Towards an improved qualitative and quantitative determination of glutathione peroxidase, selenoprotein P and selenoalbumin in human serum by HPLC coupled to ICP-MS
Petru
Jitaru
*ab,
Giulio
Cozzi
a,
Roberta
Seraglia
c,
Pietro
Traldi
c,
Paolo
Cescon
ad and
Carlo
Barbante
ad
aInstitute for the Dynamics of Environmental Processes (IDPA-CNR), Dorsoduro 2137, 30123, Venice, Italy
bInstitut Polytechnique LaSalle Beauvais, Département des Sciences et Techniques Agro-Industrielles, 19 rue Pierre Waguet BP 30313, 60026 Beauvais Cedex, France. E-mail: Petru.Jitaru@lasalle-beauvais.fr; Fax: + 33 3 44 06 25 26; Tel: + 33 3 44 06 89 72
cInstitute of Scientific and Molecular Technologies (ISTM-CNR), Corso Stati Uniti 4, 35100, Padova, Italy
dUniversity of Venice Ca' Foscari, Department of Environmental Sciences, Dorsoduro 2137, 30123, Venice, Italy
First published on 12th July 2010
Abstract
This paper deals primarily with the validation of a clean-up procedure based on anion exchange solid-phase extraction for the accurate determination of glutathione peroxidase (GPx), selenoprotein P (SelP) and selenoalbumin (SeAlb) in human serum by affinity HPLC (AF-HPLC) coupled to inductively coupled plasma-mass spectrometry (ICP-MS). In addition, the identification and the purity assessment of the GPx, SelP and SeAlb peak fractions separated by AF-HPLC is addressed by their analysis using matrix assisted laser desorption ionization-time of flight mass spectrometry.
Introduction
Serum is the bio-indicator mostly used for the assessment of selenium status in humans and the concentrations of glutathione peroxidase (GPx, isoform 3, ∼90–92 kDa) and selenoprotein P (SelP, ∼57 kDa), which are the main selenoproteins in this fluid, are commonly determined for this purpose.1 Besides GPx and SelP, selenoalbumin (SeAlb, ∼66 kDa), which is a seleno-containing protein, is also monitored because part of Se from the diet is incorporated non-specifically into serum albumin forming traces of SeAlb, and hence the level of this selenium-containing protein may provide information regarding the nutritional status of Se. More details regarding the biochemistry and essentiality of Se and its relationship with different diseases in humans can be found elsewhere.2,3The accurate determination of GPx, SelP and SeAlb (all referred here for simplicity as ‘selenoproteins’) in human serum is challenging because of their relatively low concentration and the high complexity of the serum matrix, which can lead to significant non-spectral and spectral interferences. The best method currently available for the quantification of GPx, SelP and SeAlb in serum is based on affinity high performance liquid chromatography (AF-HPLC)4–10 coupled on-line with inductively coupled plasma-mass spectrometry (ICP-MS). Apart from the fact that the ICP-MS detection of the most abundant isotope of Se (80Se) is seriously interfered by plasma argon (40Ar40Ar), the main drawback of this approach arises from the co-elution of Cl− and Br− (which are present in serum at high levels: ∼3.5 g L−1 and ∼3.5 mg L−1, respectively) with GPx (in the void volume, GPx is not-retained by AF-HPLC columns), which causes spectral interferences on several other Se isotopes, such as 40Ar37Cl on 77Se, 79Br1H on 80Se and 81Br1H on 82Se. While a collision/reaction cell ICP-quadrupole MS (CRC ICP-qMS) can theoretically be used for the detection of 80Se (the interference caused by the argon dimers is considerably alleviated), in case of the presence of Br in the sample matrix, the addition of H2 (the common reaction gas) in the CRC ICP-qMS leads to the formation of hydrides, the most critical being the formation of 79Br1H, which interferes with 80Se. Therefore, when applied to the analysis of human serum, which contains high amounts of Br− (ca 3.5 μg mL−1), the CRC cannot resolve the severe spectral interference previously mentioned. ICP-sector field-MS (sf-ICP-MS) can be used in such a situation, but, apart from its high cost, this technique suffers from low sensitivity when working in the high-resolution mode and consequently a high efficiency sample introduction device must be used.7 In this context, the interferences mentioned above can be relatively simple and efficiently alleviated by serum clean up using anion exchange solid phase extraction (AE-SPE), whose capability to remove Cl− and Br− from human serum was demonstrated elsewhere.5 In our previous work,5 the accuracy of the AE-SPE and AF-HPLC-ICP-qMS procedure was tentatively demonstrated by comparison of the levels of total protein-bound Se (GPx + SelP + SeAlb) in two CRMs (BCR-637 and BCR-639) with certified values of total Se.
