Evaluating MTBSTFA derivatization reagents for measuring naphthenic acids by gas chromatography-mass spectrometry
Rozlyn F.
Young
,
Debora L.
Coy
and
Phillip M.
Fedorak
*
Department of Biological Sciences, University of Alberta, Edmonton, Alberta, Canada T6G 2E9. E-mail: phil.fedorak@ualberta.ca; Fax: (+1 780) 492-9234; Tel: (+1 780) 492-3670
First published on 9th April 2010
Abstract
Naphthenic acids (general formula CnH2n+ZO2) are found in petroleum and oil sands deposits. Release of these acids to aquatic environments is a concern because of their potential toxicity. Naphthenic acids consist of a complex mixture of carboxylic acids, and estimating their concentrations in environmental samples is a challenge. Two recent reports have described gas chromatography-mass spectrometry (GC-MS) methods to selectively detect these acids in fish flesh and water samples. The methods use N-methyl-N-(t-butyldimethylsilyl)-trifluoroacetamide (MTBSTFA) with 1% t-butyldimethylchlorosilane (t-BDMCS) to derivatize naphthenic acids to their t-butyldimethylsilyl derivatives. Single ion monitoring (SIM) was used to detect the fragment m/z 267, which corresponds to the derivatives of one isomer class (n = 13 and Z = −4) of naphthenic acids. The SIM chromatograms give a characteristic naphthenic acids hump between retention times 15 and 20 min and a sharp peak for the t-butyldimethylsilyl derivatized surrogate standard, 9-fluorenecarboxylic acid. Integration of this hump and the peak from the surrogate standard allows quantification of the naphthenic acids. Using newly purchased MTBSTFA containing 1% t-BDMCS (from three different suppliers) yielded SIM chromatograms with one or two large contaminating peaks (eluting at 16.7 and 18.8 min) that interfered with integration of the hump, rendering the method unreliable. The contaminants were traced to the presence of t-BDMCS. Each of the three suppliers sells MTBSTFA devoid of t-BDMCS, and using MTBSTFA without 1% t-BDMCS was found to be suitable for the GC-MS method.
Introduction
Naphthenic acids, with general formula CnH2n+ZO2 (where Z is zero or a negative, even integer whose absolute value divided by 2 gives the number of rings in the compound), are commonly found in petroleum and oil sands bitumen.1 The release of these acids into the environment is a concern because they are toxic to aquatic and terrestrial organisms.1Naphthenic acids and metal naphthenates also have a wide variety of industrial uses,2 including wood and textile preservatives, paint driers, and tire manufacture. Characterization and quantification of naphthenic acids is a major analytical challenge because of the myriad of compounds present in naphthenic acids preparations obtained from petroleum. St. John et al.3 described a method in which naphthenic acids were derivatized with N-methyl-N-(t-butyldimethylsilyl)-trifluoroacetamide (MTBSTFA) with 1% t-butyldimethylchlorosilane (t-BDMCS, CAS Registry No. 18162-45-6), and the t-butyldimethylsilyl derivatives were analyzed by gas chromatography-electron impact mass spectrometry (GC-MS). Electron impact causes the t-butyldimethylsilyl derivatives to lose the t-butyl group, producing [M + 57]+ ions, where M is the molecular mass of the parent naphthenic acids, as the predominate ions3,4 in the mass spectrum. This method has been used to characterize naphthenic acids based on their n and Z values,3,5–7 and to monitor naphthenic acids biodegradation.8–10
The oil sands industry standard method for quantifying naphthenic acids in aqueous samples is based on a Fourier transform infrared (FTIR) spectroscopy method.11,12 Water samples are acidified and extracted with dichloromethane, then the absorbances of the extract are measured at 1743 and 1706 cm−1, which are characteristic of carboxylic acids. This method allows the quantification of all carboxylic acids (including naphthenic acids) that are extracted from the water sample. To improve the specificity for detection of naphthenic acids, Merlin et al.13 extracted these acids from water samples and derivatized them with a mixture of MTBSTFA with 1% t-BDMCS. The extracts were analyzed by GC-MS to produce reconstructed ion chromatograms for ions of m/z 267. These ions are specific for the t-butyldimethylsilyl derivatives of naphthenic acids with n = 13 and Z = −4, and the presence of an unresolved hump that eluted between 16 and 21 min was found to be indicative of the presence of naphthenic acids. The method of Merlin et al.13 was adapted to specifically detect naphthenic acids among the numerous and abundant natural fatty acids in fish flesh.14 Subsequently, Young et al.15 used GC-MS in the single ion monitoring (SIM, m/z 267) mode to estimate naphthenic acids concentrations in fish flesh extracts. This was done by integrating the area under the hump of m/z 267, in the SIM chromatogram, using 9-fluorenecarboxylic acid (9-FCA) as a surrogate standard which also produces an ion at m/z 267. To date, this is the only reported analytical method available to estimate naphthenic acids concentrations in fish.
