Recent organic pollution and its biosensing methods
Hideaki
Nakamura
*
School of Bioscience and Biotechnology, Tokyo University of Technology, 1404-1 Katakura, Hachioji, Tokyo, 192-0982. E-mail: nakamura@bs.teu.ac.jp
First published on 3rd March 2010
Abstract
Rapid economic growth in Japan resulted in serious organic pollution in the 1960s. In contrast, recent organic pollution is caused primarily by phosphorus-based anthropogenic eutrophication, which also induces heavy metal pollution. In the present review, causes of the recent organic pollution are briefly introduced, and our approaches, based on studies of environmental water biosensing methods as tools for estimating the degrees of the recent organic pollution, eutrophication, and heavy metal pollution, are introduced.
 Hideaki Nakamura | Hideaki Nakamura is Assistant Professor of Tokyo University of Technology. His research interests are the areas of biosensing, water analyses, and remediation of environmental waters. |
1. Introduction
Water pollution is classified into harmful substances, suspended solids (SS), organic pollution, and eutrophication. In the 1960s, serious water pollution by discharge of voluminous organic matter and following foul smell occurred in many urban and industrialized areas during the rapid economic growth in Japan (1955–1973) and other advanced countries (Fig. 1a). It is known that the limit for the occurrence of a foul smell is under 10 mg O2 L−1 biochemical oxygen demand (BOD) in environmental water, although this depends on the relationship with the dissolved oxygen (DO) concentration. In the 1960s, organic pollution was caused primarily by organic matter in domestic and industrial wastewater, which was made worse by delays in the construction of sewerage systems and wastewater treatment facilities. Furthermore, organic pollution was attributed to auxiliary agents used in synthetic detergents used in the 1970s.1 The auxiliary agent made of phosphates was employed to remove calcium ions (Ca2+) and magnesium ions (Mg2+) in tap water, although tap water in Japan is generally softened, unlike that in Europe and the U.S. Consequently, some phosphates remain in the sediments of closed water bodies. To solve the problem of water pollution, the government decreed a Water Quality Pollution Control Act in 1971 and revised the ministerial ordinance in 2006. With the ministerial ordinance, environmental water quality has gradually improved.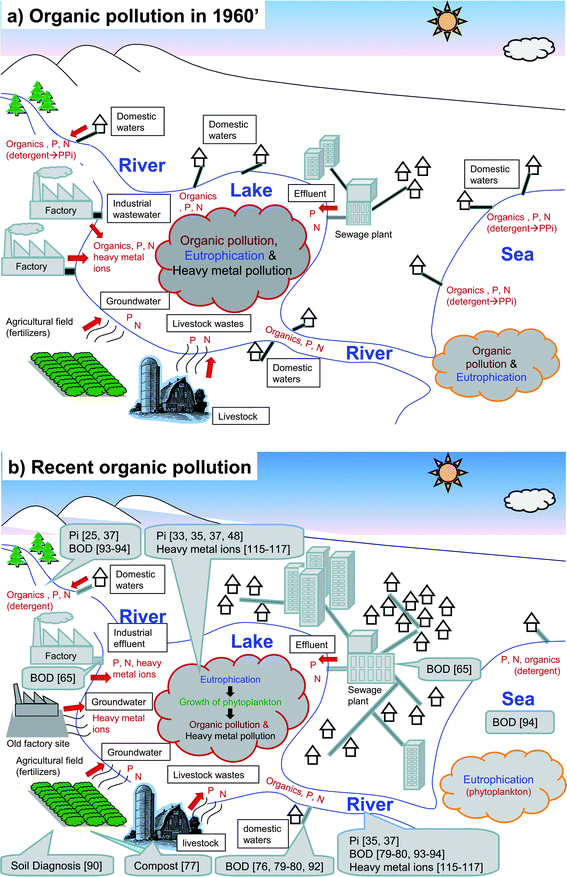 | ||
| Fig. 1 Comparison on the causes of organic pollution in the 1960s and the recent past. (a) 1960s, and (b) recent past. Examples of the application of biosensing methods are given for each category. | ||
Currently, organic pollution of environmental water is generally attributed to eutrophication (although surfactants from domestic water might be producing some of the recent organic pollution), especially in closed water bodies, such as lakes, marshes, reservoirs, and enclosed coastal seas (Fig. 1b). Eutrophication is caused by the release of nutritive salts, such as phosphates and nitrates. The nutritive salts are released from effluents after sewage treatment of domestic and industrial wastewater, livestock wastes, and ground water from fertilized agricultural fields. In these anthropogenic runoff sources, the influence of sewage effluents on the environmental water increases day by day due to the increase of the population in urban areas. In addition, these nutritive salts in wastewater cannot be easily removed by current sewage treatment techniques employing a conventional activated sludge method (ca. 50%) or an advanced treatment method (ca. 75%), although most organic material can be removed with the use of both methods.2 However, this is extremely wasteful, especially in the case of phosphorus, which is a concern worldwide. In addition, the price of phosphorus is rapidly increasing by drain of phosphate rock. Therefore, efficient recovery and recycling techniques are much in demand.
Incidentally, the minimal concentration of nutritive salts required for the occurrence of water bloom was 0.3 mg L−1 nitrogen (N), 0.02 mg L−1 phosphorus (P), 0.05 mg L−1 sulfur (S), 0.02 mg L−1 magnesium (Mg), and 0.002 mg L−1 iron (Fe) in 1977;3i.e., the amount of phosphorus required to induce eutrophication was fifteen times lower than that of nitrogen, although, the ratio is not close to the Redfield ratio in phytoplankton, i.e., C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 41
P = 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by mass.4
1 by mass.4
The phosphorus concentration in flowing water is considered to be an important indicator for estimating the degree of eutrophication,5 and phosphorus is considered as a limiting factor of eutrophication in lakes,6,7 although sunlight is considered to be a limiting factor of ecosystems in oligotrophic lakes.8 In fact, nitrogen is constantly supplied through many kinds of nitrogen-fixation microbes, such as cyanobacteria Anabaena sp. from atmospheric nitrogen molecules. Here, cyanobacteria are blue-green algae, which belong to phytoplankton. On the other hand, phosphorus, as soluble phosphates, is not naturally supplied in any great quantity; in other words, it is mostly supplied from anthropogenic sources.9 Thus, it is known that phosphates induce accelerated eutrophication involving aberrant growth of phytoplankton as a biological producer and water bloom is formed on the surface water.
The phytoplankton produces an excess of biomass by photosynthesis, involving the fixation of bicarbonate ions from atmospheric carbon dioxide. The aquatic ecosystem then deteriorates, and cyanobacteria as a principal phytoplankton produce cyanobacterial toxins (cyanotoxins), such as the neurotoxin (anatoxin-a from Anabaena sp.)10 and hepatotoxins (cylindrospermopsin from Cylindrospermopsis raciborskii11 and microcystins from Microcystis sp. and Anabaena sp.12). Further, by degradation of the biomass in the water, ill-fitted malodorous substances, such as geosmin (C12H22O, which has an earthy smell) and 2-methylisoborneol (2-MIB; C11H20O), are produced by actinomycetes (Streptomyces sp.) and cyanobacteria (Oscillatoria sp. and Phormidium sp.).13 Furthermore, excessive amounts of phytoplankton can cause death, and decomposed matter produces organic pollution. The fumes emanating from water containing decomposing organic matter are foul and contain mold, making the water not potable. Therefore, water quality in reservoirs and lakes must be carefully controlled to maintain safe supplies of drinking water.