This study completes in terms of validation the AE-SPE procedure developed previously5 for serum clean up prior to the determination of selenoproteins by AF-HPLC coupled to ICP-qMS. In this case, BCR-639 CRM, a high Se level material obtained by spiking a genuine serum (BCR-637 CRM) with inorganic selenium, was analyzed by ICP-qMS and sf-ICP-MS for total Se following the AE-SPE clean up. In this case, the clean up capability of AE-SPE was proven effective also for the removal of inorganic Se, which otherwise would bias the determination of GPx.
The identification and the purity assessment of human serum GPx, SelP and SeAlb separated by AF-HPLC (peak fraction collection) is also addressed here by analysis using matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOFMS). It is worth noting that although AF-HPLC has been successfully applied to the quantitative analysis of GPx, SelP and SeAlb in human serum, unequivocal identification of these selenoproteins separated by AF-HPLC has not yet been reported in the literature.
Experimental
Reagents and chemicals
All reagents used were of at least analytical-reagent grade purity and all solutions were prepared gravimetrically in a class 1000 clean room under a class 100 laminar flow bench.5A pooled un-diluted human serum obtained by centrifugation (3000 rev min−1 for 10 min) of freshly collected blood was used throughout for method optimization. The human serum certified reference material (CRM) BCR-639 was purchased from the Institute for Reference Materials and Measurements (IRMM), Geel, Belgium. The serums were divided into aliquots of 1.5 mL and stored in Eppendorf tubes at −20 °C until analyzed. Prior to the analysis, serum was defrosted at room temperature and then filtered using 0.45 μm cellulose acetate syringe filters (Alltech Italia Milano, Italy).
Standards of human and bovine serum albumin (≥99%), human glutathione peroxidase (100 units mg−1 protein) and bovine glutathione peroxidase (700 units mg−1 protein), immunoglobulin G (≥95%) and apo-transferrin (≥98%) from human serum were purchased from Sigma-Aldrich (Milano, Italy).
The Protein Calibration Standard II (Bruker Daltonics GmbH, Bremen, Germany) containing trypsinogen, protein A and bovine albumin was used for the external mass calibration of the MALDI-TOFMS.
Apparatus
AE-SPE was carried out using SAX Maxi-Clean™ cartridges (∼2 cm length) (Alltech Italia) packed with an anion exchange resin (0.6 g of styrene divinylbenzene with quaternary ammonium groups, particles size 45–150 μm). Microcon™ centrifugal filters (Millipore, MA, USA) with nominal molecular weight limits (NMWL) of 10 kDa were employed for serum desalting/preconcentration. 10 μL C18 ZipTip™ pipette tips (Millipore) were used for the purification of selenoproteins (separated by AF-HPLC) just prior to MALDI-TOFMS analysis. In brief, ZipTip is a 10 μL pipette tip with a 0.6 or 0.2 μL bed of chromatography media fixed at its end with no dead volume. It is suitable for concentrating and purifying proteins prior analysis by mass spectrometry, such as MALDI-TOFMS or other analytical techniques.11The separation of GPx, SelP and SeAlb from human serum by AF-HPLC (1.0 mL min−1 flow rate) was carried out using HiTrap™ Heparin HP (Heparin-Sepharose, HEP) and HiTrap™ Blue HP (BLUE) columns (2.5 cm length × 0.7 cm ID × 34 μm particle size) from GE Healthcare Europe (Milano, Italy) using the experimental set-up reported elsewhere.5 More details of the HPLC and ICP-qMS instruments used can be found in previous publications.5,6
The determination of total Se in serum by sf-ICP-MS was carried out using an Element2 instrument (Thermo Fisher Scientific, Bremen, Germany) with the experimental parameters reported elsewhere.7
MALDI-TOFMS analyses were carried out with an Ultraflex II instrument from Bruker Daltonics GmbH (Bremen, Germany).