More recently, Scott et al.16 applied this GC-MS method with SIM to specifically quantify naphthenic acids in water samples and compared this method with results from the FTIR method. In the vast majority of the samples that were analyzed, the GC-MS method gave lower concentrations of naphthenic acids than the FTIR method. This was attributed to the higher specificity of the GC-MS method.
Although our GC-MS method is more specific than the FTIR method, any compounds that elute within the retention time of the unresolved hump (typically between 15 and 20 min) and yield m/z 267 ions would be erroneously quantified as naphthenic acids. It is well known that MTBSTFA will derivatize hydroxyls (including hydroxylated naphthenic acids), thiols, and primary and secondary amines, in addition to carboxylic acids. Thus, these compounds could be potential sources of interference for the naphthenic acids analyses. However, in our experience13–16 this has not been a problem.
In the studies by Young et al.15 and Scott et al.,16 the derivatizing agent, MTBSTFA with 1% t-BDMCS (Reagent 2, Table 1) was used. After exhausting the supply of Reagent 2, Reagent 3 (Table 1) was purchased from the same supplier. When Reagent 3 was used for the derivatization, two sharp, contaminating peaks protruded from the hump of naphthenic acids. These peaks interfered with the hump integration, and precluded the use of this method to quantify naphthenic acids in fish or water samples. Thus, the objective of this study was to evaluate MTBSTFA derivatizing reagents from various sources to find those that could be used with SIM (m/z 267) to quantify naphthenic acids.
| Reagent number | Supplier | Date received | Lot number | With 1% t-BDMCS | Reported purity (%) | Contaminant Observed |
|---|---|---|---|---|---|---|
| a Milwaukee, WI. b Rockford, IL. c Beverly, MA. d Bachs, Switzerland, ordered from Sigma-Aldrich. e Ordered from Pierce. | ||||||
| 1 | Sigma-Aldricha | 2006 | 04115TC | Yes | 97 | No |
| 2 | Sigma-Aldrich | Apr 2007 | 04115TC | Yes | > 95 | No |
| 3 | Sigma-Aldrich | Nov 2007 | 05821KE | Yes | > 95 | Yes |
| 4 | Sigma-Aldrich | Feb 2008 | 08605DH | Yes | > 95 | Yes |
| 5 | Pierceb | Mar 2008 | II116494 | Yes | 98 | Yes |
| 6 | Soltech Venturesc | Jun 2008 | SV70818-01 | Yes | > 99 | Yes |
| 7 | Flukad | Jul 2008 | 1337050 | No | ≥ 97 | No |
| 8 | Thermo Scientifice | Jul 2008 | JD122782 | No | ≥ 98 | No |
| 9 | Soltech Ventures | Sept 2008 | SV040510 | No | 99 | No |
Methods
Chemicals and reagents
Derivatizing reagents were purchased from three different companies, as shown in Table 1. All of the reagents contained MTBSTFA and most contained 1% t-BDMCS. Routinely, 100 μL of derivatizing reagent was used in each derivatization reaction. Naphthenic acids were a gift from Merichem Chemicals and Refinery Services LLC (Houston, TX) and 9-FCA was purchased from Sigma-Aldrich. Chrysene, 1-methyl-1-cyclohexanecarboxylic acid, trans-1,4-pentylcyclohexanecarboxylic acid, 2-hexyldecanoic acid, dicyclohexylacetic acid, 5β-cholanic acid, 2-hydroxycyclohexanecarboxylic acid, and 2-hydroxydodecanoic acid were also purchased from Sigma-Aldrich. Eicosanoic acid was obtained from Applied Sciences Labs, State College, PA.Extraction and analyses of naphthenic acids from fish and water