In the process of organic pollution, water goes from the oxidation to the reduction state, which leads to anoxia. Harmful heavy metal ions are then dissolved into the water from the sediments. Thus, heavy metal pollution occurs. Such polluted areas are referred to as “dead zones”.
In summary, the causes of recent organic and heavy metal pollution following eutrophication were briefly discussed above. Subsequently, recent biosensing methods for such water pollution are introduced along with our research strategies and experiences with each type of pollution. In reviews, general biosensing methods that include fundamentals and applications were thoroughly summarized by Nakamura and Karube in 2003.14 Other reviews relevant to biosensing methods for environmental monitoring were published by Lelie et al. in 1994,15 Barcelo in 2005,16 and Rogers in 2006.17
2. Estimation methods for eutrophication
2.1. Conventional measurement methods
The degree of eutrophication is generally determined with an algal growth potential (AGP) test, a standard method used by the U.S. Environmental Protection Agency (USEPA) in 1971.18,19 Although the bioassay method requires long-term incubation to obtain the results (around 1 or 3 weeks), it is very important because it allows the determination of the bioavailability of algal growth-limiting nutrients. Therefore, the requirement for studies on biosensing methods, namely, simple and rapid measurement methods, as described later in detail, has increased.As described above, phosphorus recently became known as a limiting factor of eutrophication.6,7 In fact, from a biological viewpoint, phosphorus is an essential element for living organisms to form nucleic acid, phospholipids, adenosine 5'-triphosphate (ATP), and bone (vertebrates). Thus, phosphates in man-made sources are eutrophied in environmental water, and then anthropogenically accelerated eutrophication is induced. The Organization for Economic Cooperation and Development (OECD) classified lakes according to their trophic conditions into ultra-oligotrophic (4.0 mg m−3 and below), oligotrophic (10.0 mg m−3 and below), mesotrophic (10–35 mg m−3), eutrophic (35–100 mg m−3), and hypertrophic (100 mg m−3 and above), as the total-P (T–P).20 In Japan, 20 mg m−3 T–P is an indication of eutrophication,13 and the maximal permissible concentration in lakes is 0.01 mg L−1 T–P (0.03 mg L−1; 0.32 μmol L−1 orthophosphate ion; PO43−; Pi) for drinking and 0.1 mg L−1 T–P (0.3 mg L−1; 3.2 μmol L−1 Pi) for environmental protection.
Phosphorus in environmental water is classified into an inorganic form (further classified into an orthophosphate form and condensed phosphates) and an organic form, and each form can be separated by filtration into a dissolved form and a suspended form. On the other hand, phosphorus exists almost solely as phosphates, which are classified into Pi, condensed phosphates (pyro-, meta-, and other polyphosphates), and organic phosphates. In the measurement method, phosphorus is classified into Pi (mg L−1 PO4–P), acid-hydrolysable phosphates, and T–P (mg L−1 P). In general, Pi is measured as inorganic phosphates in environmental water.
For Pi determination, the conventional spectrophotometric molybdenum blue method “JIS K 0102” was established by the Japanese Industrial Standard (JIS) Committee as the official method for industrial wastewater in 1993.21 This method requires the use of strong acid and heavy metal ions. Therefore, the amount of waste, such as environmentally loading chemicals, increases with the number of measurements when the spectrophotometric molybdenum blue method is employed. The use of such chemicals is subject to limitation when applied to on-site monitoring and continuous monitoring because leakage of the chemicals into the environment has to be prevented.
In addition, this method has a poor detection limit of 10 μg L−1 T–P (0.32 μmol L−1 Pi). This value is insufficient for the estimation of eutrophication, which is classified into five categories (between around 1.0 and 100 μg L−1 T–P (0.032–3.2 μmol L−1 Pi)) in lakes.
For these reasons, highly sensitive Pi biosensing methods, namely, simple, safe, and ecological methods, have been studied. Such studies have been reviewed in several articles.14,22–26 Our goal is to realize the practical use of Pi biosensing systems.
2.2. Recent biosensing methods for eutrophication
Several enzymatic Pi biosensing methods have been studied. In 1975, Guilbalt et al. studied an enzymatic Pi biosensing method which was based on the inhibition of alkaline phosphatase activity by Pi.27 This method lacked selectivity and sensitivity. In 1990, D'Urso et al. studied a two-enzyme Pi biosensing method employing nucleoside phosphatase and xanthine oxidase.28 In 1992, the two-enzyme system was improved to a flow injection analysis (FIA) system by Wollenberger et al.29 However, these measuring systems were not for practical use due to their short lifetimes.Karube et al. have studied several kinds of enzymatic Pi biosensing systems using pyruvate oxidase (PO) from Pediococcus sp. (POPsp)30–33 and genetically engineered PO from Lactobacillus plantarum (POLp),34Aerococcus viridans (POAv),35 and maltose phosphorylase (MP).36,37 These studies were performed for the practical use of an automatic FIA system (desktop-type)35,37 and an on-site Pi monitoring system (submersible buoy-type).33
The Pi biosensing system employing PO is superior to other systems because it requires only one step of a catalytic reaction for selective Pi detection. Using POPsp, the first Pi biosensing system was studied by Kubo et al. in 1991.30 The system employed an electrochemical reaction for DO, which is consumed by the POPsp catalytic reaction under the existence of two cofactors (thiamin pyrophosphate; TPP and flavin adenine dinucleotide; FAD) and an enzyme activator (Mg2+). However, the detection limit of 12 μmol L−1 was insufficient for water quality control.
In 1996, Ikebukuro et al. examined the combination of a luminol chemiluminescence (CL) reaction and a FIA system for Pi biosensing (CL-FIA system).31 In fact, the CL reaction is known as a highly sensitive biocatalytic reaction,38–42 and the FIA system is also known as a highly reproducible sensing system.24,43–45 Furthermore, CL-FIA biosensing systems have been widely studied and reviewed.46
In the POPsp catalytic reaction, hydrogen peroxide was produced and subsequently consumed by a luminol-peroxidase reaction (from horseradish, HRP). The CL light resulting from the presence of Pi was detected at a photomultiplier tube (PMT). Then, TPP was purified by chromatography to remove residual phosphates.32 As a result, a sufficient sensitivity of 74 nmol L−1 Pi was obtained, although the TPP purification was unsuitable for practical use.
In 1997, Nakamura et al. employed highly sensitive luminol catalyzing peroxidase from Arthromyces ramosus (ARP).33 This CL-FIA system was improved to a remote-controlled continuous Pi monitoring system and compactly integrated into a submersible buoy14 for quality monitoring of dam water for drinking.33 Chitin-chitosan beads immobilized POPsp were filled in a column. In this study, 160 nmol L−1 Pi was detected without purification of TPP, and 320 nmol L−1 Pi was detected for 48 days. This result could yield a practical use of the CL-FIA system for Pi monitoring. However, the manufacture of POPsp was unfortunately stopped.
In 1998, Suzuki et al. studied a reagent-less electrochemical Pi biosensing system using genetically engineered POLp to save co-enzymes and activator.34 This system had sufficient sensitivity of 15 nmol L−1 Pi; however, its lifetime was not practical.