Procedures
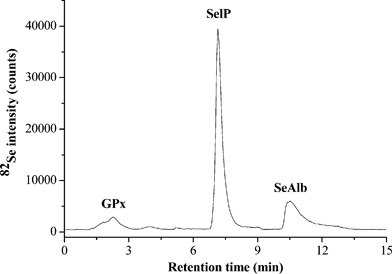 | ||
| Fig. 1 Typical chromatogram obtained by analysis of un-diluted human serum by AF-HPLC coupled to ICP-qMS (82Se monitored) following AE-SPE clean up. A flow rate of 1.0 mL min−1 and 100 μL injection loop were used throughout the AF-HPLC separations; the chromatographic details can be found elsewhere.5 | ||
The sample spots for MALDI-TOFMS analysis of selenoproteins separated by AF-HPLC were prepared by mixing 5 μL of sample with 5 μL of matrix solution (saturated solution of sinapinic acid in H2O–acetonitrile 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v:v with 0.1% trifluoroacetic acid); 1 μL of the resulting mixture was deposited onto a stainless steel holder and allowed to dry before its introduction into the MALDI-TOFMS instrument. Molecular ions were generated by a pulsed UV nitrogen laser (λ = 337 nm). In order to improve the signal-to-noise ratio, the spectra obtained by 100 laser shots were averaged. External mass calibration was achieved on the basis of the average values of [M + H]+ for trypsinogen (m/z = 23,982), protein A (m/z = 44,613) and bovine albumin (m/z = 66,431) and [M + 2H]2+ for protein A (m/z = 22,306) and bovine albumin (m/z = 33,216).
50 v:v with 0.1% trifluoroacetic acid); 1 μL of the resulting mixture was deposited onto a stainless steel holder and allowed to dry before its introduction into the MALDI-TOFMS instrument. Molecular ions were generated by a pulsed UV nitrogen laser (λ = 337 nm). In order to improve the signal-to-noise ratio, the spectra obtained by 100 laser shots were averaged. External mass calibration was achieved on the basis of the average values of [M + H]+ for trypsinogen (m/z = 23,982), protein A (m/z = 44,613) and bovine albumin (m/z = 66,431) and [M + 2H]2+ for protein A (m/z = 22,306) and bovine albumin (m/z = 33,216).
