A practical method for determination of molybdenite Re-Os age by inductively coupled plasma-mass spectrometry combined with Carius tube-HNO3 digestion
Yali
Sun
*a,
Peng
Xu
a,
Jing
Li
a,
Ke
He
b,
Zhuyin
Chu
c and
Christina Yan
Wang
a
aKey Laboratory of Isotope Geochronology and Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Wushan, Guangzhou 510640, China. E-mail: yalisun@gig.ac.cn
bCollege of Resource Science, University of Chang'an, China
cInstitute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, China
First published on 10th February 2010
Abstract
A simplified method for the determination of molybdenite Re-Os ages using inductively coupled plasma-mass spectrometry (ICP-MS) is presented. By the means of Carius tube method, molybdenite and pyrite were digested using concentrated HNO3, and were then changed into MoO3 and Fe(NO3)3 precipitates, respectively. Rhenium was determined directly by ICP-MS after removal of Os by heating for the molybdenite supernatant or by cation-exchange purification for the pyrite supernatant. Osmium distilled as OsO4 from the supernatant was trapped using pure water and could be directly analyzed by ICP-MS. This method was validated using two molybdenite reference materials, GBW 04435 and GBW 04436, and their Re-Os ages obtained are 220.3 ± 1.1 Ma (1.0%, 2s), and 140.5 ± 0.9 Ma (1.2%, 2s), respectively, consistent with literature values. The proposed Re-Os dating method was applied to molybdenite and pyrite sampled from a porphyry Mo-deposit. The results show that this deposit is the oldest Mo-deposit so far found in China.
Introduction
The Re-Os isotopic system, based on the β−1 decay of 187Re to 187Os has been widely applied to isotopic and chronological studies for decades.1–4 Both Re and Os are siderophile/chalcophile elements and tend to concentrate in sulfides during melting events, so sulfides are useful for direct dating with the Re-Os system. Among them, molybdenite (MoS2) usually has high Re concentration (ppm to %) because of the same valence and similar ionic radii for Mo and Re. Moreover, the structure of molybdenite excludes common Os. Consequently, the quantity of initial common Os is negligible in molybdenite,5 and the 187Os concentration is almost totally radiogenic and can be determined by using natural Os as a spike tracer. Re-Os ages can be calculated using the contents of 187Re and 187Os. To obtain the analyte contents, molybdenite is decomposed using either alkaline fusion6,7 or acid digestion.8–10 The acid digestion with aqua regia (HNO3-HCl) in sealed glass tubes has been widely used because of its low reagent contamination, complete isotope equilibrium and no volatile loss of Os.11 After this acid digestion, Os is separated from the sample matrix via distillation12 or solvent extraction.10 Rhenium is separated via solvent extraction-cation exchange12 or anion exchange.8,9 Using the aqua regia digestion, however, there is considerable amount of NOx and Cl2 formed, which can be absorbed by a trapping solution during distillation of Os. If using a water solution for trapping, the acidity is too high for ICP-MS analysis and so the solution has to undergo another distillation.12,13 In addition, lower yield of Os was also noted for the aqua regia medium.14 As it is well-known, sulfide minerals are easily decomposed by concentrated HNO3. During the decomposition, molybdenite (MoS2) can be changed into insoluble MoO3, effectively removing the Mo matrix. Therefore HNO3 was used instead of aqua regia in this study, aiming to simplify the separation of Re and Os for molybdenite samples.For molybdenite Re-Os dating, analyte concentrations are commonly determined by either inductively coupled plasma-mass spectrometry (ICP-MS)8,9,12 or negative thermal ionization mass spectrometry (NTIMS).10,13,15 Although both the isotopic precision and sensitivity by ICP-MS analysis are not as good as that by NTIMS, ICP-MS technique has been applied to rapid and accurate determination of Os when OsO4 in pure water is measured, because of high sensitivity,16 or OsO4 in digested solution is directly sparged into ICP.17 In addition to molybdenite itself, there is essentially no common Os to take in account, and the determined Re and Os concentrations can be used to calculate the Re-Os age (t) from the basic age equation of 187Os = 187Re (eλt − 1).8,10,12 Therefore ICP-MS technique can meet the analytical requirement of Re and Os concentrations for molybdenite Re-Os dating. Other advantages of ICP-MS technique over NTIMS are higher sample throughput for analysis and higher matrix permitted in analytical solutions. In this study, mass fractionations of ICP-MS measurements were systematically evaluated, aiming to establish a reasonable method for correction of the fractionations and to obtain high quality Re and Os ratios. The objective was to develop a simple and reliable method for determination of molybdenite Re-Os ages by routinely used ICP-MS combined with HNO3-digestion.