In 1999, Nakamura et al. reexamined the development of the CL-FIA system for Pi biosensing using a new enzyme, POAv, which was purified from recombinant Escherichia coli.35 A desktop-type automatic CL-FIA system involving a precise system for temperature control was constructed. POAv was immobilized to an N-hydroxysuccinicacidimido (NHS) gel. The results showed, with sufficient performance at a short measurement time of 2 min, a wide linear range of calibration (96 nmol L−1 and 32 μmol L−1 Pi) with an average relative standard deviation (RSDav) of 2.3% (8 points, n = 5) at 25.0 °C. In addition, this system kept making calibration curves from 0.16 to 32 μmol L−1 Pi (5 points, n = 3; averaged correlation, r = 1.00) for at least 2 weeks. Thus, the practicability of the Pi biosensing system was demonstrated.
On the other hand, Conrath et al. studied a new electrochemical Pi biosensing method using an analyte recycling system in 1995 consisting of four enzymes, maltose phosphorylase (MP), acid phosphatase (AcP), mutarotase (MUT), and glucose oxidase (GOD).47 The complicated system enabled the successful detection of 10 nmol L−1 Pi. However, the system was too complicated, and we thought it would be difficult to replicate in a manufacturing environment. In 1999, Nakamura et al. modified the MP system and applied it to our CL-FIA biosensing system using a trienzymatic reaction of MP-MUT-GOD without the analyte recycling by AcP.36 The same results with Conrath et al. were obtained with a detection limit of 10 nmol L−1 Pi. An excellent calibration between 10 nmol L−1 and 30 μmol L−1 Pi was obtained. Stability to detect 1.0 μmol L−1 Pi was observed for at least 2 weeks.
In 2003, Nakamura et al. improved the MP-MUT-GOD system as an automatic CL-FIA system,37 and thirty river water samples were applied to the system. The results were compared with those from the conventional molybdenum blue spectrophotometric method:21 the value from the conventional method was 2.78 times higher than that from the Pi biosensing system. One reason for this outcome was the large difference in the reacting conditions between the biosensing (natural pH) and the conventional method, which required acidic conditions to make a complex between molybdenum and phosphate. Therefore, enzymatic Pi biosensing could be used to determine a more accurate Pi concentration than that achieved with the conventional method.
Furthermore, Nakamura et al. studied a pyrophosphate ion (PPi) biosensing system using the automatic CL-FIA system in 2004.48 In general, condensed phosphates, such as PPi, exist in anthropogenically eutrophied water and cause organic pollution as well as Pi; therefore, the PPi concentration can be an indicator for the estimation of eutrophication and organic pollution. For enzymatic PPi biosensing, inorganic pyrophosphatase (IP) was added to the POAv reaction. A calibration curve was then obtained from 100 nmol L−1 to 100 μmol L−1 PPi (7 points, r2 = 0.9997), and the applicability of this PPi biosensing system to environmental water was shown.
In another study, Nakamura et al. examined the development of a disposable electrochemical Pi biosensing chip using an available self-monitoring blood glucose (SMBG) chip.49 Pi was measured with a POAv or several MP systems coupled with ferricyanide ion (Fe3+) as a mediator. This system is able to apply to on-site Pi monitoring.
As described above, we studied many types of Pi biosensing systems for the estimation of organic pollution following anthropogenically accelerated eutrophication, and two practical Pi biosensors equipped the automatic CL-FIA system and the precise temperature controlling system were successfully developed with wide determination ranges and stabilities35,37 (Table 1).
| Enzyme/s | System | Transducer | Immobilization of enzyme/s | Sample volume/μL | Cariblation range/μmol L−1 | Detect limit/μmol L−1 | Meas. Time (ca min) | Stability | Published in | Ref. | Authors | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Response times. | ||||||||||||
| II. 2. Recent biosensing methods for eutrophication | ||||||||||||
| POPsp | electrochemical (batch) | DO electrode | Photo-crosslinkable resin | — | 12–80 | 12 | 5a | 7 days | 1991 | 30 | I. Kubo, I. Karube et al. | The first Pi biosensing system using PO. |
| POPsp | optical (CL;FIA) | PMT | CPG | 25 | 4.8–160 | 3.2 | 3 | 4 days | 1996 | 31 | K. Ikebukuro, I. Karube et al. | CL-FLA type biosensing system using. |
| POPsp | optical (CL;FIA) | PMT | CPG | 500 | 0.37–7.4 | 0.074 | 3 | 2 weeks | 1996 | 32 | K. Ikebukuro, I Karube et al. | Improvement of [15]. |
| POPsp | optical (CL;FIA) | PMT | Chitin-chitosan beads | 500 | 0.16–32 | 0.16 | 3 | 48 days | 1997 | 33 | H. Nakamura, I. Karube et al. | Submersible busy type. |
| POLp | electrochemical (batch) | H2O2 electrode | CPG | 500 | 0.015–0.050 | 0.015 | 80a | 9 days | 1998 | 34 | M. Suzuki, I. Karube et al. | Regent-less POLp reaction was employed |
| POAv | optical (CL;FIA) | PMT | NHS gel | 500 | 0.096–32 | 0.096 | 2 | At least 2 weeks | 1999 | 35 | H. Nakamura, I. Karube et al. | Automatic CL-FLA system using PO. |
| MP-MUT-GOD | optical (CL;FIA) | PMT | NHS gel | 100 | 0.01–30 | 0.01 | 3 | At least 2 weeks | 1999 | 36 | H. Nakamura, I Karube et al. | CL-FLA system using MP system. |
| MP-MUT-GOD | optical (CL;FIA) | PMT | NHS gel | 100 | 0.1–30 | — | 1a | At least 2 weeks | 2003 | 37 | H. Nakamura, I. Karube et al. | Automatic CL-FLA system using MP react system. |
| IP-POAv | optical (CL;FIA) | PMT | NHS gel | 100 | 0.1–100 (PPi) | 0.1 (PPi) | 0.5a | At least 2 weeks | 2004 | 48 | H. Nakamura, I. Karube et al. | Automatic CL-FLA system for PPi. |
Subsequently, recent studies on enzymatic Pi biosensing methods by other groups were introduced. In 2005, Kwan et al. studied a screen-printed Pi biosensing system employing PO. PO was immobilized in a nafion matrix that was spread over the working Pt electrode. Hydrogen peroxide produced by the PO reaction was electrochemically detected. This system had a linear calibration range from 75 to 625 μmol L−1.50 However, as described above, the use of PO has a potential problem, namely, the requirement of two co-enzymes and an activator for a Pi catalytic reaction. In 2001, Mousty et al. studied an electrochemical trienzymatic Pi biosensing system employing an MP-MUT-GOD reaction system.51 They were successfully removed by AP reaction which was required in the study by Conrath et al.47 Three enzymes were entrapped in inorganic laponite clay, which was spread on the platinum electrode surface. Hydrogen peroxide was detected as a product of the MP-MUT-GOD reaction. This system had a linear calibration range from 1 to 50 μmol L−1 and was stable for at least 2 weeks. In 1998, Fernández et al. studied another type of electrochemical trienzymatic Pi biosensing system employing phosphorylase A, phosphoglucomutase, and glucose 6-phosphate dehydrogenase.52 Three enzymes, a substrate, a cofactor, and a mediator, were co-immobilized in a carbon paste electrode and covered with hydrogels. By incorporation of all reagents into hydrogels, this system enabled reagentless biosensing. However, the principle for Pi biosensing is quite complicated, and this system had an insufficient detection limit of 2 mmol L−1. Thus, all of the enzymatic Pi biosensing methods had insufficient sensitivity for water quality control (below 0.32 μmol L−1 Pi as determination limit is required for practical use). The difficulty with developing a practical Pi biosensing system is clearly evident.