Rainbow trout (Oncorhynchus mykiss) was purchased from a local supermarket and 5 g portions of the flesh were spiked with various concentrations of naphthenic acids. After addition of 1 μg of the surrogate standard, 9-FCA, the samples were homogenized, extracted, cleaned up and derivatized.15 A groundwater sample was taken from a domestic well 40 km northwest of Edmonton, Alberta in July 2008. This sample was designated well #1313 and a previous GC-MS analysis of this sample showed it contained 25 μg naphthenic acids L−1.16 The water sample was extracted, cleaned up and derivatized as outlined by Scott et al.16 with the following modifications: (a) the volume of water extracted was 250 mL (rather than 1 L), (b) 2.5 μg (instead of 10 μg) of 9-FCA was added to the sample, (c) 38 g (rather than 150 g) of NaCl was dissolved in the water sample, and (d) this was extracted three times with 40 mL portions (instead of 60 mL portions) of chloroform.GC-MS analyses
The descriptions of the instrument and the method used for most of the SIM (m/z 267) GC-MS analyses have been reported previously.15 On a few occasions, a similar instrument in the Biogeochemical Analytical Laboratory in the Department of Biological Sciences at the University of Alberta was used to help trace the source of the contaminating peaks in a derivatized sample of fish extract.Preparation of calibration plots
Appropriate volumes of a stock solution of Merichem naphthenic acids in dichloromethane were dispensed into each of five vials giving 0, 1, 2, 5, or 10 μg of these acids in the vials. Each portion received 1 μg of 9-FCA and the mixture was made to 100 μL with dichloromethane. These were derivatized with Reagent 8 and analyzed by GC-MS (SIM, m/z 267). A calibration plot was prepared by plotting the ratio of the integrated area of the naphthenic acids hump to the area of the 9-FCA peak vs. the amount of naphthenic acids in each solution.15 A second set of five solutions was prepared in the same manner, but these were derivatized with Reagent 5 prior to GC-MS analyses. A second calibration plot was prepared from these data.Comparing the derivatization effectiveness of Reagents 5 and 8
Approximately equal masses (10 mg) of chrysene and each the of nine model carboxylic acids listed in Table 2 were dissolved as a mixture in 100 mL dichloromethane. Six 100-μL aliquots of this mixture, containing 1 μg of each compound, were dispensed into individual vials. Three of these vial received 100 μL of Reagent 5 (which contained 1% t-BDMCS), while the other three received 100 μL of Reagent 8 (which was devoid of t-BDMCS). All of the vials were sealed and the contents were reacted at 60 °C for 20 min.3 Chrysene served as the internal standard because it does not react during this treatment. The derivatized solutions were analyzed by GC-MS and total ion chromatograms were collected. From each analyses, the peak areas of each derivatized carboxylic acid were normalized to the area of the chrysene peak. A t-test was used to compare the mean normalized area for each carboxylic acid derivatized by Reagent 5 with the corresponding normalized area obtained from derivatization by Reagent 8.| Compound | Reagent 5 with t-BDMCS | Reagent 8 without t-BDMCS | ||
|---|---|---|---|---|
| Mean normalized ratioa | Std Dev | Mean normalized ratio a | Std Dev | |
| a Mean of three replicates. b Mass spectrum of product showed that both the hydroxy and carboxy groups were derivatized with t-butyldimethylsilyl moieties. | ||||
| 1-methyl-1-cyclohexane-carboxylic acid | 0.14 | 0.16 | 0.28 | 0.09 |
| 5-β-cholanic acid | 1.52 | 0.71 | 0.71 | 0.03 |
| 2-hydroxycyclohexane-carboxylic acid b | 0.05 | 0.02 | 0.04 | 0.01 |
| trans-1,4-pentylcyclohexane-carboxylic acid | 1.68 | 0.39 | 1.93 | 0.03 |
| dicyclohexylacetic acid | 1.72 | 0.26 | 1.94 | 0.02 |
| 2-hexyldecanoic acid | 2.14 | 0.03 | 2.18 | 0.01 |
| 9-FCA | 1.70 | 0.08 | 1.82 | 0.01 |
| 2-hydroxydodecanoic acid b | 3.45 | 0.07 | 3.36 | 0.02 |
| eicosanoic acid | 1.69 | 0.03 | 1.68 | 0.01 |
Results and discussion