Experimental
Instrumentation
A type of XSeries-7 quadrupole ICP-MS (Thermo Scientific, USA) with glass spray chamber and concentric glass nebulizer at the College of Resource Science, University of Chang'an was employed for Re and Os analyses. In order to obtain high quality Re and Os isotopic ratios, the ICP-MS was tuned for better precision instead of higher sensitivity using a solution of 10 ng ml−1 for Ir and Re, whose mass range can cover the analyte isotopes. In general, the precision for isotopic measurements is better than 0.2% (2s, n = 15), and details of running conditions are summarized in Table 1. The isotopes of 187Os, 188Os, 189Os, 190Os and 192Os were measured for Os, and 185Re and 187Re were measured for Re plus 190Os, which was used to monitor potential isobaric interference of 187Os with 187Re.| ICP-MS (ThermoElectron, USA) | |
|---|---|
| Forward power | 1200 W |
| Ar cooling gas flow rate | 14 l min−1 |
| Ar auxiliary gas flow rate | 0.8 l min−1 |
| Ar nebulizer gas flow rate | 0.67 l min−1 |
| Nebulizer pressure | 1.5 bar |
| Analyzer pressure | 3.2 × 10−7 mbar |
| Expansion pressure | 2.0 mbar |
| Ni sampling cone orifice | 1.0 mm |
| Ni skimmer cone orifice | 0.7 mm |
| Acquisition mode | Peak jumping |
| Number of sweeps | 150 |
| Channel dwell time per sweep | 10000 μs |
| Channel per mass | 3 |
| Channel spacing | 0.02 |
| Number of repeats | 10 |
| Measured isotopes | 185Re, 187Re and 190Os for Re; and 187Os, 188Os, 189Os, 190Os and 192Os for Os |
Standards and reagents
A standard stock solution of 1000 μg g−1 for Os in 6 M HCl was prepared with Specpure (NH4)2OsCl6 (Johnson Matthey Chemicals, UK), in which the Os weight fraction is indistinguishable from the ideal value observed based on the thermogravimetric analysis of Markey et al.10 In the course of this work, the fraction of Os in the ammonium salt was considered to be stoichiometric when preparing the standard stock solution of Os. Prior to weighing, this ammonium salt was carefully dried at 70 °C for 2 h under N2 flow, and then stored in a desiccator. Using the stock solution, a series of Os standard solutions in 3 M HCl were prepared by stepwise dilution. The individual isotopic compositions of the Os standards were determined using NTIMS and they are 0.018% for 184Os, 1.595% for 186Os, 1.437% for 187Os, 13.304% for 188Os, 16.227% for 189Os, 26.398% for 190Os and 41.021% for 192Os, respectively.190Os- and 185Re-enriched spikes were used, and their individual isotopic compositions are 0.001% for 184Os, 0.020% for 186Os, 0.037% for 187Os, 0.339% for 188Os, 0.969% for 189Os, 96.968% for 190Os and 1.666% for 192Os, and 96.648% for 185Re and 3.352% for 187Re, respectively.
High purity water (Milli-Q) and HNO3 (Electronic industry grade, Beijing Institute of Reagents, Beijing) were used. Carius tubes used in this work were fabricated from thick-walled borosilicate glass tubing and have a 220 mm long body [25 mm inner diameter (ID) and 30 mm outer diameter (OD)] and a small U-shape funnel with a 50 mm long neck (5 mm ID, 10 mm OD). The small funnel designed could be used easily for sample introduction. For safety and to minimize cross-contamination, Carius tubes were used only one time.
Sulfide samples
Two Chinese molybdenite reference materials, GBW 04435 (HLP molybdenite) and GBW 04436 (JDC molybdenite) were used for validation of accuracy and precision. These materials have also been used internationally as an inhouse/external control of absolute Re-Os age and reproducibility in some laboratories.7,9,15,18 Other sulfide samples were fine-grained (∼80 mesh) molybdenite (LHS0801mo and LHS0823mo) and pyrite (LHS0834py), which had been handpicked under microscopes to remove any visible impurities. The molybdenite and pyrite were freshly collected from the same vein of a porphyry Mo-deposit in Henan province, China. For this deposit (associated geological background could be found in the report of Wei et al.19), molybdenite Re-Os ages have been determined using alkaline fusion and ID-ICP-MS, and the ages varied between 1868–2044 Ma, suggesting it is the oldest Mo-deposit found in China. However, the molybdenite ages were determined using ∼10 mg of sample, which is not large enough, so Re-Os dating was further attempted using the three sulfide samples in this study.Sample preparation
By the means of Carius tube method, each sample was digested using concentrated HNO3. To a Carius tube, 185Re spike, natural Os standard solutions and molybdenite (0.025–1 g) were weighed accurately and added. The lower half of the tube was immersed in an ethanol-liquid nitrogen slush. To this 10 ml concentrated HNO3 was added. The sample-bearing tube was handled carefully for sealing and opening in a fashion similar to that described by Shirey and Walker.11 The sealed tube was heated at 225–230 °C for 24 h. After sample digestion, the tube was refrozen, and then opened. From the opened tube, an approximate amount of the supernatant, depending on the estimated Re concentration in the molybdenite was transferred to a 30 ml quartz beaker and heated to dryness at 150 °C. 0.5 ml concentrated HNO3 was added and dried-down. This step was repeated twice to ensure the removal of Os as OsO4, and finally diluted to a volume of 10 ml using 2% HNO3 for Re determination by ICP-MS. The remaining supernatant was directly poured into a 50 ml distillation flask redesigned after Sun et al.16 and distilled at 110 °C for 20 min for Os, which was trapped using 5 ml of H2O or HBr chilled in a water-ice bath. The H2O solution was used for determination of Os by ICP-MS. The HBr solution, which can reduce Os to a nonvolatile bromide species, was concentrated for further purification of Os using a micro-distillation method to obtain Os for NTIMS determination.20For pyrite, 185Re, 190Os spike solutions and 1 g-sample were weighed and added to a Carius tube, and then the tube was treated like molybdenite. The total of the supernatant was distilled for Os, then heated, and finally changed to a volume of 2 ml using 2% HNO3. This solution was loaded onto a 2 ml cation-exchange resin column (AG 50W-X12, 200–400 mesh, which was cleaned by 50% HCl and H2O, and conditioned by 2% HNO3 prior to use), and then the column was continuously washed twice using 4 ml of 2% HNO3 (2 × 2 ml). Since Re is not sequestered by cation resin, all of the eluant passing through the column was saved and directly determined for Re by ICP-MS.