Using a phosphate-binding protein (PBP), Pi biosensing systems have been studied. In 2002, Kubo et al. applied PBP to an electrochemical system. PBP is a component of a phosphate transport system of E. coli and a highly selective recognition element that was immobilized on a nitrocellulose membrane. Potentiometric determination of phosphate binding was performed in a range from 0.1 to 1.5 mmol L−1.53 In 2004, Lyndon et al. applied a mutant PBP (MPBP) to fluorometric Pi biosensing. Fluorophore was site-specifically labeled to MPBP. The change in fluorescence intensity caused by the addition of Pi could be measured with a fluorometer, a microtiter plate reader, and a fiber optic sensing system. This system showed excellent selectivity and detected around sub-μmol L−1 Pi.54 Thus, these PBP biosensing systems for Pi showed high potential regarding their selectivity, although the stability of PBP should be addressed.
As another type of Pi-recognizing element, the ionophore has been employed for selective Pi biosensing employing gravimetry by quartz crystal microbalance, voltammetry, and fluorometry.55 The ionophore is a lipophilic molecule synthesized by microorganisms to transport ions across the lipid bilayer of the cell membrane. In 2008, Wygladacz et al. studied a fluorescence-based Pi biosensing employing uranyl salopene ionophore. An H+-selective fluoroionophore was used as a reference chromoionophore.56 These ionophores were co-immobilized to sensing film or microspheres. Pi was measured by changing the fluorescence intensity of the chromoionophore after binding Pi to the ionophore. This system obtained a linear calibration range from 1.0 μmol L−1 to 2.5 mmol L−1 Pi. However, by continuous contact with the Pi-containing sample, the lifetime was shortened to the order of days.
In 2001, Schreiter et al. studied a Pi bioavailability assay using a luminescent cyanobacterial reporter strain for the replacement of the conventional AGP test. The method enabled the detection of Pi from 0.3 to 8 μmol L−1 at only 8 h of incubation.57 In 2003, Dollard and Billard studied phoA::lux-based bacterial sensors using a Pi-sensing plasmid that demonstrated the possibility for the assessment of Pi bioavailability.58 However, the use of genetically engineered biosensing elements has problems associated with leakage of these elements into environment.
As described above, several phosphate biosensing methods and Pi bioavailability assays have been developed. Further studies will be required to develop a practical Pi biosensing method, although research activities are now less vigorous than they were 10 years ago. However, necessities of Pi biosensing for environmental monitoring will be increased by realization of practical sensitivity (less than 10 nmol L−1 Pi), disposable chip, and mobile meter. The analyte recycling techniques will become key technologies for highly sensitive Pi biosensing,47 and the SMBG techniques should be studied extensively for on-site Pi monitoring.17
2.3. Water quality estimations in an anthropogenically eutrophied storage reservoir
Since 2006, we have been investigating the recent organic pollution caused by anthropogenic eutrophication. We have circumstantially investigated the water quality of a closed and anthropogenically polluted water system (a water-storage pond in TUT) by both fortnightly measurements (organic pollution; DO, chemical oxygen demand; COD, and total organic carbon; TOC, eutrophication; Pi, silicate ion, NO3−, NO2−, NH4+, and inorganic N, cationic ions; Na+, K+, Ca2+, and Mg2+, others; temperature, pH, conductivity, salinity, DO, oxidation-reduction potential; ORP, transparency, turbidity, and chromaticity, atmosphere; whether, temperature, pressure, humidity, and wind-force) and continuous monitoring (once every hour; temperature, pH, conductivity, DO, and ORP).59In these water quality investigations, the characteristics of polluted water were progressively understood. Now, we are studying the application of these characteristics for the improvement of recent organic pollution. For example, we are studying a simultaneous Pi and phytoplankton recovery system and effective applications of the recovery materials. The detailed results will be published elsewhere.
3. Estimation methods for organic pollution
3.1. Conventional measurement methods
To estimate the degree of organic pollution, several indicators have been employed; they include TOC, COD, BOD, and DO. Furthermore, COD is mainly employed for closed water bodies of both natural water and seawater, and BOD is used for flowing water, such as rivers. The differences in the usage of COD and BOD estimation methods are simply determined by the presence or absence of the flux, which depends on the decomposition rate of organics dissolved in the water body by aerobic microbes, although they are often employed as references in each field.Of the estimation methods for organic pollution, only BOD involves the results obtained by a biological reaction; thus, in some cases, abbreviation of the BOD is derived from the biological oxygen demand. The BOD estimation method was introduced as a conventional test method by the American Public Health Association Standard Methods Committee in 1936 and by the JIS Committee as the official method for industrial wastewater, JIS K 0102, in 1964.21 The conventional method is referred to as the 5-day BOD (BOD5) method. It requires 5 days of incubation to obtain the results. The BOD value (mg O2 L−1) is calculated from the amount of DO consumed by the microbial aerobic decomposition of organics during incubation (primary fermentation). According to this principle, BOD is rarely called biochemical oxygen consumption.
3.2. Brief history of early biosensing methods for organic pollution
At the peak of organic pollution in advanced countries in the 1960s, the official or conventional method for BOD was limited to the BOD5 method.21,60 The BOD5 method has several problems that prevent it satisfying the needs for practical use in wastewater control, i.e., this method is time-consuming, tedious, and requires a high degree of proficiency from the person conducting the test. Therefore, a method that can be used to monitor the BOD value in real time or continually was urgently needed.In 1977, Dr Karube studied a practical microbial biosensing system.61 The key technique was the immobilization of microbes to a thin collagen membrane (thickness; 50 μm). The membrane-immobilized microbe was put onto the surface of a DO electrode. With the addition of a sample solution into a batch system, microbial respiration was activated by the decomposition of organics, and the degree of DO consumption by immobilized microbes was determined by the DO electrode. The microbial BOD biosensing method indicating DO consumption (BODDO) could successfully determine the BOD value at dramatically shortened incubation and measurement times (ca. 30 min). With this study, the possibilities for solving the problems of wastewater control were enhanced.
In 1979, Hikuma and Karube developed a flow system of the BODDO biosensing method.62 The microbes were immobilized onto a microporous membrane and attached onto the surface of an oxygen electrode. Based on the study, a flow-type BODDO biosensing system was available for practical applications. Both the desktop-type for rapid measurements and the installation-type for continuous monitoring were sold by the Central Kagaku Corporation in 1983.14,63,64 These BODDO biosensing systems enabled wastewater control by both real-time measurement and continuous monitoring at sewage plants and factories. The BODDO biosensing method was established as one of the JIS methods (JIS K 3602) in 1990.65
Since the first microbial biosensing method was reported in 1977, many such methods have been studied for not only environmental applications but also food applications, including fermentation. These studies on both environments and foods by microbial biosensing methods were reviewed by Bousse in 1996,66 D'Souza in 2001,67 Nakamura and Karube in 2003,14 Baronian in 2004,68 Nakamura and Karube in 2005,63 Lei in 2006,69 and Nakamura, Shimomura-Shimizu, and Karube in 2008.64
In addition, several articles describing microbial biosensing methods for environmental monitoring were published in 1995 by Lazarova and Manem70 and in 2003 by Belkin.71 Articles describing BOD biosensing methods were also introduced by Liu and Mattiasson in 200272 and Bourgeois et al. in 2003.73
3.3. Recent biosensing methods for organic pollution
3.3.1.1. Three remarkable generations. Since 1977, BOD biosensing methods associated with microbes, biosensing systems, and principles have been widely studied.14,63,64 In the JIS K 3602 method, the stable, omnivorous, and facultative anaerobic yeast Trichosporon cutaneum (eukaryotic microbe; IFO-10466) was employed as a desirable microbe for BOD estimation.65 Three historically important developments related to BOD biosensing methods are shown in Fig. 2. In addition, the characteristics of BOD biosensing systems developed by our group in recent years are summarized in Table 2.
