Spontaneous water cleanup using an epoxy-based polymer monolith†
Takuya
Kubo
*,
Yuichi
Tominaga
,
Koji
Yasuda
,
Soryu
Fujii
,
Fuminori
Watanabe
,
Tomoko
Mori
,
Yuzuru
Kakudo
and
Ken
Hosoya
Graduate School of Environmental Studies, Tohoku University, Aoba 6-6-20, Aramaki, Aobaku, Sendai, 9808579, Japan. E-mail: kubo@mail.kankyo.tohoku.ac.jp; Fax: +81-22-795-7410; Tel: +81-22-795-7410
First published on 11th March 2010
Abstract
Spontaneous cleanup processes based on capillary action for natural water containing humic acids were carried out using an epoxy-based polymer monolith. We were able to control the microstructures of monoliths by changing the polymerization conditions, including the curing agent/monomer/porogenic solvent ratio and the polymerization temperature. Furthermore, we confirmed that updrawing of water based on capillary action was highly dependent on the microstructures of the monolith, i.e., whether they are structures of particle aggregates, non-porous structures, or three-dimensional monolithic structures. The epoxy-based monoliths have a unique ability to adsorb hydrophobic and anionic compounds because the monoliths are prepared using epoxy monomers and curing agents with amines, and therefore, the monoliths can be used for the selective adsorption of polyphenols such as humic acids. In fact, we used a siphonic system to completely remove the humic acids in water samples and for the spontaneous cleanup of real pond water via spontaneous updrawing of water by “J”-shaped monoliths.
1. Introduction
In separation science, monolithic materials that comprise three-dimensional (3D) co-continuous structures have been widely developed.1–3 The most important feature of monolithic materials is the high permeability owing to their unique structure in which the skeleton and the through-pore are combined. Hence, monolithic materials have been utilized in chromatographic separation with high efficiency under high flow-rate conditions.4–6 Silica-based monolithic materials have been the main topic of study, and a silica-based monolith column is commercially available for use in liquid chromatography (LC).7–9 On the other hand, organic polymer-based monoliths have also been studied since 1996.1,10–12 Some organic polymer monoliths have been utilized for separating biomolecules because the polymer is relatively more stable to changes in pH than silica-based materials. However, in general, the polymer-based monolithic materials are present as aggregates, and hence, their separation efficiencies are lower than those of silica-based monoliths; nothing but polymers are used as compensates for silica ones.Recently, some researchers have reported well-controlled polymer monoliths using epoxy-based polymers.13–15 In these polymers, phase separation based on spinodal decomposition was easily controlled because the polymerization mechanism is different from the commonly adopted radical polymerization. Unfortunately, these epoxy-based polymer monoliths have to be prepared at temperatures up to 100 °C, and therefore, it is very difficult to realize the repeatability of homogeneous 3D structures. Therefore, another monolith which can be prepared by a more simple method such as lower temperature conditions and with higher repeatability is required. In this study, we report a simple preparation method which is modified from the previously reported procedures.16
Here, most of previously reported monolithic materials were utilized as separation columns on LC. In LC separations, the mobile phase is eluted into the column by pump. On the other hand, we believed that the separation abilities would also be performed on the spontaneous updrawing by capillary action based on the microstructures of monoliths, and the monoliths can be used for spontaneous cleanup of environmental water.
In this paper, we describe a simple and definite preparation method for well controlled monolithic materials; our method involves the use of epoxy-based resins. We also examined the spontaneous updrawing of water arising from the microstructures of monoliths. Furthermore, we demonstrate the use of the monolith in spontaneous water cleanup for a saturated humic acid aqueous solution and real environmental water.