For the whole procedure as described above, the average blanks and standard deviation from 15 analyses are 2.8 ± 1.1 pg for Re, and 0.7 ± 0.3 pg for common Os, respectively. These blanks have negligible effect on the measured Re and Os abundances.
Due to the significant memory effect of Os in ICP-MS sampling system, its determination was paid much attention. As reported by Sun et al.,21 the ICP-MS sampling system was flushed using 0.5% H2NNH2·H2O in 10% ethanol and 5% HNO3, alternately until the count of 190Os drops to background level after any solutions of Os were introduced.
Results and discussion
The effect of matrix separation
As a hardly soluble compound, MoO3 is precipitated when concentrated HNO3 is used for digestion of molybdenite. The remaining Mo in the supernatant was examined for different amounts of molybdenite from 0.1–1.0 g. As shown in Table 2, the concentrations of Mo are within the range of 114 μg ml−1 to 237 μg ml−1 with an average value of 155 μg ml−1. Almost all of Mo is precipitated as MoO3. The Mo matrix over 98% and 99% is separated for the quantity of ∼0.1 g and >0.25 g molybdenite, respectively. The larger the sample size, the better separation effectiveness. Because molybdenite is rich in Re, just a small portion of the 10 ml supernatant is satisfactory for determination of Re. For the GBW 04435 and GBW 04436 molybdenite containing 283.8 μg g−1 and 17.39 μg g−1 Re, respectively, when 0.025 g and 0.15 g were digested, 0.14 ml and 0.38 ml of the supernatant could provide the concentration level of 10–15 ng ml−1 Re for accurate ICP-MS determination. In the analytical solutions, the concentration of Mo is around 2 μg ml−1 and 6 μg ml−1, respectively. For molybdenite with Re higher than GBW 04435, smaller sample size is enough for accurate Re determination but over 25 mg is also required in order to overcome the spatial decoupling of Re and 187Os, which has been noted and further demonstrated by Stein et al.5,22 This decoupling phenomenon is related to a smaller ionic size of the radiogenic Os than Re(IV), arising from the high mobility of Os compared to Re in molybdenite crystals.23 In order to obtain representative Re-Os age data, the sample size must be large enough so that the Re-187Os natural decoupling is not captured.23–25 Consequently, the level of Mo concentrations in the last analytical solutions will be lower than 2 μg ml−1 for this kind of molybdenite containing higher Re concentrations than GBW 04435. For low Re molybdenite like GBW 04436, a larger sample size is needed in order to obtain satisfactory analyte data, so a small digestion supernatant is also enough for accurate determination of Re. The level of Mo concentrations is generally around 5 μg ml−1. Compared with the level of 1‰ salt limit of ICP-MS analysis, the effect of the remaining Mo is insignificant. Using concentrated HNO3 instead of aqua regia for digestion of molybdenite, Mo matrix was changed to MoO3 precipitate and effectively removed. As a result, there is no need to further purify Re for ICP-MS analysis. For pyrite digested with concentrated HNO3, Fe is effectively precipitated as Fe(NO3)3 due to its low solubility in HNO3 medium. The remaining quantity of metal cations is readily separated by a small cation-exchange resin column, resulting in a ReO4− solution suitable for ICP-MS direct determination.| No | Mass/g | Mo in liquid and solid phase | Acidity of H2O solution trapping OsO4/HNO3 (%) | |
|---|---|---|---|---|
| Supernatant/μg ml−1 | MoO3 precipitate (%) | |||
| 1 | 0.1068 | 114 | 98.21 | 0.27 |
| 2 | 0.1038 | 125 | 98.15 | 0.33 |
| 3 | 0.2518 | 146 | 99.11 | 0.65 |
| 4 | 0.2512 | 117 | 99.28 | 1.18 |
| 5 | 0.5000 | 168 | 99.48 | 0.31 |
| 6 | 0.5045 | 179 | 99.45 | 0.41 |
| 7 | 1.0024 | 237 | 99.63 | 0.52 |
The acidity of water solution trapping OsO4