Results and discussion
Accuracy assessment of the AE-SPE clean up procedure in combination with AF-HPLC-ICP-qMS for the determination of GPx, SelP and SeAlb in human serum
In this study, efficient separation of GPx, SelP and SeAlb in undiluted human serum was obtained with a total chromatographic time under 15 min (see Fig. 1) using a double column AF-HPLC system exploiting the proteins' selectivity towards HEP and BLUE stationary phases, the separation principle is described elsewhere.7 A serum clean up procedure based on AE-SPE using a styrene divinylbenzene resin with quaternary ammonium functional groups was developed in a previous study in order to efficiently remove Cl− and Br− which interfere with Se determination by ICP-qMS.5 Several isobaric interferences that can potentially interfere with the determination of Se were monitored before and after clean up of the serum by AE-SPE. As can be seen in Table 1, apart from the complete removal of Cl− and Br− (which are the main interfering elements) the levels of 34S, 31P and several metals (Cr, Co, Fe, Ni, Cu and Zn), which could lead to spectral interferences on 77Se and 82Se decreased considerably following AE-SPE serum clean up. This indicates that the clean up procedure for human serum proposed here may improve the accuracy of the determination of selenoproteins by AF-HPLC-ICP-qMS, particularly of GPx, given that it co-elutes with most of the interfering elements listed in Table 1.| Isotope monitored | Possible spectral interference | Potentially interfered isotope | Interfered co-elution peak | ||
|---|---|---|---|---|---|
| GPx | SelP | SeAlb | |||
| 31P | 31P32S14N | 77Se | 10 | 90 | 90 |
| 31P35Cl16O | 82Se | ||||
| 34S | 34S16O16O16O | 82Se | 30 | 60 | 30 |
| 34S16O16O16O | 82Se | ||||
| 35Cl | 40Ar37Cl | 77Se | 0 | not present | not present |
| 37Cl | 35Cl35Cl12C | 82Se | |||
| 53Cr | 50Cr32S, 50Cr31P1H | 82Se | 0 | not present | not present |
| 59Co | 59Co18O | 77Se | 70 | 100 | 100 |
| 57Fe | 56Fe14N12C | 82Se | 50 | 100 | not present |
| 60Ni | 60Ni16O1H, 61Ni16O | 77Se | 40 | not present | not present |
| 65Cu | 63Cu14N, 65Cu12C | 77Se | 10 | 100 | 30 |
| 66Zn | 66Zn16O | 82Se | 30 | 80 | 80 |
| 81Br | 81Br1H | 82Se | 0 | not present | not present |
The accuracy assessment of the AE-SPE procedure for alleviating spectral interferences prior to the analysis of GPx, SelP and SeAlb by AF-HPLC-ICP-qMS was carried out by analysis of a CRM for total Se, BCR-639 human serum (certified concentration = 133 ± 12 ng mL−1). The CRM was produced (by the manufacturer) by spiking a genuine human serum certified for total Se (BCR-637 CRM, certified Se level of 81 ± 7 ng mL−1) with 51 ng mL−1 inorganic Se (sodium selenate, SeO42−). BCR-639 was chosen in this study in order to evaluate the efficiency of AE-SPE for the removal of Cl− and Br− (the main interfering anions in Se determination) as well as the inorganic selenium (SeO42−), which biases the determination of GPx (both co-elute in the void volume). Assuming that AE-SPE efficiently removes Cl−, Br− and SeO42−, the purified BCR-639 serum would contain a level of total Se corresponding to that of the genuine serum (BCR-637 before being spiked with SeO42−), namely 81 ± 7 ng mL−1. In this case, analysis of a serum following the AE-SPE clean up proposed will ensure accurate quantification of the selenoproteins (GPx, SelP and SeAlb).
Analysis of BCR-639 for total Se, either before or after AE-SPE clean up was carried out by ICP-qMS and sf-ICP-MS (high-resolution mode). For this purpose, the serum was diluted 10- and 50-fold, respectively with 1% HNO3; three internal standards (IS), namely Y, Rh and In were employed to alleviate the non-spectral interferences (only the results closest in agreement with the certified values obtained using IS are reported in Table 2); a mixture of the three ISs was spiked at a level of 0.2 ng mL−1 in blanks, standards and samples. In order to ensure comprehensive data comparison, ICP-qMS and sf-ICP-MS analyses were carried out in the same way and using the same solutions (blanks, standards and samples).