2. Experimental
2.1 Preparation of monoliths
The epoxy-based monolithic materials were prepared with a diglycidyl ether of bisphenol A (DGEBA, Epicote 828®, Japan Epoxy Resin) as an epoxy component, polyaminoamide (Tohmide® 245S, Fuji Kasei) as a curing agent, and poly(ethylene glycol) 200 (MW = 200) as a porogenic solvent, which were completely mixed at several compositions and degassed in vacuum. The mixture was poured into a PTFE tube (150 mm × 17 mm i.d.) and reacted at various temperatures for 18 h. After polymerization, the solidified materials were squeezed out of the PTFE tube and reacted in boiling water for absolute curing and removal of the porogenic solvent. These two processes including long time polymerization and curing as well as rough washing are different from previous studies.15,16 Finally, in order to wash the unreacted reagent completely, the materials were washed in acetone with ultrasonication. After drying, stick-type epoxy-based polymers with porous structures were obtained. Fig. 1 shows the SEM images of each structure that was obtained by changing the polymerization conditions such as the monomer to porogen ratio, content of the curing agent, and polymerization temperature. These images clearly show that the microporous structures can be easily controlled by simply changing the monomer compositions and polymerization temperature. These changes can be completely understood by considering the phase-separation rate or polymerization rate. For further investigation, we originally categorized the structures as follows: a) particle aggregation structures, b) transition structures, c) 3D network structures, d) thick skeleton structure, e) non-porous structures, and f) gel structures. The regulations of these categories are summarized in Table 1. Through these detailed examinations, we prepared well-controlled monoliths under mild conditions (at 50 °C) by long-time polymerization. Furthermore, the reproducibility of monolithic structures across different days or batches was extremely good. Additionally, in order to compare the microstructure and updrawing of water, the other monoliths including hydrophilic or hydrophobic properties were also prepared.17–19 These polymers were prepared from polyethylene glycol dimethacrylate (9G),17 ethylene dimethacrylate-glycidyl methacrylate (EDMA-GMA),18 and styrene-divinylbenzene (ST-DVB).19 The SEM images are shown in Fig. 2.| Name of structure | Pore size |
|---|---|
| Particle aggregation structure | 1.0 to 20.0 μm |
| Transition structure | 1.0 to 5.0 μm |
| 3D Network structure | 0.5 to 3.0 μm |
| Skeleton thickly structure | 0.1 to 0.8 μm |
| Non-porous structure | < 0.1 μm |
| Gel structure | — |
 | ||
| Fig. 1 SEM images of porous structures of epoxy-based polymer monoliths under different conditions. (A) Various monomer ratios: the weight proportions of monomer components including epoxy components to the curing agent versus the total amount involving porogenic solvent (PEG) are shown. The ratio of epoxy component to the curing agent was fixed at 7/3 (wt/wt). (B) Various amine ratios: the weight proportions of curing agents versus total amount are shown. The weight proportion of the monomer component to porogenic solvent was fixed at 8/2. (C) Various reaction temperatures: the weight proportion of the epoxy component/curing agent/porogenic solvent was fixed at 7/3/40. | ||
 | ||
| Fig. 2 SEM images of other polymer monoliths. (A): 9G based polymer, (B): EDMA-GMA based polymer, (C): ST-DVB based polymer. | ||
2.2 Spontaneous water updrawing
Porous materials can be used for spontaneous updrawing of water by capillary action. We expected that the epoxy-based monolith would also be effective for updrawing of water. Water absorption capabilities were examined using definite-sized epoxy-based polymer monoliths with several porous structures. Completely dried and definite-sized polymers (17 mm diameter × 100 mm) were infiltrated in pure water (40 mL) in an upright posture. Here, only a part of the monolith was immersed in water. In other words, the weight of the monoliths was not observed to change unless water was drawn upward by spontaneous capillary action. Then, the weight of each monolith was measured at regular time intervals.2.3 Spontaneous water cleanup
Firstly, we demonstrated the spontaneous water cleanup for the saturated humic acids (Wako Chemicals, Osaka, Japan) aqueous solution. The “J”-shaped polymer monolith was prepared using a silicon tube to achieve spontaneous cleanup of siphonic water without additional energies. The monolith treated and untreated water samples were evaluated by an UV-VIS spectrophotometer (UV-1700, Shimadzu Co., Kyoto, Japan).Furthermore, we carried out the spontaneous cleanup of real environmental water. We used pond water (Goshikinuma, Sendai, Japan) as an environmental water sample. The cleanup procedures were same as above the evaluations for the humic acids sample. Similarly, the water samples were analyzed by an LC-photo diode array (LC-PDA system, Shimadzu Co., Kyoto, Japan).