The chromatograms in Fig. 1 are from the SIM (m/z 267) analyses of the extracts of 5-g samples of purchased fish that were spiked with 1 μg of Merichem naphthenic acids. This amount is equivalent to 0.2 mg naphthenic acids kg−1, near the detection limit of ∼0.1 mg kg−1 reported by Young et al.15Fig. 1a shows a typical hump eluting between 15 and 20 min, which is indicative of naphthenic acids with n = 13 and Z = −4. With this type of chromatogram, naphthenic acids are quantified by integrating the area of the hump eluting between 15 and 20 min, and by integrating the area of the surrogate standard peak.15,16 Then the ratio of the area of the naphthenic acids hump to the area of the 9-FCA peak is compared to the ratios used to generate a calibration curve.15,16Fig. 1b shows the SIM chromatogram from the analysis of the extract of a sample of the same fish spiked with the same amount of Merichem naphthenic acids and surrogate standard after derivatization with newly purchased Reagent 3. Using this reagent produced two large contaminating peaks with retention times of 16.7 and 18.8 min. The areas of these two peaks accounted for 53% of the total area integrated between retention times 15 to 20 min in Fig. 1b. Similarly, when extracts of water from well #13 were derivatized with Reagent 3, the two contaminating peaks were detected (data not shown).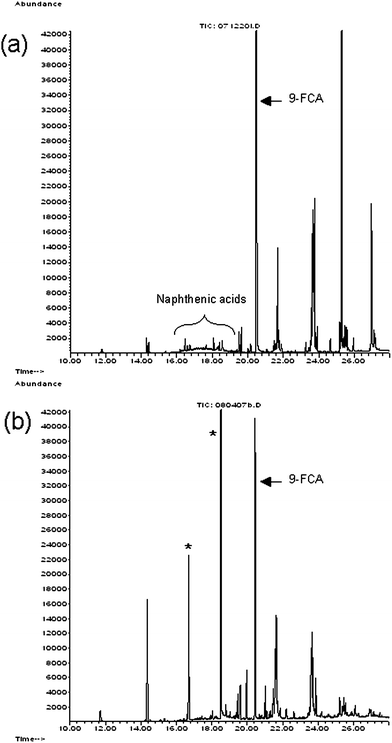 | ||
| Fig. 1 SIM (m/z 267) chromatogram of extracts of fish flesh samples that were spiked with 1 μg of Merichem naphthenic acids and 1 μg of the surrogate standard 9-FCA. These extracts were derivatized with Reagent 2 (a) and Reagent 3 (b), MTBSTFA with 1% t-BDMCS. The naphthenic acids elute between 15 and 20 min, where two large peaks (*) appear at 16.7 and 18.8 min in panel (b). The standard 9-FCA elutes at 20.5 min. | ||
Examination of reconstructed ion chromatograms (m/z 267) from archived GC-MS analyses obtained using Reagent 1, (purchased before Reagent 2 with the same lot number shown in Table 1) revealed no contaminating peaks in the region 15 to 20 min. These archived chromatograms were essentially the same as Fig. 1a, suggesting that Reagent 3 was the source of the contaminating peaks. Subsequently, we purchased Reagents 4, 5 and 6 (MTBSTFA with 1% t-BDMCS) from a variety of suppliers (Table 1) and analyzed extracts from fish spiked with naphthenic acids. Each of these analyses showed the contaminating peaks, although only one major peak was associated with Reagent 5. In addition, when extracts of water from well #13 were derivatized with Reagents 4, 5 or 6, the contaminating peaks were detected.
Although all of the SIM chromatograms collected suggested that the contaminants originated from the derivatizing reagents, several other sources of the unidentified contaminants were considered. In attempts to isolate the source of the contaminating peak, blank extractions without fish flesh were also analyzed using SIM GC-MS. The extraction process was stopped at various stages and samples were derivatized with Reagent 3 to determine if one of the solvents or reagents was responsible for the contamination. However, in each case, the SIM chromatogram contained the contaminant peaks. Increasing the volume of derivatizing reagent used in the absence of fish extract, increased the relative abundance of the contaminating peaks to that of the peak from a constant amount of surrogate standard. These observations also suggested that the contaminant came from the derivatizing agent, not from the fish extract. To ensure that these peaks were not originating from some contaminant in our instrument, selected derivatized samples were analyzed using a similar GC-MS instrument located in the Biogeochemical Analytical Laboratory. This instrument had never been used for analyzing naphthenic acids. Nonetheless, the contaminating peaks were also observed in each analysis, thereby ruling out our instrument as the source of the problem.