| Dilution | Total Se/ng mL−1 | ||||
|---|---|---|---|---|---|
| ICP-qMS | sf-ICP-MS | ||||
| No AE-SPEa | AE-SPEb | No AE-SPEa | AE-SPEb | ||
| a Certified value: 133 ± 12 ng mL−1. b Indicative value: 81 ± 7 ng mL−1 (the total Se level in BCR-637 serum CRM). c Internal standardization using 89Y. d Internal standardization using 103Rh. | |||||
| 1/50 | no IS | 138±7 | 83±4 | 143±7 | 86±5 |
| IS | 134±6d | 72±3d | 130±6c | 76±4c | |
| 1/10 | no IS | 167±8 | 86±4 | 173±9 | 84±4 |
| IS | 156±7c | 80±4c | 136±6d | 89±5d | |
As shown in Table 2, good recovery for total Se in BCR-639 was obtained by ICP-qMS analysis after its AE-SPE clean up regardless of the dilution factor and the presence or absence of an IS. While, after 50-fold dilution, good agreement with the certified result was obtained when the serum was analyzed without AE-SPE clean up, the analysis of a 10-fold diluted serum turned out to be highly biased. Given that selenoproteins are commonly analyzed without diluting the serum (because of the low concentrations of GPx and SeAlb, see Fig. 1), these results demonstrate not only the efficiency of AE-SPE to remove the main (spectral) interfering elements such as Cl− and Br− but its potential to remove free SeO42− also present in serum, which biases in particular the determination of GPx.
The results obtained by sf-ICP-MS are comparable in all cases to those obtained by ICP-qMS; it is worth noting here that in high-resolution mode, accurate results were obtained, except in the case when no IS was used for the analysis of the 10-fold diluted serum.
The levels of total protein-bound Se in BCR-639 serum obtained in the previous work5 by means of AE-SPE and AF-HPLC-ICP-qMS agrees very well with those obtained in this study by ICP-qMS and sf-ICP-MS (Table 2), hence confirming that AE-SPE can be used as an efficient serum clean up procedure for the determination of total Se and selenoproteins in human serum.
Identification of selenoproteins separated by AF-HPLC
It is interesting to note that so far there is no published information regarding the identification of GPx, SelP and SeAlb separated by double column AF-HPLC. In addition, whereas GPx and albumin (which contains traces of SeAlb) standards are commercially available, their analysis by AF-HPLC-ICP-MS was not found in the literature; so far, the identification of GPx, SelP and SeAlb proteins separated by AF-HPLC has been performed on the basis of their (chromatographic) behavior towards the HEP and BLUE affinity media, as initially reported by Deagen et al.13Analysis of GPx and SeAlb standards by AF-HPLC-ICP-qMS
Identification of GPx and SeAlb in human serum was attempted first by analyzing commercial standards of GPx (∼50 units mL−1) and albumin (∼100 mg mL−1) obtained from human blood (a SelP standard is not commercially available yet) and the chromatograms obtained by AF-HPLC-ICP-qMS are shown in Fig. 2a–c. As can be seen in Fig. 2a, the analysis of the albumin standard confirmed (retention time matching exactly) the presence of SeAlb in the fraction separated by AF-HPLC and also the relatively high purity of this standard. Conversely, a low-intensity peak was obtained for the analysis of the GPx standard; moreover, the analysis of this standard revealed a predominant peak that can be attributed to SelP (based on the retention time matching SelP in the serum) and a less intense peak for SeAlb (see Fig. 2b), which indicates low purity of the GPx standard. These experiments were repeated using bovine standards and the same results were obtained. In addition, solutions of GPx and albumin (with the same concentration) prepared from standards purchased from a different stock solution were analyzed by microbore AF-HPLC hyphenated to CRC ICP-qMS as described elsewhere7; as can be seen in Fig. 2d–e, similar chromatograms were obtained. It therefore appears difficult to assess unequivocally the identity of GPx by AF-HPLC-ICP-qMS analysis of commercial standards. In addition, given that purified standards for SelP are not yet commercially available, the assessment of the identity and purity of selenoproteins separated by AF-HPLC should be performed using a complementary method such as MALDI-TOFMS. | ||