3. Results and discussion
3.1 Capability of updrawing water
The increasing amount for various structures of monoliths at each point in time is shown in Fig. 3. These results clearly indicate that the updrawing water capability depended on the size and shape of the porous structures. In particular, the effect of pore size was observed. Interestingly, although the porosities of these monoliths were practically equal because the compositions of the monomer component and porogenic solvent were identical, the absolute amounts of water absorption (at 240 min) for the different monoliths differed greatly. Consequently, it appears that the continuity of through-pores was lost in smaller porous structures; therefore, the monoliths could not be filled with water. At the same time, the updrawing abilities of other polymer monoliths (9G, EDMA-GMA, ST-DVB) were also evaluated. As a result, all polymers involving 9G, EDMA-GMA, and ST-DVB indicated the similar updrawing ability with the epoxy-based monolith having a 3D network structure. According to these results, we expect that these polymers could also be applied to spontaneous water cleanup. However, it was difficult to apply them to additional approaches because of their less physical strength and formability which are described in the after-mentioned discussion. | ||
| Fig. 3 Spontaneous updrawing water effect of epoxy-based monoliths. The representations in each plot are as follows: ♦, particle aggregation structure; ■, transition structure; ▲, 3D network structure; ●, skeleton thickly structure; ×, non-porous structure. | ||
Furthermore, we confirmed that the physical strength of monoliths with larger pores, especially that of particle aggregates, was significantly lower. In fact, the measured maximum bending stress for definite-sized monoliths (40 mm square by 30 mm deep) was 2.60 MPa for monoliths with particle aggregates, while higher values were observed for the other monoliths, i.e., 13.2 MPa and 13.5 MPa for monoliths with the 3D network structure and skeleton thickly structure, respectively. These differences can be explained by the structural features of each monolith: particle aggregates are formed by the particles connected at points on the structure, while the skeleton is integrated in the case of the 3D network structure. Thus, on the basis of the updrawing water abilities and physical strength, we conclude that the 3D network structure and transition structure are suitable for spontaneous updrawing on water cleanup processes.
On the other hand, EDMA-GMA and ST-DVB monoliths had much lower physical strength; both monoliths were easily broken up by weak tension. Therefore, we considered that these monoliths cannot be suitable for molded forms although they can be used for packed columns such as liquid chromatographic columns. In contrast, 9G monolith had higher flexibility than epoxy-based monolith, but spontaneous water cleanup could not be achieved as shown in the below discussion.
3.2 Spontaneous water cleanup
As shown in Fig. 4(A), the brown-colored water sample containing saturated humic acids was completely cleaned up and appeared clear. Here, humic acids are well known to be residues from natural water and act as contaminants in quantitative analyses or purification of drinking water. The photo and UV spectra show the prospects for spontaneous water cleanup. The mechanism of the cleanup process is easily explained: humic acids including several polyphenols are simply adsorbed on the surface of polymer monoliths because of the presence of amino groups and a certain level of hydrophobicity. On the other hand, when we prepared 9G based “J”-shaped monoliths and carried out similar cleanup procedures for humic acid solutions, the spontaneous water drops could not be observed in spite of long time evaluation. Therefore, the water cleanup was not achieved on 9G monolith.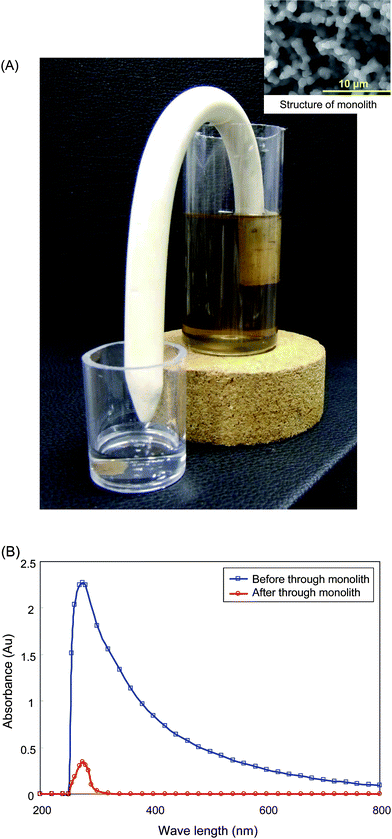 | ||
| Fig. 4 Demonstration of spontaneous water cleanup using “J”-shaped monolith. (A): Water was spontaneously drawn up by capillary action using a siphonic system, (B): UV-VIS absorption spectra. □, water sample containing humic acids before it was passed through the monolith; ○, after it was passed through the monolith. | ||
The real water samples before and after spontaneous water cleanup were analyzed by LC-PDA. The chromatograms of samples are shown in Fig. 5. These results also suggested that the real water sample was mostly cleaned by passing through the monolith. In this case, we used the water sample without additional purifications such as filtration for removal of insoluble particles. In general, filtration is a fundamental procedure for analyses and purifications of water samples. However, we can utilize the sample directly in this system because the insoluble particles are settled down by itself without passage through the monolith. It is also one of the advantages of this system.
 | ||
| Fig. 5 PDA chromatograms of the pond sample before and after treatment with the monolith. LC conditions: column: mightysil RP (150 mm × 4.6 mm i.d., Kanto Chemical Co.Inc.), mobile phase: MeOH–H2O = 6/4, flow-rate: 1.0 mL min−1, detection: UV 210 nm, temperature: 40 °C, injection volume 10 μL. | ||
On the basis of this evaluation, we expect that spontaneous updrawing of water and cleanup for multiplicity of natural water can be carried out by changing the chemical properties of the monoliths; the properties can be varied by selecting different monoliths or by their chemical modification.