The catalyzing agent t-BDMCS is added to MTBSTFA preparations to enhance the derivatization of hindered alcohols and amines.17 Each of the suppliers sells MTBSTFA without t-BDMCS (Table 1). To determine whether the t-BDMCS was the source of the contaminating peaks, identical solutions containing 1 μg of Merichem naphthenic acids in 100 μL dichloromethane were prepared. One solution was reacted with 100 μL of Reagent 5 (which contained 1% t-BDMCS) and the other solution was reacted with 100 μL of Reagent 8 (which did not contain t-BDMCS). Fig. 2 shows the SIM (m/z 267) of these two analyses. These two chromatograms clearly indicate that the contaminating peaks were only found when t-BDMCS was in the derivatizing reagent. Similarly, when extracts of water from well #13 were derivatized with Reagent 8, no contaminating peaks eluted between 15 and 20 min. However, when the extract of this water was reacted with Reagent 5, the contaminating peak with retention time 16.7 min was present, giving a SIM (m/z 267) chromatogram that was similar to that in Fig. 2a. SIM analyses of extracts of other samples derivatized with Reagents 7 or 9 (both lacking t-BDMCS) were devoid of the contaminating peaks at retention times 16.7 and 18.8 min. These results clearly demonstrated that t-BDMCS was the source of the contaminating peaks.
 | ||
| Fig. 2 SIM (m/z 267) chromatogram of Merichem naphthenic acids derivatized with Reagent 5, containing 1% t-BDMCS (a) or with Reagent 8, without t-BDMCS (b). | ||
The calibration plot prepared with Merichem naphthenic acids derivatized with Reagent 8 (devoid of t-BDMCS) yielded a line with a R2 of 0.998 (Fig. 3). The SIM (m/z 267) chromatograms showed no contaminating peaks between 15 and 20 min. However, when an identical set of Merichem naphthenic acids was derivatized with Reagent 5 (containing 1% t-BDMCS), each of the chromatograms showed a contaminating peak with retention time 16.7 min. The calibration line that resulted from integrating the hump and including the area of the contaminating peak had a R2 value of only 0.907 (Fig. 3). Statistical comparisons18 showed that the slopes and y-intercepts of these lines were significantly different (p < 0.0001). The y-intercept was closer to zero when Reagent 8 was used for the derivatizations. Using Reagent 2, which did not give the contaminating peaks, Young et al.15 generated a calibration line with R2 = 0.999, similar to that observed with Reagent 8 (Fig. 3).
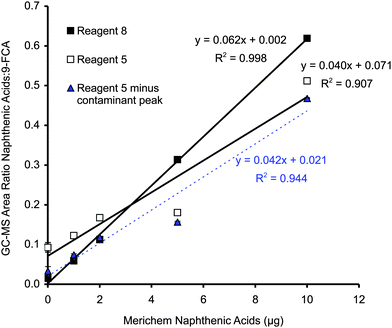 | ||
| Fig. 3 Calibration plots generated from solutions of Merichem naphthenic acids containing 1 μg of 9-FCA. One calibration line was prepared with Reagent 8 (devoid of 1% t-BDMCS) and one was prepared with Reagent 5 (containing 1% t-BDMCS). Ratios of the integrated total area under the hump between 15 and 20 min and the area under the 9-FCA peak were used to generate these plots. A third calibration line was generated by subtracting the area of the contaminating peak from the total area of the hump between 15 and 20 min. Each point is the mean of three analyses and the error bars, which are usually smaller than the symbol, represent one standard deviation. | ||
A third calibration line was generated using the integrations obtained from the analyses of the solutions derivatized with Reagent 5. Subtracting the area of the contaminating peak from the total area of the hump between 15 and 20 min gave the data shown as triangles in Fig. 3. The regression line for these data gave R2 = 0.944. The slope of this line (0.042/μg) was indistinguishable from that obtained with Reagent 5 without this manipulation (0.040/μg), but the y-intercepts were statistically different (p < 0.05). Subtracting the area of the contaminating peak gave a y-intercept that was indistinguishable from that obtained with Reagent 8, but the slopes of these two lines were significantly different (p < 0.0001). Overall, the calibration plot generated with MTBSTFA, devoid of 1% t-BDMCS, was superior.