As mentioned above, Os was separated by distillation from the digested supernatant and trapped by pure water. This trapping solution was titrated using 0.1 M NaOH. As shown in Table 2, the acidity, equivalent to the amount of HNO3, is within the range from 0.1% to 1.2%, which can be directly analyzed by ICP-MS. Conversely, when aqua regia is used for digestion, HCl and HNO3 react intensely, and produce considerable Cl2 and NOx, which are sealed in glass tubes. During distillation of Os, some of them were certainly absorbed by trapping solutions. When ICP-MS is used for measurements, water as a trapping solution is preferred. However, the acidity of the trapping solution is too high for ICP-MS analysis, so the solution had to be redistilled.13 In contrast to HNO3 digestion, the acidity of the Os trapping solution from the first-stage distillation is very low (Table 2), suitable for ICP-MS direct analysis.Correction of mass fractionation
Because of mass fractionation, isotope ratios measured by mass spectrometers are different from their true values. In practice, the mass fractionation is generally corrected using a fractionation factor of a measured isotopic ratio to its true value. Os is comprised of seven naturally occurring isotopes, i.e.184Os, 186Os, 187Os, 188Os, 189Os, 190Os and 192Os. Because both 186Os and 187Os are radiogenic, the absolute abundances of these isotopes vary in nature. However, the abundance ratios between the four stable isotopes, 188Os, 189Os, 190Os and 192Os, are constant. Consequently, they are commonly used to correct mass fractionations, for example using a 192Os/188Os ratio of 3.08271.26 For molybdenite, natural Os standards can be used as a spike for Re-Os dating because the level of common Os is negligible.8–10 As reported by Suzuki et al.8 for the measured isotopic ratios by ICP-MS, mass fractionations could be corrected on-line based on the assumption of a linear relationship between mass fractionation factor and mass number. In the course of this work, the ratios (Rm) of the four stable isotopes to 190Os were measured individually and the mass fractionation factors (F) were calculated relative to their true values (Rt), i.e. F = Rm/Rt. These factors versus their corresponding mass number were regressed and show a good linear relationship (Fig. 1) as previously noted by Hirata et al.27 This linearity is as good as that of NTIMS although the fractionation factors are much different between the measurements of ICP-MS and NTIMS (Fig. 1). According to the linear equation, the fractionation factor of 187Os/190Os ratio can be obtained. Combined with the measured value of 187Os/190Os, a true value of 187Os/190Os can be derived. On-line correction of mass fractionations was achieved for determination of 187Os in molybdenite. This on-line correction method is validated using any one set of the measured ratio data for 188Os/190Os, 189Os/190Os and 192Os/190Os obtained from the molybdenite samples during ICP-MS running period. Fig. 2 just shows the corrected results of 188Os/190Os, and their mean value is 0.5050 ± 0.0009 (2s, n = 36). As shown in Fig. 2, the corrected ratio data are less variable than the measured data. The relative standard deviation (RSD, 2s) is 0.18% and 0.42%, respectively. After this on-line correction of mass fractionation, both accuracy and reproducibility were much improved.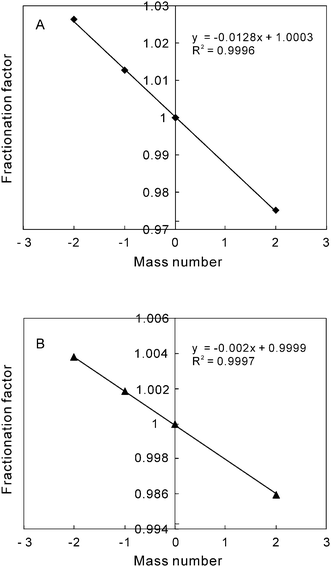 | ||
| Fig. 1 Comparison of mass fractionation factors between the measurements of ICP-MS (A) and NTIMS (B). | ||
 | ||
| Fig. 2 Comparison of the variation in the corrected 188Os/190Os ratios (Rt) with that in their corresponding measured 188Os/190Os ratios (Rm). | ||
This correction method was applied to both ICP-MS and NTIMS measurements for determination of 187Os in GBW 04436 molybdenite. As shown in Table 3, the results of ICP-MS are well consistent with those of NTIMS. For ICP-MS measurements, however, Os could be directly analyzed using the trapping solution at the first-stage distillation and there was no need in further chemical purification compared to NTIMS measurements. In addition, ICP-MS analysis also has much higher sample throughput than NTIMS analysis.