| Type | Generation | Indicator | System | Transducer | Microbial domain | Microbial species (configuration) | Immobilization of microbe | Sample volume | Standard solution | Caliblation range (mg O2/L) | Detect. limit (mg O2/L) | Meas. time/min | Stability | Publish ed in | Ref. | Authors | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a GGA was prepared by artificial seawater. Remarkable points were shown by Bold Style. | |||||||||||||||||
| III.3.1.1. Comparison of three generations | |||||||||||||||||
| BODDO-Tc | 1st | DO | electrochemical (flow) | DO electrode | Eukarya (yeast) | T. cutaneum (mycelia) | porous membrane | 5 mL | GGA | 10–40 | 10 | < 18 | 17 days (400 tests) | 1979 | 62 | M. Hikuma, I. Karube et al. | The research product was finally realized practical BOD biosensor. |
| BODDO-Tc | 1st | DO | electrochemical (flow) | DO electrode | Eukarya (yeast) | T. cutaneum (mycelia) | porous membrane | — | GGA | 2–50 | 2 | 1 h | 1∼3 months | (Sold since) 1983 | — | — | The newest model; Quick BOD α 1000, aveirable at Central Kagaku Co. |
| BODSM-Pf | 2nd | SM | electrochemical (batch) | disposable chip | Bacteria | P. fluorescens biovar V (rod-shaped) | photo-crosslinkable resin | — | OSS | 15–200 | 15 | 15 | at least 1 week | 2000 | 75 | N. Yoshida, H. Nakamura, I. Karube et al. | Mostly the first study on a BODSM biosensor. |
| BODSM-Pf | 2nd | SM | electrochemical (batch) | disposable chip | Bacteria | P. fluorescens biovar V (rod-shaped) | porous membrane | 2.7 mL | OSS | 15–260 | 15 | 15 | > 35 days | 2001 | 76 | N. Yoshida, H. Nakamura, I. Karube et al. | The first study on a palm-sized mobile BODSM meter. |
| BODSM-Pf | 2nd | SM | electrochemical (batch) | disposable chip | Bacteria | P. fluorescens biovar V (rod-shaped) | calcium arginate gel | < 600 μL | OSS, SES | 10–120 (SES), 25–250 (OECD) | 10 | — | — | 2001 | 77 | N. Yoshida, H. Nakamura, I. Karube et al. | The first study on compost monitoring applying BODSM biosensor. |
| BODDM-Sc | 3rd | DM | electrochemical (batch) | disposable chip | Eukarya (yeast) | S. cerevisiae (monocellular) | (suspension) | 350 μL | GGA | 6.6–220 | 6.6 | 15 | at least 14 days | 2007 | 79 | H. Nakamura, I. Karube et al. | The first study on BODDM method. |
| BODDM-Sc | 3rd | DM | electrochemical (batch) | disposable chip | Eukarya (yeast) | S. cerevisiae (monocellular) | (suspension) | 350 μL | GGA | 5.5–220 | 5.5 | 10 | at least 10 days | 2008 | 80 | H. Nakamura, I. Karube et al. | Improvement of the BODDM biosensor. |
| III.3.1.2. Other sensing methods employing color indicator | |||||||||||||||||
| BODRCI-Pf | — | RCI | optical (absorption; batch) | PMT | Bacteria | P. fluorescens biovar V (rod-shaped) | (suspension) | < 160 μL | OSS | 50–430 | 50 | 20 | Low | 2002 | 91 | N. Yoshida, H. Nakamura, I. Karube et al. | The first study using a microplate reader and a 96 well microplate. |
| BODRCI-Pf | — | RCI | optical (absorption; batch) | PD | Bacteria | P. fluorescens biovar V (rod-shaped) | photo-crosslinkable resin | < 700 μ L | OSS | 50–3000 | 50 | 10 | Low | 2001 | 92 | N. Yoshida, H. Nakamura, I. Karube et al. | A mobile type meter and a disposable optical cell chip. |
| BODRCI-Sc | — | RCI | optical (absorption; batch) | PD | Eukarya (yeast) | S. cerevisiae (monocellular) | (suspension) | 600 μL | GGA | 1.1–22 | 1.1 | 10 | at least 36 days | 2007 | 93 | H. Nakamura, I. Karube et al. | A temperature controlled three-consecutive-stir system was employed. |
| Sea-BODRCI-Sc | — | RCI | optical (absorption; batch) | PD | Eukarya (yeast) | S. cerevisiae ARIF KD-003 (monocellular) | (suspension) | 400 μL | GGA ASW | 0.33–22 | 0.33 (3σ = 0.07) | 10 | at least 4 weeks | 2008 | 94 | H. Nakamura, I. Karube et al. | The lowest detection limit was marked. |
| III.3.1.3. Other sensing methods employing chemiluminescence reaction | |||||||||||||||||
| BODCL-Sc | — | CL | optical (CL; batch) | PMT | Eukarya (yeast) | S. cerevisiae (monocellular) | (suspension) | 350 μL | GGA | 11–220 | 5.5 | 5 | at least 24 days | 2007 | 104 | H. Nakamura, I. Karube et al. | The first study on BODCL method. |
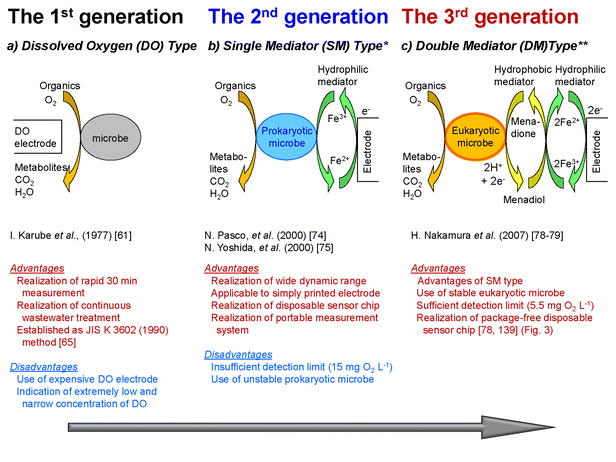 | ||
| Fig. 2 History of typical BOD biosensor developments. *The illustrations of the principle are modified from those in ref. 75. **The schematic diagram is from ref. 75, 81 and 83. | ||
The advantages of the BODDO biosensing system are explained above (also Fig. 2a); however, the method requires a highly sensitive but expensive DO electrode to determine the precise DO concentration (8.84 mg O2 L−1 at 1 atm, 20 °C). In addition, the DO electrode is difficult to employ for single use. There are limitations for applications with a portable apparatus. Therefore, as one of the research strategies, other indications replacing that of the DO concentration have been studied.