Comparisons of the relative amounts of derivatized products of nine model carboxylic acids formed by Reagent 5 (containing the catalyst t-BDMCS) or Reagent 8 (devoid of t-BDMCS) are summarized in Table 2. The peak areas of each derivatized compound were normalized to the area of the chrysene peak. When compared by a t-test, the mean normalized ratio for each compound derivatized by Reagent 5 was not significantly different from the mean normalized ratio for that compound derivatized by Reagent 8 (p > 0.05). However for each carboxylic acid, the reproducibility of reaction with Reagent 8 was better (e.g. the standard deviation was lower) than with Reagent 5. For example, the standard deviation of the triplicate reactions for 1-methyl-1-cyclohexanecarboxylic acid with Reagent 8 was 0.09 whereas the standard deviation for the reaction with Reagent 5 was 0.16 (Table 2). The results in Table 2 demonstrate that the absence of the catalyst t-BDMCS does not significantly reduce the yields of the derivatized model carboxylic acids.
In the application of our methods,15,16 there is no need for the catalyst t-BDMCS to enhance reactions with hindered alcohols and amines for the following reasons. First, hydroxylated naphthenic acids do not fit the general formula for naphthenic acids (CnH2n+ZO2), because they contain three oxygen atoms. Thus, they are not targeted as part of this SIM GC-MS method. Second, any amines that might be present in fish or aqueous extracts would be separated from the naphthenic acids during the clean up step in which the extract is passed through a strong anion exchange (SAX) column.15,16,19 Therefore, the derivatization of amines is not required.
The goal of this study was to determine the source of the contaminants in the derivatizing agents, and to modify the analytical method to eliminate the contamination problem. We were not interested in identifying the contaminants. However, Fig. 4 shows the mass spectra of the two contaminants so that readers can recognize these if they occur in future studies. These mass spectra could not be matched to any compounds in the mass spectral library (NIST Mass Spectral Search Program Version 2.0a, build July 1, 2002) on our Agilent ChemStation. The m/z 267 ions are not prominent in Fig. 4, yet they were sufficient to produce the large peaks with retention times 16.7 and 18.8 min in Fig. 1b.
 | ||
| Fig. 4 Mass spectra of contaminants attributed to t-BDMCS. Contaminant with retention time 16.7 min (a), contaminant with retention time 18.8 min (b). | ||
Our observations demonstrate that many commercially available preparations of the reagent MTBSTFA plus 1% t-BDMCS contain contaminants that interfere with the SIM GC-MS method developed to quantify naphthenic acids in fish flesh and water samples. During the development of these analytical methods, it was simply fortuitous that Reagent 2 did not contain these contaminants. The results reported in this communication demonstrate that the methods of Young et al.15 and Scott et al.16 can still be used for naphthenic acids quantification if MTBSTFA devoid of t-BDMCS is used as the derivatizing reagent.
Acknowledgements
Funding was provided by the Canadian Water Network, NSERC, and by the CONRAD Wetlands and Aquatic Working group - Fish Tainting Committee, with contributions from Albian Sands Inc., Canadian Natural Resources Ltd., Fort Hills Energy LP, Imperial Oil Resources Limited, Suncor Energy Inc., Syncrude Canada Ltd., Total E&P Canada Ltd. We thank M. Ma in the Biogeochemical Analytical Laboratory for access to their GC-MS, and D. M. Grewer for technical assistance. S. Ebert provided the well water sample.References
- J. S. Clemente and P. M. Fedorak, A review of the occurrence, analyses, toxicity, and biodegradation of naphthenic acids, Chemosphere, 2005, 60, 585–600 CrossRef CAS.
- J. A. Brient, P. J. Wessner and M. N. Doyle, in Encyclopedia of Chemical Technology, ed. J. I. Kroschwitz, John Wiley & Sons, New York, 4th edn, 1995, pp. 1017–29 Search PubMed.
