reason, it is necessary to investigate the interference of these species in the determination of zinc concerning the proposed system.Considering the composition of the samples to be analyzed, the effects of other metallic ions, K, Ca, Na, Mn and Mg, were also investigated. In this study solutions containing 7.5 μg of Zn were spiked with different concentrations of the foreign ions. When studied separately the ions showed no interference at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (Zn
10 (Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metallic ion). When a mixture of large excess of potentially interfering ions was used a white precipitate was formed due to iron hydroxide formation at pH 8.0, leading to the loss of zinc during filtration, and a consequent decreased recovery as can be seen in Table 1. A mixture of ions in concentrations based on the mineral composition of beverages was used and also showed no interference under the studied conditions.
metallic ion). When a mixture of large excess of potentially interfering ions was used a white precipitate was formed due to iron hydroxide formation at pH 8.0, leading to the loss of zinc during filtration, and a consequent decreased recovery as can be seen in Table 1. A mixture of ions in concentrations based on the mineral composition of beverages was used and also showed no interference under the studied conditions.
| Interferent | Zn recovery (%) |
|---|---|
| Cu | 97 |
| Fe | 89 |
| Co | 97 |
| Ni | 103 |
| Mn | 97 |
| Mg | 103 |
| Ca | 97 |
| Na | 106 |
| K | 101 |
| Mixture a | 90 |
| Mixture b | 75 |
Figures of merit
Considering that it is possible to retain 0.5 μg of zinc from 300 mL of solution passing through a column (250 × 4 mm) filled with 200 mg of Dowex 1X8 resin modified with zincon, the elution of the resulting complex with 10 mL of 0.1 mol L−1 HNO3, at a flow rate of 3 mL min−1, gives a limit of determination of 1.7 μg L−1 and an enrichment factor of 25. The precision, expressed as a relative standard deviation (RSD, n = 10) is 1.2%. The limit of detection, calculated as three times the blank standard deviation was 0.5 μg L −1. The accuracy of the proposed method was evaluated by means of zinc determination in two certified samples: “Mixed Food Diet” (High Purity Standards, CRM-MFD Lot123215) and “Natural Water” (NIST, SRM 1640 LotH-483). The concentrations found were in reasonable agreement with the certified values (Table 2) showing that it is possible to use the proposed system for the determination of zinc.Determination of Zinc
Due to accurate results obtained, the method was then applied for the determination of zinc in concentrated fruit juices. Table 3 shows the concentration of zinc obtained with the proposed method, which have no statistical difference (at 95% confidence level) with the results obtained by ICP OES (Inductively Coupled Plasma Optical Emission Spectrometry). In order to confirm these results some samples were enriched with the analyte and their recoveries were also evaluated. For all the juices analyzed, the recovery was quantitative (90–110%) attesting that the proposed method is adequate for this determination.| Juice | Concentration of zinc/mg kg−1 | |
|---|---|---|
| FAAS | ICP OES | |
| Cashew | 0.97 ± 0.05 | 0.9 ± 0.1 |
| Passion Fruit | 1.4 ± 0.1 | 1.5 ± 0.3 |
| Grape | 0.46 ± 0.02 | 0.5 ± 0.1 |
| Guava | 0.52 ± 0.04 | 0.6 ± 0.2 |
| Pineapple | 0.3 ± 0.1 | 0.6 ± 0.2 |
| Mango | 0.52 ± 0.02 | 0.5 ± 0.1 |
Conclusions
This study showed that zincon is an appropriate reagent for preconcentration of zinc and the proposed method is simple, sensitive and of low cost considering that the sorption material can be reutilized at least 15 times. The preconcentration of zinc is effective in the presence of a potassium dihydrogen-phosphate/sodium hydroxide buffer solution in the pH range of 7.0 to 8.0 and its elution is quantitative using 10 mL of 0.1 mol L−1 HNO3. The determination of zinc in certified reference materials showed adequate accuracy. Although it was only applied for samples of fruit juices, the enrichment factor of 25 obtained using the optimized conditions shows the potential use of zincon as an agent for preconcentration of zinc.Acknowledgements
The authors gratefully acknowledge Dr Carol H. Collins for assistance with English in this manuscript, as well as the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support of this work.References
- D. F. Shriver, P. W. Atkins, C. H. Langford, Inorganic Chemistry, 2nd ed.; W.H. Freeman and Co., New York, 1994 Search PubMed.
- R. E. Black, American Journal of Clinical Nutrition, 1998, 68, 476S–479S Search PubMed.