4. Conclusion
We established a simple preparation method of novel epoxy-based monoliths whose structures could be controlled, and we demonstrated that microstructure alternation of the monoliths results in an increase in their spontaneous updrawing water capability. Furthermore, we achieved the removal of humic acids and cleanup of the environmental water by the spontaneous updrawing-dropping of water using the “J”-shaped monolith. These demonstrations carried out in this study are valid for other water purification processes by optimizing the chemical properties and the scale. For practical applications, the chemicals and chemical engineering processes relevant to water purification must be studied in further detail.Acknowledgements
This study was partly supported by the Nanotechnology Project of the Ministry of the Environment, Japan.References
- F. Svec and J. M. J. Frechet, Science, 1996, 273, 205 CrossRef CAS.
- F. M. Sinner and M. R. Buchmeiser, Angew. Chem., Int. Ed., 2000, 39, 1433 CrossRef CAS.
- A. Rosenflanz, M. Frey, B. Endres, T. Anderson, E. Richards and C. Schardt, Nature, 2004, 430, 761 CrossRef CAS.
- I. Gusev, X. Huang and C. Horvath, J. Chromatogr., A, 1999, 855, 273 CrossRef CAS.
- C. D. Liang, S. Dai and G. Guiochon, Anal. Chem., 2003, 75, 4904 CrossRef CAS.
- S. Eeltink, J. M. Herrero-Martinez, G. P. Rozing, P. J. Schoenmakers and W. T. Kok, Anal. Chem., 2005, 77, 7342 CrossRef CAS.
- K. Nakanishi, H. Minakuchi, N. Soga and N. Tanaka, J. Sol–Gel Sci. Technol., 1997, 8, 547 CAS.
- N. Tanaka, H. Kobayashi, N. Ishizuka, H. Minakuchi, K. Nakanishi, K. Hosoya and T. Ikegami, J. Chromatogr., A, 2002, 965, 35 CrossRef CAS.
- T. Hara, H. Kobayashi, T. Ikegami, K. Nakanishi and N. Tanaka, Anal. Chem., 2006, 78, 7632 CrossRef CAS.
- E. C. Peters, F. Svec and J. M. J. Frechet, Adv. Mater., 1997, 9, 630 CrossRef CAS.
- A. Castellanos, S. J. DuPont, A. J. Heim, G. Matthews, P. G. Stroot, W. Moreno and R. G. Toomey, Langmuir, 2007, 23, 6391 CrossRef CAS.
- J. W. Kim, J. K. Taki, S. Nagamine and M. Ohshima, Langmuir, 2009, 25, 5304 CrossRef CAS.
- K. Hosoya, N. Hira, K. Yamamoto, M. Nishimura and N. Tanaka, Anal. Chem., 2006, 78, 5729 CrossRef CAS.
- A. M. Nguyen and K. Irgum, Chem. Mater., 2006, 18, 6308 CrossRef CAS.
- N. Tsujioka, N. Ishizuka, N. Tanaka, T. Kubo and K. Hosoya, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3272 CrossRef CAS.
- N. Tsujioka, Thesis for a doctorate, Kyoto Institute of Technology, 2009, pp. 78–102, http://repository.lib.kit.ac.jp/dspace/handle/10212/1930 Search PubMed.
- T. Mori, T. Takahashi, T. Shiyama, A. Tanaka, N. Hira, N. Tanaka and K. Hosoya, Bioorg. Med. Chem., 2006, 14, 5549 CrossRef CAS.
- F. Svec and J. M. J. Frechet, Anal. Chem., 1992, 64, 820 CrossRef CAS.
- Q. C. Wang, F. Svec and J. M. J. Frechet, J. Chromatogr., A, 1994, 669, 230 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1. Preparation procedures of monoliths. Fig. S2. Physical appearance of monolith. Fig. S3. Structural changing on polymerization temperature. Fig. S4. Structural changing on ratio of curing agent. Fig. S5. SEM images of reproducibility on day-to-day, batch-to-batch. See DOI: 10.1039/c0ay00102c |
| This journal is © The Royal Society of Chemistry 2010 |