In 2000, Pasco et al. and Yoshida et al. reported the second generation of a single mediator-type BOD biosensing (BODSM) method using the pair of ferricyanide as a hydrophilic mediator and a prokaryotic microbe74,75 (Fig. 2b). We then employed the bacteria Pseudomonas fluorescens biovar V, which was isolated from activated sludge sampled from a municipal sewage treatment plant.75 In the study, the high solubility to water and low applied potential for the amperometry of ferricyanide resulted in a disposable microbial electrode chip. The study was expanded to include the development of a palm-sized portable apparatus for on-site monitoring76 and a compost monitoring system.77 In the former study, a calibration curve was obtained between the output current and OECD standard solutions from 15 to 260 mg O2 L−1 BOD. The low sensitivity was a disadvantage for natural water; however, the BOD biosensing system was successfully adopted for domestic water. Incidentally, according to the investigation by the Japan Sewage Works Association (JSWA) in 2006, the adoption rate of sewage was ca. 70% (the rate was within the average of advanced countries); therefore, we considered that the portable BOD biosensing system could be applicable for the on-site monitoring of domestic water. However, bacteria are unstable in general and not easy to manage, although it is difficult to obtain electrical signals from eukaryotic microorganisms, such as yeast, with a single mediator system due to the thick outer wall of the eukaryote cells. To overcome this problem, seven additional years of study were required to develop a new BOD biosensing principle.
In 2007, 40 years after the first BOD biosensing method was reported, we developed a third generation of the BOD biosensing method using a double mediator (DM) system, which consists of a hydrophilic mediator (ferricyanide) and a hydrophobic mediator (menadione)78–80 (Fig. 2c). In this study, we refer to several studies concerning the DM system by Rabinowitz et al.,81 Baronian et al.,82 and Heiskanen et al.,83 and we employed baker's yeast Saccharomyces cerevisiae (budding yeast) as a eukaryote microbe for BODDM biosensing. In principle, organics were assimilated by S. cerevisiae, and a lipophilic mediator, menadione, penetrating the outer cell membrane, was then changed to menadiol. Subsequently, the ferricyanide ion (Fe3+) was reduced to the ferrocyanide ion (Fe2+). The amounts of Fe2+ were determined by chronoamperometry.
In this study, a rationally designed disposable microbial electrode chip was constructed as a small batch system (inner volume; 563 μL); the design included a micro-stirrer system. The chip enabled the storage of wet microbes (Fig. 3).79,84 In related studies, we designed many types of biosensing chips and applied for more than 100 patents in Japan and about 30 worldwide. For example, simple designs for disposable biosensing chips85,86 and a package-free disposable biosensing chip87,88 were completed. Several biosensing chips will be applicable to microbial biosensing.
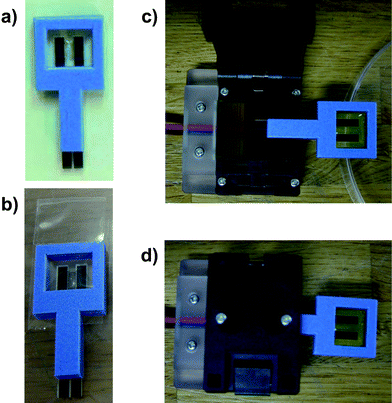 | ||
| Fig. 3 Package-free disposable biosensor chip.78–80,87,139 a) Addition of microbial cells into a chip. b) Packaged chip containing wet microbial cells. c) An open connector and a chip. d) Chip connected to the connector. | ||
In general, in biosensing techniques, microbial cells are immobilized to a carrier material and used in repetitive measurements. The variance of the microbial cell amounts and their activity entrapped in each membrane could be corrected by taking the calibration. On the other hand, in a study on BODDM biosensing, the use of S. cerevisiae cells led to success.79S. cerevisiae can be suspended uniformly as a single cell in solution; therefore, the S. cerevisiae suspension can be dispensed quantitatively and accurately in a unit volume. The feature was used to achieve highly repeatable BODDM biosensing, and the high repeatability led to highly sensitive biosensing. Incidentally, T. cutaneum could not be used in this study because the microbe formed mycelia (fungal filaments) and flocked in solution.
Thus, the features of BODDM methods are described above. The DM system was also applied to the direct determination of ethanol concentration in alcoholic beverages in 2009.89 The technique can be applied to bioethanol determination in an environmental field. In addition, we recently studied a method for rapid agricultural soil diagnosis on the basis of the BODDO biosensing method.90 The study was a collaboration with a nursery company, the Sakata Seed Co., Ltd. In general, soil diagnosis has been performed by a pot test, which requires several weeks to obtain the results; however, using a dual microbial electrode system, soil diagnosis was concluded in 30 min. One electrode was an immobilized antagonist, and the other was an immobilized plant pathogen. In this study, microbial electrodes were constructed using 13 different microbes. Measurements were performed by immersing two microbial electrodes in a soil extract. The ratio between the responses of the two electrodes correlated with symptoms. Thus, the soil biosensing system determined whether or not the soil was diseased. In this study, the possibility to determine cross-interaction between different microbes was anticipated.
3.3.1.2. BOD biosensing methods employing a color indicator. Utilizing the feature of uniformly suspended microbes, new BOD estimation methods using prokaryote or eukaryote microbes have been studied; these methods employ 2,6-dichlorophenolindophenol (DCIP; λmax = 600 nm) as a redox color indicator (BODRCI method).91–93 First, a simple, multiple simultaneous spectrophotometric biosensing method was studied using a 96-well microplate absorbance reader in 2002.91 In this study, P. fluorescens biovar V was employed as a prokaryote microbe. In principle, organics were assimilated by P. fluorescens biovar V, and an oxidized type of DCIP (DCIPOx; deep blue) was reduced and decolorized. A reduced type of DCIP (DCIPRed) was then formed. The BODRCI-Pf measurement was simply carried out by mixing a P. fluorescens biovar V cell suspension, a DCIPOx solution, and a sample containing organics. The amount of the reduction was proportional to the amount of organics dissolved in a sample.
On the basis of this principle, a portable-type BODRCI-Pf biosensing system and a disposable optical cell chip were developed in 2001.92 The chip was designed to have three-consecutive-cell units, and each cell contained P. fluorescens biovar V cells and DCIPOx. The portable-type BODRCI-Pf biosensing system was equipped with three absorbance meters consisting of three pairs with a light-emitting diode (LED) and a photodiode (PD). However, the prokaryote microbe is generally unstable and difficult to manage; therefore, we applied baker's yeast S. cerevisiae as a stable, safe, easily available, and easily manageable eukaryote microbe.
Utilizing the feature of the S. cerevisiae cell suspension, we fabricated a temperature-controlled three-consecutive-stir system for a BODRCI-Sc biosensing in 2007.93 The BODRCI-Sc measurement was simply performed by mixing the S. cerevisiae cell suspension, DCIPOx, and a sample solution. A change in absorbance between before and after incubation of the mixture for precisely 10.0 min was measured using a spectrophotometer. As a result, a highly sensitive BODRCI-Sc determination was achieved by highly repeatable measurements.
In recent years, the requirements of BOD measurements for seawater have been increased. We tried to apply the BODRCI-Sc method to seawater measurements employing salt-tolerant yeast S. cerevisiae ARIF KD-003.94S. cerevisiae ARIF KD-003 was isolated from a cold district of the Shirakami mountains in Akita Prefecture by the Akita Research Institute of Food and Brewing (ARIF). In this study, the GGA standard solution was prepared using artificial seawater (GGAASW) to reduce the difference in the constituents between the standard solution and the real sample solution. As a result, the Sea-BODRCI-Sc method yielded two calibration curves between absorption and BOD value from 0.33 to 22 mg O2 L−1 using normal GGA or GGAASW, and the performance of this method was superior to that of the available BODDO biosensor (2–50 mg O2 L−1 BOD). In addition, the 3σ lower detection limit was calculated to be 0.07 mg O2 L−1 BOD (normal GGA), which is the lowest value in the literature of BOD biosensing methods. Thus, the Sea-BODRCI-Sc method demonstrated its practicability in seawater and extremely pure fresh water, such as ultra-oligotrophic water. Sufficient storage stability was also obtained with this method for at least 4 weeks.