| No | ICP-MS | NTIMS | ||
|---|---|---|---|---|
| Mass/g | 187Os/ng g−1 | Mass/g | 187Os/ng g−1 | |
| 1 | 0.1490 | 25.02 | 0.1024 | 24.86 |
| 2 | 0.1008 | 25.23 | 0.1024 | 24.94 |
| 3 | 0.0753 | 25.87 | 0.1020 | 24.89 |
| 4 | 0.1028 | 24.82 | 0.06467 | 25.07 |
| 5 | 0.1040 | 24.81 | 0.05325 | 24.95 |
| 6 | 0.05027 | 24.76 | 0.0602 | 25.15 |
| 7 | 0.08394 | 25.16 | 0.05941 | 25.02 |
| 8 | 0.0991 | 24.72 | 0.0600 | 25.21 |
| Mean ± s | 25.02 ± 0.36 | 25.01 ± 0.12 | ||
Because Re has only two naturally occurring isotopes of 185Re and 187Re, on-line correction is unreliable for their mass fractionation. For the measured ratios of 185Re/187Re, the mass fractionation was corrected using an external standard of natural Re, which was run repeatedly to obtain a corresponding fractionation factor. For each of the samples, two analyses of the Re standard bracketing the sample were carried out and they were used to calculate an averaged fractionation factor for correction of the measured 185Re/187Re ratio of the sample. During ICP-MS running period for determination of Re, the repeated analyses of the natural Re standard indicate this long-term reproducibility for the fractionation factors of 185Re/187Re ratios is at the level of 0.12% (RSD, 2s, Fig. 3). After correction with the averaged fractionation factor of 0.9803, the measured 185Re/187Re ratios give a mean value of 0.5974 ± 0.0008 (2s, n = 42). This good reproducibility means the external correction method for Re isotopic ratios is applicable to accurate determination of Re contents in sulfide samples.
 | ||
| Fig. 3 Variations in 185Re/187Re fractionation factors against ICP-MS working time. | ||
Analysis of sulfide samples
The applicability of this improved method is validated with the molybdenite materials mentioned above. As shown in Table 4, the mean and standard deviation of the calculated ages are 220.3 ± 1.1 Ma (0.5%, 1s) for HLP molybdenite, and 140.5 ± 0.9 Ma (0.6%, 1s) for JDC molybdenite, respectively. Concentration data for Re and 187Os are reproducible to within 0.9% and 1.0% for JDC molybdenite or within 1.0% and 1.4% for HLP molybdenite. These results are very well consistent with the published data of Markey et al.7 So far, the molybdenite materials have been used internationally in several laboratories, so our data can be compared with those obtained from highly precise NTIMS. As shown in Table 4, although the concentration data of Re and Os determined for HLP molybdenite are somewhat different between laboratories, the calculated Re-Os ages are consistent. All uncertainties are given as absolute amounts at the 2σ level. Uncertainties in spike calibration, weighing for spike and sample, and mass spectrometry measurements for isotope ratio and mass fractionation factor are included in the uncertainty on Re and Os data. The calculated Re-Os age uncertainty includes the uncertainties of Re, 187Os data and the decay constant of 187Re. The relative standard deviation for Re data is larger than that for Os data, and this is attributed to larger error magnification for Re than for Os. Internal analytical precision for Re and Os concentration data using NTIMS is significantly better than that using ICP-MS measurements. The uncertainty for our Re concentration data is about six times as much as that for the Re data of Stein et al.,15 and about three times as much as that of Markey et al.7 The uncertainty for our Os