| Fig. 2 Chromatograms obtained for the analysis of human albumin (a) and GPx (b, which is zoomed in c) standards by AF-HPLC-ICP-qMS (monitoring 82Se) and by microbore HPLC coupled to CRC ICP-qMS (80Se), with (d) for GPx and (e) for SeAlb. Note: The microbore AF-HPLC system consisted of HEP and BLUE columns of 1.0 mm ID (5 cm length). 5 μL of sample was injected onto the microbore AF-HPLC system and a flow rate of 0.3 mL min−1 was used, as described in detail elsewhere.7 | ||
MALDI-TOFMS analysis of GPx, SelP and SeAlb fractions separated by AF-HPLC
The peak fractions of GPx, SelP and SeAlb separated by AF-HPLC were analyzed by MALDI-TOFMS following their preconcentration and purification as described in the experimental section. As can be seen in Fig. 3a, positive identification was obtained for SeAlb: the ion at m/z 68,404 corresponds to [M + H]+ of SeAlb, while the ions at m/z 34,207 and 22,805 correspond to [M + 2H]2+ and [M + 3H]3+, respectively. | ||
| Fig. 3 MALDI-TOFMS spectra obtained for the analysis of SeAlb (a), SelP (b) and GPx (c) peak fractions separated by (double column) AF-HPLC. | ||
Identification of GPx and SelP by MALDI-TOFMS was not achieved in this study (the mass spectra did not contain any peak at the theoretical mass of these proteins). It is worth noting that the (sulfur) albumin is the most abundant protein in human serum (25–50 mg mL−1)14 and accounts for ∼60% of the total mass of proteins in this fluid, whereas the levels of SelP and GPx are considerably lower, which may explain the difficulty of their detection by MALDI-TOFMS. Indeed, an average concentration of ∼60 ng mL−1 of Se in SelP corresponds to a level of ∼4 μg mL−1 SelP (as protein); similarly, for an average GPx level of ∼10–15 ng mL−1 (as Se) (one Se atom per molecule) the concentration of GPx (as protein) in human serum is ∼10 μg mL−1. Therefore, compared to albumin (∼50 mg mL−1), the levels of SelP and GPx are ∼104 and 5 × 103 times lower, respectively. Correlated with the high complexity of the serum, this largely explains the difficulty in MALDI-TOFMS detection of GPx and SelP obtained after their separation by AF-HPLC.
As can be seen in Fig. 3b, the MALDI-TOFMS spectrum obtained for the analysis of the SelP consists of two ions at m/z = 83,000 and 154,000, respectively. An ion at m/z∼80,000 was also detected in the fraction corresponding to GPx (see Fig. 3c). Most likely these peaks are attributed to apo-transferrin (AT) (Mr ≅ 76–81 kDa) and immunoglobulin G (IgG, Mr = 150 kDa), double ionized (m/z ∼75–80,000), as both these proteins are highly abundant in the human serum (albumin and IgG represent more than 75% of all serum proteins).15 The presence of IgG and AT in the fractions of GPx and SelP was confirmed by AF-HPLC-ICP-qMS analysis of IgG and AT standards (chromatograms are shown in Fig. 4a–b). The analysis of an AT standard led to two peaks, one at the retention time corresponding to GPx (tGPx) and one to SelP (tSelP), which may explain the presence of this protein in the fractions of GPx and SelP. The analysis of IgG showed a single peak in the fraction corresponding to SelP, which may also confirm the presence of IgG (m/z∼150,000) in the fraction of SelP, accordingly to what was found by MALDI-TOFMS analysis.