- W. P. St. John, J. Rughani, S. A. Green and G. D. McGinnis, Analysis and characterization of naphthenic acids by gas chromatography–electron impact mass spectrometry of t-butyldimethylsilyl derivatives, J. Chromatogr., A, 1998, 807, 241–51 CrossRef.
- J. S. Clemente and P. M. Fedorak, Evaluation of the analyses of t-butyldimethylsilyl derivatives of naphthenic acids by gas chromatography-electron impact mass spectrometry, J. Chromatogr., A, 2004, 1047, 117–28 CrossRef CAS.
- F. M. Holowenko, M. D. MacKinnon and P. M. Fedorak, Characterization of naphthenic acids in oil sands wastewaters by gas chromatography-mass spectrometry, Water Res., 2002, 36, 2843–55 CrossRef CAS.
- J. S. Clemente, N. G. N. Prasad, M. D. MacKinnon and P. M. Fedorak, A statistical comparison of naphthenic acids characterized by gas chromatography-mass spectrometry, Chemosphere, 2003, 50, 1265–74 CrossRef CAS.
- V. Nero, A. Farwell, L. E. J. Lee, T. Van Meer, M. D. MacKinnon and D. G. Dixon, The effects of salinity on naphthenic acid toxicity to yellow perch: Gill and liver histopathology, Ecotoxicol. Environ. Saf., 2006, 65, 252–64 CrossRef CAS.
- J. S. Clemente, M. D. MacKinnon and P. M. Fedorak, Aerobic biodegradation of two commercial naphthenic acids preparations, Environ. Sci. Technol., 2004, 38, 1009–16 CrossRef CAS.
- L. F. Del Rio, A. K. M. Hadwin, L. J. Pinto, M. D. MacKinnon and M. M. Moore, Degradation of naphthenic acids by sediment micro-organisms, J. Appl. Microbiol., 2006, 101, 1049–61 CrossRef CAS.
- O. V. Biryukova, P. M. Fedorak and S. A. Quideau, Biodegradation of naphthenic acids by rhizosphere microorganisms, Chemosphere, 2007, 67, 2058–64 CrossRef CAS.
- M. N. Jivraj, M. MacKinnon and B. Fung, Naphthenic Acids Extraction and Quantitative Analyses With FT-IR Spectroscopy. Syncrude Analytical Methods Manual, 4th ed. Syncrude Canada Ltd. Research Department, Edmonton, AB 1995 (unpublished) Search PubMed.
- F. M. Holowenko, M. D. MacKinnon and P. M. Fedorak, Naphthenic acids and surrogate naphthenic acids in methanogenic microcosms, Water Res., 2001, 35, 2595–2606 CrossRef CAS.
- M. Merlin, S. E. Guigard and P. M. Fedorak, Detecting naphthenic acids in waters by gas chromatography-mass spectrometry, J. Chromatogr., A, 2007, 1140, 225–29 CrossRef CAS.
- R. F. Young, E. A. Orr, G. G. Goss and P. M. Fedorak, Detection of naphthenic acids in fish exposed to commercial naphthenic acids or oil sands process-affected water, Chemosphere, 2007, 68, 518–27 CrossRef CAS.
- R. F. Young, W. V. Wismer and P. M. Fedorak, Estimating naphthenic acids concentrations in laboratory-exposed fish and in fish from the wild, Chemosphere, 2008, 73, 498–505 CrossRef CAS.
- A. C. Scott, R. F. Young and P. M. Fedorak, Comparison of GC-MS and FTIR methods for quantifying naphthenic acids in water samples, Chemosphere, 2008, 73, 1258–64 CrossRef CAS.
- Regis Technologies, Inc. http://www.registech.com/Library/Catalog/GC_Derivatization_2008.pdf Accessed Jan 2, 2010.
- D. G. Kleinbaum and L. L. Kupper, Applied Regression Analysis and other Multivariable Methods, Duxbury Press, North Scituate, Massachusetts 1978, ch 8, pp. 95–122 Search PubMed.
- D. M. Jones, J. S. Watson, W. Meredith, M. Chen and B. Bennett, Determination of naphthenic acids in crude oils using nonaqueous ion exchange solid-phase extraction, Anal. Chem., 2001, 73, 703–7 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