concentration data is about 2–3 times as much as that for the Os data of Stein et al.15 or Markey et al.7 At the error level, the calculated Re-Os ages certainly involved larger uncertainty for ICP-MS measurements than for NTIMS measurements (Table 4). However, when the uncertainty in the decay constant of 187Re are considered for calculating Re-Os ages, it has very significant effect on NTIMS Re-Os age data even using the uncertainty of 0.31% just accepted in the best Re-Os dating laboratories.5 When the decay constant uncertainty of 1.02% recommended strongly by Smoliar et al.28 is used, the uncertainties on NTIMS Re-Os ages are expected to be slightly smaller than those on ICP-MS Re-Os ages. In most laboratories, the routinely used ICP-MS should have much potential for molybdenite Re-Os dating.| Mass/g | Re/μg g−1 | 187Os/ng g−1 | T/Ma | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Found | 2σ | Found | 2σ | Found | 2σ (λ uncertainty) | ||||
| 0% | 0.31% | 1.02% | |||||||
| a The values in italics are derived from the published uncertainty in Re-Os age data including 0.31% uncertainty for the decay constant of 187Re. b The unit: μg g−1; model age calculation: T = λ−1 × ln(1 + 187Os/187Re), where λ is 187Re decay constant of 1.666 × 10−11 yr−1.28 c Uncertainty in Re and Os concentration data includes the uncertainties in spike calibration, weighing for spike and sample, and mass spectrometry measurements for ratio and mass fractionation factor. d Uncertainty in Re-Os ages includes uncertainties in Re and 187Os concentration data and in the decay constant of 187Re. | |||||||||
| This study/ICP-MS-Carius tube digestion for HLP molybdenite | |||||||||
| 1 | 0.02651 | 279.0 | 3.2 | 643.2 | 4.4 | 219.8 | 2.9 | 3.0 | 3.7 |
| 2 | 0.02585 | 277.1 | 3.2 | 641.5 | 6.0 | 220.7 | 3.3 | 3.4 | 4.0 |
| 3 | 0.02946 | 280.2 | 3.8 | 651.4 | 4.2 | 221.6 | 3.3 | 3.4 | 4.0 |
| 4 | 0.03126 | 273.0 | 3.3 | 626.3 | 4.0 | 218.7 | 3.0 | 3.1 | 3.7 |
| 5 | 0.03094 | 275.5 | 4.6 | 638.2 | 6.4 | 220.8 | 4.3 | 4.4 | 4.9 |
| Markey et al.7/NTIMS-alkaline fusion for HLP molybdenite | |||||||||
| M-34 | 278.1 | 1.22 | 645 | 3.2 | 221.1 | 0.9 | 2.4 | ||
| M-35 | 285.3 | 1.09 | 662 | 2.9 | 221.1 | 0.9 | 2.4 | ||
| M-36 | 289.1 | 1.19 | 672 | 3 | 221.6 | 0.93 | 2.4 | ||
| M-37 | 281.8 | 1.16 | 656 | 3 | 221.1 | 0.91 | 2.4 | ||
| M-38 | 284.4 | 1.14 | 662 | 3 | 222 | 0.92 | 2.4 | ||
| M-39 | 282.7 | 1.17 | 657 | 3.1 | 221.5 | 0.9 | 2.4 | ||
| M-60 | 284.5 | 1.17 | 661 | 3.1 | 221.5 | 0.91 | 2.4 | ||
| M-76 | 282 | 1.17 | 654 | 3.1 | 221 | 0.89 | 2.4 | ||
| M-79 | 286.4 | 0.93 | 660 | 2.6 | 219.7 | 0.88 | 2.4 | ||
| M-87 | 283.9 | 1.2 | 662 | 3.2 | 221 | 0.88 | 2.4 | ||
| M-104 | 284.5 | 1.67 | 661 | 3.7 | 222.2 | 1.09 | 2.5 | ||
| M-106 | 283.7 | 1.56 | 657 | 3.4 | 220.8 | 1.08 | 2.5 | ||
| M-107 | 287 | 1.4 | 657 | 3 | 218 | 1.07 | 2.5 | ||
| M-109 | 285.3 | 2.05 | 661 | 4.6 | 220.7 | 1.09 | 2.5 | ||
| M-112 | 286.4 | 1.48 | 658 | 3.2 | 219.1 | 1.07 | 2.5 | ||
| M-124 | 285.7 | 1.41 | 660 | 3.1 | 220.4 | 1.08 | 2.5 | ||
| M-141 | 283.7 | 1.24 | 659 | 3.1 | 221.3 | 0.96 | 2.5 | ||
| M-143 | 282.8 | 1.23 | 657 | 3 | 221.5 | 0.97 | 2.5 | ||
| M-148 | 286.9 | 1.22 | 667 | 3 | 221.5 | 0.96 | 2.5 | ||
| Stein et al.15/NTIMS-alkaline fusion for HLP molybdenite | |||||||||
| M-34 | 278.1 | 0.5 | 645 | 2 | 221.1 | 0.8 | 0.9 | 2.4 | |
| M-35 | 285.3 | 0.5 | 662 | 2 | 221.1 | 0.8 | 0.9 | 2.4 | |