Other groups have studied several Sea-BOD biosensing systems.95–100 In 1999, Chan et al.,95 Lehmann et al.,96 and Tag et al.97 developed several Sea-BODDO biosensing systems using salt-tolerant yeast Arxula adeninivorans LS3 for seawater. A. adeninivorans LS3 is mycelia, as is T. cutaneum, and has a broad substrate range. A. adeninivorans LS3 was immobilized onto the surface of a DO electrode using a hydrogel. The Sea-BODDO-Aa biosensing system was optimized and characterized95 and applied to real samples.96 The results obtained by this system agreed with those obtained by the BOD5 method with a wide measurable range between approximately 3 and 165 mg O2 L−1 using salted sewage samples.
Subsequently, Lin et al.,98 Jiang et al.,99 and Xin et al.100 have developed several fiber-optic Sea-BODDO biosensing systems using three kinds of seawater microorganisms, Bacillus licheniformis, Dietzia maris, and Marinobacter marinus. The first study on a fiber-optic BODDO biosensor probe was carried out by employing an available fluorescence DO probe by Chee et al.101 Lin et al. developed an oxygen-sensing film that consisted of an organically modified silicate (ORMOSIL) film containing a ruthenium complex.98 The oxygen-sensing film was applied to optical fiber Sea-BODDO biosensing. This system was improved to an automatic sensing system99 and further on-line robotized for automatic flow sampling.100 In studies on Sea-BOD biosensing by two groups,95–100 the standard solution was prepared using pure water. For future studies, artificial seawater will be required for the preparation of the standard solution.94
In another remarkable study of a BOD biosensing method, Pang et al. (2007) studied a high-throughput spectrophotometric BODDO determination method using a polystyrene microtiter plate coated with a crack-free ORMOSIL oxygen-sensing film embedding a ruthenium dye.102Stenotrophomonas maltophilia was used as an aerobic biosensing element. The method enabled a high-throughput BODDO determination, similar to what we had achieved by the BODRCI method in a previous study,91 and drastically simplified measurements that concluded by mixing microbes and sample on a microtiter plate.
In this section, remarkable optical BOD biosensing methods for both natural water and seawater, including wastewater, are introduced. In the future, the BODRCI biosensing method can be improved to a disposable colorimetric method as a simple and universal method. ORMOSIL oxygen-sensing materials might be future trends in BODDO biosensing studies as similar-principle methods with the conventional BODDO sensor.
3.3.1.3. BOD biosensing methods employing a chemiluminescence reaction. Since the first report of a BOD biosensor in 1977, several principles have been studied, for example, biofuels, DO, mediators, and bioluminescence.103 However, chemiluminescence (CL) has not been studied in microbial biosensing methods; nevertheless, the CL reaction is used extensively in other biosensing methods in general.
Using our knowledge of CL biosensing techniques, we applied the principle of the microbial DM system to luminol chemiluminometric BOD (BODCL-Sc) biosensing using S. cerevisiae in 2007.104 This was the first report applying CL to BOD biosensing. In this study, we employed a coupling of organic assimilations by S. cerevisiae and a ferricyanide catalyzing a CL reaction through a lipophilic mediator of menadione. The measurement was concluded in a transparent test tube for CL detection. In the BODCL-Sc method, a linear calibration curve was obtained from 11 to 220 mg O2 L−1 with a detection limit of 5.5 mg O2 L−1, although the dynamic range was insufficient for application to environmental water. Thus, we successfully developed a new BODCL-Sc biosensing method.
The BOD biosensing methods have become diversified due to their simplicity, stability, portability, high sensitivity, and high throughput. In particular, highly sensitive BOD biosensing is urgently needed to overcome organic pollution worldwide. Then, broad assimilability of organic substances, including refractory organic substances, will be required in the future.
4. Estimation methods for heavy metal pollution
4.1. Introduction and conventional measurement methods
By accelerating eutrophication, DO is aerobically consumed by aerobic organisms in the water, and primary fermentation is finished. Subsequently, anaerobes anaerobically catabolize organics that are deposited as sediments and are supplied from dead cells of phytoplankton after accelerating growth. The lake beds and sea beds then change to anaerobic conditions, and several harmful substances, such as heavy metal ions, are dissolved from the sediments. Thus, the influence of heavy metal toxicity on living organisms cannot be disregarded as a pollution source during organic pollution following eutrophication. Heavy metal ions have been also found in environmental waters from anthropogenic sources, such as industrial wastewater and groundwater from industrial sites.As toxic substances, heavy metal ions have been measured by the conventional JIS K 0102 method, such as flame atomic absorption spectrometry (FAAS), electrothermal atomic absorption spectrometry (ET-AAS), inductively coupled plasma-atomic emission spectrometry (ICP-AEC), and ICP mass spectrometry (ICP-MS) in 1993.21 However, these methods require expensive instruments, and their installation space, maintenance, and measurements are tedious and time-consuming. In addition, the measurement of toxic substances cannot estimate the toxicity to living organisms or aquatic ecosystems. To estimate such toxicity, the use of living organisms is the best solution.
As a traditional method for the estimation of heavy metal ions, the algal growth inhibition (AGI) assay method has been most conventionally adopted.105 The AGI assay method makes it possible to estimate the influence of water pollution contamination on the growth rate of algae; however, such assay methods require time-consuming incubation to obtain the results. Therefore, simple and rapid biosensing methods have been widely studied.
4.2. Recent biosensing methods for heavy metal pollution
Thus, the biosensing method, which uses living organisms, such as mammals, fish, and microbes, has been widely studied as a tool in analytical toxicology. In particular, microbial toxicity biosensing methods have many advantages. They are easy to manage and measure, and the equipment is portable. Several review articles on microbial toxicity biosensing methods were published in 1996 by Tothill and Turner,106 in 1998 by Blaise,107 in 2000 by Ikebukuro et al.,108 in 2003 by Beelen,109 in 2007 by Barcelo,110 and as described above.14,63,64The microbial toxicity biosensing methods are categorized into two types, respiratory inhibition (RODTOX™ method)111 and metabolic inhibition (Micredox™, CellSense™, and Microtox™ methods).112–114 The RODTOX™ method requires highly sensitive oxygen electrodes because the solubility of oxygen is very low (8.8 mg O2 L−1 at 20 °C, 1 atm). The indication of microbial metabolic inhibition is categorized into two types: mediator methods and bioluminescence methods. The former methods are the Micredox™ and CellSense™ methods, which require sensitivity to toxicity and are inferior to the bioluminescent methods.112,113 The latter bioluminometric method is the Microtox™, which is known to be common and practical,114 although prokaryotes are used.
In 2007, Nakamura et al. also studied the microbial toxicity biosensing methods.115–117 First, we applied an RCI-Sc biosensing method93 for the toxicity detection of heavy metal ions (Cu2+, Mn2+, Zn2+, Cr3+, and Fe3+) as substances of water pollution.115 For an accurate assay, the repeatability should be improved. In this study, a highly repeatable colorimetric toxicity assay method was proposed, which can be easily improved to a disposable toxicity assay kit or a portable toxicity assay system, using a thermo-stable three-consecutive-stir unit and baker's yeast. S. cerevisiae is eukaryote as well as mammals; therefore the toxicity to S. cerevisiae can be estimated not only for environmental safety but also for human safety. With regard to the other advantages, safety, availability, easy handling, and stability of the microbe, they are present in the use of S. cerevisiae.