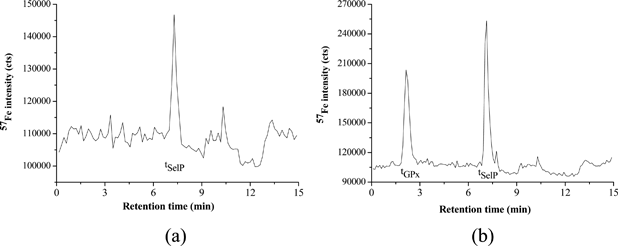 | ||
| Fig. 4 Chromatograms obtained for the analysis of immunoglobulin G (a) and apo-transferrin (b) standards by AF-HPLC-ICP-qMS (57Fe monitored) using the chromatographic details described elsewhere.5 | ||
Conclusions
This study demonstrates the suitability of a clean up procedure based on AE-SPE for the elimination of the major interfering elements (Cl−, Br− and inorganic Se) from human serum, making it suitable for the accurate determination of GPx, SelP and SeAlb in this human fluid by AF-HPLC-ICP-qMS. While the identification of selenoproteins turned out to be uncertain using analysis of commercial GPx and albumin standards alone, MALDI-TOFMS analysis of GPx and SelP fractions purified by AF-HPLC was challenging because of their relative low level in serum. Work is in progress to develop a more efficient procedure for the purification and preconcentration of the selenoproteins separated by AF-HPLC, in order to achieve the sensitivity required for their positive identification by MALDI-TOFMS.Acknowledgements
In Italy, this study was a contribution to the Marie-Curie Intra-European Fellowship Project (MEIF-CT-2006-024156/ELSA-BIM) funded by the European Commission. The authors thank Marco Prete (University of Venice Ca' Foscari) and Laura Molin (ISTM-CNR) for laboratory assistance.References
- H. Koyama, K. Omura, A. Ejima, Y. Kasanuma, C. Watanabe and H. Satoh, Anal. Biochem., 1999, 267, 84–91 CrossRef CAS.
- Selenium: Its Molecular Biology and Role in Human Health, ed. D. L. Hatfield, M. J. Berry and Gladyshev V N, Springer, New York, 2nd edn, 2006 Search PubMed.
- E. Dumont, F. Vanhaecke and R. Cornelis, Anal. Bioanal. Chem., 2006, 385, 1304–1323 CrossRef CAS.
- L. Hinojosa Reyes, J. M. Marchante-Gayon, J. I. Garcia Alonso and A. Sanz-Medel, J. Anal. At. Spectrom., 2003, 18, 1210–1216 RSC.
- P. Jitaru, M. Prete, G. Cozzi, C. Turetta, W. Cairns, R. Seraglia, P. Traldi, P. Cescon and C. Barbante, J. Anal. At. Spectrom., 2008, 23, 402–406 RSC.
- P. Jitaru, G. Cozzi, A. Gambaro, P. Cescon and C. Barbante, Anal. Bioanal. Chem., 2008, 391, 661–669 CrossRef CAS.
- P. Jitaru, M. Roman, G. Cozzi, P. Fisicaro, P. Cescon and C. Barbante, Microchim. Acta, 2009, 166, 319–327 CrossRef CAS.
- P. Jitaru, M. Roman, C. Barbante, S. Vaslin-Reimann and Paola Fisicaro, Accredit. Qual. Assur., 2010, 15, 343–350 CrossRef CAS.
- P. Jitaru, H. Goenaga-Infante, S. Vaslin-Reimann and P. Fisicaro, Anal. Chim. Acta, 2010, 657, 100–107 CrossRef CAS.
- M. Xu, L. Yang and Q. Wang, J. Anal. At. Spectrom., 2008, 23, 1545–1549 RSC.
- http://www.millipore.com/catalogue/module/c5737 .
- R. Seraglia, et al, J. Mass Spectrom., 2005, 40, 123–126 CrossRef CAS.
- J. T. Deagen, J. A. Butler, B. A. Zachara and P. D. Whanger, Anal. Biochem., 1993, 208, 176–181 CrossRef CAS.
- D. L. Mendez, R. A. Jensen, L. A. McElroy, J. M. Pena and R. M. Esquerra, Arch. Biochem. Biophys., 2005, 444, 92–99 CrossRef CAS.
- T. C. Petric, P. Brne, B. Gabor, L. Govednik, M. Barut, A. Strancar and L. Z. Kralj, J. Pharm. Biomed. Anal., 2007, 43(1), 243–249 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