| M-36 | 289.1 | 0.6 | 672 | 2 | 221.6 | 0.8 | 0.9 | 2.4 | |
| M-37 | 281.8 | 0.5 | 656 | 2 | 221.9 | 0.8 | 0.9 | 2.4 | |
| M-38 | 284.4 | 0.5 | 662 | 2 | 222.0 | 0.8 | 0.9 | 2.4 | |
| M-39 | 282.7 | 0.5 | 657 | 2 | 221.5 | 0.8 | 0.9 | 2.4 | |
| M-60 | 284.5 | 0.5 | 661 | 2 | 221.5 | 0.8 | 0.9 | 2.4 | |
| Selby and Creaser18/NTIMS-Carius tube digestion for HLP molybdenite | |||||||||
| HLP 5-1 | 265.7 | 611.6 | 219.4 | 0.2 | 0.70 | 2.2 | |||
| HLP 5-2 | 268 | 619.6 | 220.4 | 0.2 | 0.72 | 2.3 | |||
| HLP 5-3 | 256.4 | 593.5 | 220.5 | 0.2 | 0.70 | 2.2 | |||
| HLP 5-4 | 240.4 | 555.6 | 220.3 | 0.2 | 0.71 | 2.3 | |||
| HLP 5-5 | 260.7 | 598.3 | 218.8 | 0.1 | 0.69 | 2.2 | |||
| This study/ICP-MS-Carius tube digestion for JDC molybdenite (GBW 04436) | |||||||||
| 1 | 0.0783 | 17.05 | 0.26 | 25.08 | 0.16 | 140.3 | 2.3 | 2.4 | 2.7 |
| 2 | 0.0934 | 16.89 | 0.10 | 25.06 | 0.08 | 141.5 | 0.9 | 1.0 | 1.7 |
| 3 | 0.0992 | 16.90 | 0.22 | 24.97 | 0.14 | 140.9 | 2.0 | 2.0 | 2.5 |
| 4 | 0.1008 | 17.04 | 0.10 | 25.29 | 0.12 | 141.6 | 1.1 | 1.2 | 1.8 |
| 5 | 0.1022 | 16.69 | 0.06 | 24.35 | 0.14 | 139.1 | 0.9 | 1.0 | 1.7 |
| 6 | 0.1006 | 16.84 | 0.16 | 24.67 | 0.04 | 140.3 | 1.3 | 1.4 | 2.0 |
| 7 | 0.1009 | 16.68 | 0.20 | 24.79 | 0.16 | 141.7 | 1.9 | 2.0 | 2.4 |
| 8 | 0.1001 | 17.06 | 0.16 | 24.86 | 0.04 | 139.5 | 1.3 | 1.4 | 1.9 |
| 9 | 0.1259 | 16.69 | 0.16 | 24.63 | 0.04 | 141.3 | 1.4 | 1.4 | 2.0 |
| 10 | 0.1998 | 17.05 | 0.18 | 25.00 | 0.12 | 139.9 | 1.6 | 1.7 | 2.2 |
| 11 | 0.2507 | 16.94 | 0.26 | 24.96 | 0.20 | 140.5 | 2.4 | 2.5 | 2.8 |
| 12 | 0.3018 | 17.10 | 0.16 | 25.07 | 0.08 | 139.9 | 1.4 | 1.4 | 2.0 |
| Stein et al.15/NTIMS-alkaline fusion for JDC molybdenite | |||||||||
| M-111 | 17.33 | 0.05 | 25.13 | 0.09 | 138.3 | 0.6 | 0.8 | 1.6 | |
| M-113 | 17.40 | 0.05 | 25.26 | 0.09 | 138.4 | 0.6 | 0.8 | 1.6 | |
| This study/ICP-MS-Carius tube digestion for sulfides from a porphyry Mo deposit | |||||||||
| LHS0834py | 0.5540 | 0.232 | 0.004 | 4.70 | 0.024 | 1906.0 | 33.8 | 34.3 | 38.9 |
| LHS0823mo | 0.0418 | 1130 | 27 | 22.83b | 0.08b | 1898.3 | 45.3 | 45.7 | 49.2 |
| LHS0801mo | 0.0416 | 503.7 | 7.6 | 10.16b | 0.06b | 1896.5 | 30.4 | 30.9 | 35.9 |
This proposed method was applied to pyrite (LHS0834py) and molybdenite (LHS0801mo and LHS0823mo) from a porphyry Mo-deposit mentioned above. The sample of LHS0834py spiked with enriched 190Os spike was determined for both total 187Os and common Os, and the results are 4.7 ng g−1 and 12 pg g−1, respectively. Clearly, the common Os content is very low relative to the total 187Os, so the Re-Os age was calculated using the total 187Os. As shown in Table 4, the calculated Re-Os ages are 1906 Ma for LHS0834py, 1896 Ma for LHS0801mo and 1898 Ma for LHS0823mo, respectively. Although the contents of Re and Os in LHS0834py are much lower than those in LHS0801mo and LHS0823mo, these ages are well comparable. The uncertainty in the calculated Re-Os ages is compared and shows that the uncertainty for the three samples is slightly higher than that for HLP and JDC molybdenite. The reason is likely due to the contents of Re and Os being too low for LHS0834py, and too high for LHS0801mo and LHS0823mo, being out of the optimal signal range for ICP-MS measurements. Compared with the molybdenite Re-Os age range of 1868–2044 Ma obtained using alkaline fusion for the same Mo-deposit,19 our age data confirm that this deposit is the oldest Mo-deposit so far reported in China.