- C. J. A. Van de Hamer and C. Cornelisse, Sci. Total Environ., 1985, 42, 83–89 CrossRef.
- D. L. Tsalev, Z. K. Zaprianov, Atomic Absorption Spectrometry in Occupational and Environmental Health Practice, vol. 1, CRC Press Inc., Boca Raton, Florida, 1984 Search PubMed.
- P. C. Onianwa, I. G. Adetola, C. M. A. Iwegbue, M. F. Ojo and O. O. Tella, Food Chem., 1999, 66, 275–279 CrossRef CAS.
- C. Terrés, M. Navarro, F. Martin-Lagos, R. Giménez, H. López and M. C. López, Food Addit. Contam., Part A, 2001, 18, 687–695 Search PubMed.
- T. Soulis, E. Kavlentis and I. Arvanitoyannis, J. Sci. Food Agric., 1988, 45, 373–377 CAS.
- N. A. R. Pedro, E. Oliveira and S. Cadore, Food Chem., 2006, 95, 94–100 CrossRef CAS.
- Z. Marczenko, Spectrophotometric Determination of Elements, Ellis Harwood Limited, Chichester, 1976 Search PubMed.
- S. Cadore, R. D. Goi and N. Baccan, J. Braz. Chem. Soc., 2005, 16, 957–962 CAS.
- A. P. dos Anjos, L. Cornejo-Ponce, S. Cadore and N. Baccan, Talanta, 2007, 71, 1252–1256 CrossRef.
- H. S. Ferreira, A. C. N. Santos, L. A. Portugal, A. C. S. Costa, M. Miro and S. L. C. Ferreira, Talanta, 2008, 77, 73–76 CrossRef CAS.
- J. Naozuka and P. V. Oliveira, J. Braz. Chem. Soc., 2007, 18, 1547–1553 CAS.
- J. H. Yoe and R. M. Rush, Anal. Chim. Acta, 1952, 6, 526–527 CrossRef CAS.
- R. M. Rush and J. H. Yoe, Anal. Chem., 1954, 26, 1345–1347 CrossRef CAS.
- F. S. Sadek, R. W. Schmid and C. N. Reilley, Talanta, 1959, 2, 38–51 CrossRef CAS.
- K. S. Anand, P. Dayal and O. N. Anad, Fresenius' Z. Anal. Chem., 1968, 239, 33–36 CrossRef CAS.
- M. A. Dosal Gomez and J. A. Perez Bustamante, Analytical Chemistry, 1977, 73, 968–977 CAS.
- M. A. Koupparis and P. I. Anagnostopoulous, Analyst, 1986, 111, 1311–1315 RSC.
- H. E. Mabrouk, M. Gaber and A. Z. Elsonbati, Transition Metal Chemistry, 1992, 17, 1–4 Search PubMed.
- R. M. Liu, D. J. Liu, A. L. Sun and G. H. Liu, Analyst, 1995, 120, 569–572 RSC.
- M. R. Macnair and N. Smirnoff, Commun. Soil Sci. Plant Anal., 1999, 30, 1127–1136 CrossRef CAS.
- R. Kocjan, Analyst, 1994, 119, 1863–1865 RSC.
- I. Oehme, S. Prattes, O. S. Wolfbeis and G. J. Mohr, Talanta, 1998, 47, 595–604 CrossRef CAS.
- R. Kocjan, I. Sowa and E. Blicharska, Chemia Analyticzna, 2002, 47, 219–227 Search PubMed.
- Z. H. Li, X. J. Chan, Z. Hu, X. P. Huang, X. J. Zou, Q. Wu and R. Nic, J. Hazard. Mater., 2009, 166, 133–137 CrossRef CAS.
- P. Richter, M. I. Toral, A. E. Tapia and E. Fuenzalida, Analyst, 1997, 122, 1045–1048 RSC.
- J. Ghasemi, Sh. Ahmadi and K. Torkestani, Anal. Chim. Acta, 2003, 487, 181–188 CrossRef CAS.
- C. E. Sabel, J. M. Neureuther and S. Siemann, Anal. Biochem., 2010, 397, 218–226 CrossRef CAS.
- G. G. Bortoleto, G. T. Macarovscha and S. Cadore, J. Braz. Chem. Soc., 2004, 15, 313–317 CAS.
- M. Thompson, S. L. R. Ellison, A. Fajgelj, P. Willetts and R. Wood, Pure Appl. Chem., 1999, 71, 337–348 CAS.
| This journal is © The Royal Society of Chemistry 2010 |