Conclusions
Using HNO3 for digestion of pyrite and molybdenite, effective separation of matrix has been achieved, making subsequent chemical purification of Re and Os very simple. After removal of Os in the digested supernatant by heating, Re can be determined for molybdenite by ICP-MS without any chemical separation. Pure water for trapping Os at the first-stage distillation has very low acidity and can be directly analyzed by ICP-MS. Related to the proposed method, only HNO3 is used, making the whole procedural blank very low. By on-line correction of mass fractionations, both reproducibility and accuracy of Os isotopic ratios are highly improved.Acknowledgements
This study was financially supported by the National Basic Research Program of China (973 Program: 2007CB411300), the CAS/SAFEA International Partnership Program for Creative Research Teams, CAS project (KZCX2-YW-JS103) and NSFC project (No. 90714008). This is contribution No. IS-1156 from GIGCAS. Comments from anonymous reviewers are gratefully appreciated, which helped to improve the paper. The authors thank Professor Weidong Sun for polishing the English.References
- S. B. Shirey and R. J. Walker, Annu. Rev. Earth Planet. Sci., 1998, 26, 423–500 CrossRef CAS.
- G. Ravizza and B. P. Peucker-Ehrenbrink, Science, 2003, 302, 1392–1395 CrossRef CAS.
- J. A. Baker and K. K. Jensen, Earth Planet. Sci. Lett., 2004, 220, 277–286 CrossRef CAS.
- A. D. Brandon and R. J. Walker, Earth Planet. Sci. Lett., 2005, 232, 211–225 CrossRef.
- H. J. Stein, R. J. Markey, J. W. Morgan, J. L. Hannah and A. Schersten, Terra Nova, 2001, 13, 479–486 CrossRef CAS.
- H. He, A. Du, X. Zou, Y. Sun and N. Yin, Chinese J. Anal. Chem., 1994, 22, 109–114 Search PubMed.
- R. Markey, H. Stein and J. Morgan, Talanta, 1998, 45, 935–946 CrossRef CAS.
- K. Suzuki, H. Shimizu and A. Masuda, Geochim. Cosmochim. Acta, 1996, 60, 3151–3159 CrossRef CAS.
- D. Malinovsky, I. Rodushkin, D. Baxter and B. Öhlander, Anal. Chim. Acta, 2002, 463, 111–124 CrossRef CAS.
- R. Markey, H. J. Stein, J. L. Hannah, A. Zimmerman, D. Selby and R. A. Creaser, Chem. Geol., 2007, 244, 74–87 CrossRef CAS.
- S. B. Shirey and R. J. Walker, Anal. Chem., 1995, 67, 2136–2141 CrossRef CAS.
- W. J. Qu and D. A. Du, Rock Mineral Anal., 2003, 22, 254–257 Search PubMed 262.
- A. Du, S. Wu, D. Sun, S. Wang, W. Qu, R. Markey, H. Stein, J. Morgan and D. Malinovsky, Geostand. Geoanal. Res., 2004, 28, 41–52 CrossRef CAS.
- W. Qu, A. Du and J. Ren, Chinese J. Anal. Chem., 2008, 36, 223–226 Search PubMed.
- H. J. Stein, R. J. Markey, J. W. Morgan, A. Du and Y. Sun, Econ. Geol., 1997, 92, 827–835 CrossRef CAS.
- Y. Sun, M.-F. Zhou and M. Sun, J. Anal. At. Spectrom., 2001, 16, 345–349 RSC.
- D. R. Hassler, B. Peucker-Ehrenbrink and G. E. Ravizza, Chem. Geol., 2000, 166, 1–14 CrossRef CAS.
- D. Selby and R. A. Creaser, Econ. Geol., 2001, 96, 197–204 CrossRef CAS.
- J. Wei, J. Yao, T. Zhao, Y. Sun, J. Li, Z. Yuan and B. Qiao, Acta Petrolog. Sinica, 2009, 25, 2747–2751 Search PubMed.
- J. L. Birck, M. Roy-Barman and F. Capmas, Geostand. Newslett., 1997, 20, 9–27.
- Y. Sun, Z. Chu, M. Sun and X. Xia, Appl. Spectros., 2009, 63, 1232–1237 CAS.
- H. Stein, A. Scherstén, J. Hannah and R. Markey, Geochim. Cosmochim. Acta, 2003, 67, 3673–3686 CrossRef CAS.
- Y. Takahashi, T. Uruga, K. Suzuki, H. Tanida, Y. Terada and K. H. Hattori, Geochim. Cosmochim. Acta, 2007, 71, 5180–5190 CrossRef CAS.
- D. Selby and R. A. Creaser, Geochim. Cosmochim. Acta, 2004, 68, 3897–3908 CrossRef CAS.
- H. J. Stein, Lithos, 2006, 87, 300–327 CrossRef CAS.
- J. M. Luck and C. J. Allègre, Nature, 1983, 302, 130–132 CrossRef CAS.
- T. Hirata, H. Shimizu, T. Akagi and A. Masuda, ICP Inf. Newslett., 1988, 13, 731–735.
- M. I. Smoliar, R. J. Walker and J. W. Morgan, Science, 1996, 271, 379–396.
| This journal is © The Royal Society of Chemistry 2010 |