Recently, Nakamura and Suzuki studied a novel toxicity biosensing method based on an entirely new concept.116–117 For the ideal detection of toxicity in the environment, biosensing methods should be able to measure simultaneously both specific and non-specific responses from living organisms. In addition, it is also desirable that the scale of the enhancement by coexisting substances in a real sample (synergism) and the chemical or physical conditions of the real sample can be measured simultaneously. However, traditional methods utilizing non-specific biosensing by natural microbes (e.g., respiration activity) or specific biosensing by genetically engineered microbes (GEMs; e.g., bioluminescent intensities) were limited to only one indication and unable to realize the ideal detection of toxicity in the environment.
To satisfy these requirements, the specific, non-specific, synergic, chemical, and physical responses should be simultaneously obtained from living organisms used as the most reasonable biological recognition element. Therefore, we tried to apply non-linear phenomena in physiological dynamics. A metabolic oscillation phenomenon has been observed in S. cerevisiae.118 The glycolytic oscillation phenomena in yeast cells were induced by the function of two allosteric enzymes, i.e., 6-phosphofructo-1-kinase (PFK) and pyruvate kinase (PK), in glycolysis. We employed multiple indexes obtained from the wave shape of the damped glycolytic oscillation induced in S. cerevisiae-starved living cells. Transient changes based on metabolic activities in the cells were observed as a time course in the fluorescent intensity of the reduced nicotinamide adenine dinucleotide (phosphate); NAD(P)H level.
In the first step, we optimized reproducible inducing conditions of the damped glycolytic oscillation phenomenon in S. cerevisiae cells and characterized them for simultaneous qualitative and quantitative measurements of toxicity.116 Under optimum inducing conditions, the wave shapes of the damped glycolytic oscillations were changed by the instantaneous addition of both glucose and chemicals and the change in the chemical concentration. The changes in the oscillation wave shapes as six indexes, i.e., the number of wave cycles, maximum amplitude, oscillation frequency, attenuation coefficient, initial peak height, and non-steady-state time, were estimated for toxicity biosensing.
In the second step, we applied the damped glycolytic oscillation phenomenon to the simultaneous qualitative and quantitative measurements of toxicity in a sample.117 As a result, 0.01–100 mmol L−1 of HCl as an inorganic acid, 0.01–50 mmol L−1 of citric acid as an organic acid, 0.001–50 mmol L−1 of KOH as a base, 1–1000 mg L−1 of Cu2+, Pb2+, Cd2+, and Hg2+ as heavy metal ions, 3–500 mg L−1 of NaN3 as respiratory inhibitors, 10–300 mg L−1 of NaSO3 as a DO remover, 10–200 mg L−1 of benzalkonium chloride as surfactants, and 10–1000 mg L−1 of acetaldehyde showed characteristic patterns depending on each chemical and its concentration. These significant results demonstrated the potential of new methods for simultaneous toxicity qualification and quantification.
4.3. Recent biosensing methods for other types of water pollution
Biosensing methods for the estimation of water pollution can be divided into two types, e.g., toxicity measurement and toxic substance measurement. In the former, a biological defense mechanism by entire organisms, such as microbial cells (described above), or inhibition of an enzymatic reaction, has been employed. As an enzyme inhibition, acetylcholinesterase (AChE) has been widely employed. The studies have been most actively performed by Marty et al. and have been reviewed in many articles relevant to the field of the enzyme inhibition method.119–121In the latter cases, toxic substances are specifically measured by a molecular recognition element (MRE) having specific space structure and binding portion(s) to the toxic substance. As an example, the antibody,122,123 a porphin derivative,124 a nucleic-acid aptamer (DNA or RNA),125,126 and a molecularly imprinted polymer (MIP)127–130 have been employed for this purpose.
On the other hand, agrochemicals (pesticides), including herbicides,131 respiratory inhibitors,108 cyanotoxins,132,133 allelochemicals,134 odor substances,135 and endocrine disrupters,136 are known as other water-polluting substances with the exception of heavy metal ions, and these substances have been estimated by toxicity measurement or toxic substance measurement.
For on-site monitoring, miniaturization of the measurement equipment will be required. To realize the purpose, further studies on a disposable biosensing chip will be needed; e.g., screen-printing techniques will become a key technique for disposable electrochemical sensor chip developments.137,138 Then, our package-free chip techniques will be required for the realization of cost-effective measurement.84,87,88,139 In addition, the practical use of micro-fluidic devices, such as a micro-total analysis system (μ-TAS), will be required for on-site monitoring;44–46,140–142 in addition, application techniques for a real sample to the biosensing devices will be important subjects.143 Furthermore, application studies of nano-materials, such as carbon nanotubes (CNTs), to water pollution biosensing methods are also important as future possibilities.144–146
5. Conclusions
In the present review article, recent organic pollution and heavy metal pollution following eutrophication were explained in the Introduction, and subsequently, the biosensing methods for three types of water pollution were described.For the estimation of eutrophication, enzyme-specific Pi biosensing methods will be significant techniques due to their measurability of orthophosphate ion as a bioavailable, naturally dissolved, and assimilable substance, which might be different from the conventional spectrophotometric molybdenum blue method due to the complex forming reaction of the molybdenum-phosphate under acidic conditions.
For the estimation of organic pollution, several BOD biosensing methods, including the method for seawater, were introduced in this article. In the future, BOD biosensing methods might be expanded for the estimation of the degree of organic pollution occurring in closed water as a new assimilable-organics estimation method.
For the estimation of heavy metal pollution, as one possibility, the biosensing method based on damped glycolytic oscillation was introduced. Such multiple-index biosensing methods enabling simultaneous heavy metal toxicity qualification and quantification will be a key technique for heavy metal toxicity biosensing in the field of analytical toxicology.
In conclusion, multidirectional estimation methods employing multiple biosensing techniques will be required to assess the recent organic pollution in both closed and flowing water.
Acknowledgements
This article is dedicated to Professor Dr Isao Karube in recognition of his accession to the presidency of Tokyo University of Technology on June 1st, 2008. His mentoring in the field of biosensors is greatly acknowledged.References
- White Paper on Environment; Shiga Prefecture: Japan, 2009 Search PubMed.
- White Paper on Environment; The Ministry of the Environment: Japan, 2002 Search PubMed.
- S. Ojima, K. Sudo, T. Sakurai and T. Matsumoto, In Action for Eutrophication and Technologies for Denitrification and Dephosphorization (in Japanese); Sadao Ojima, Ed.; IPC Co.: Tokyo, 1977 Search PubMed.
- A. C. Redfield, Am. Sci., 1958, 46, 205–221 CAS.
- L. E. Keup, Phosphorus in flowing waters, Water Res., 1968, 2, 373–386 CrossRef.
- D. W. Schindler, Eutrophication and Recovery in Experimental Lakes: Implications for Lake Management, Science, 1974, 184, 897–899 CrossRef CAS.
- D. W. Schindler, R. E. Hecky, D. L. Findlay, M. P. Stainton, M. J. Parker, B. R. Paterson, K. G. Beaty, M. Lyng and S. E. M. Kasian, Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11254–11258 CrossRef CAS.
- J. Karlsson, P. Byström, J. Ask, L. Persson and M. Jansson, Light limitation of nutrient-poor lake ecosystems, Nature, 2009, 460, 506–509 CrossRef CAS.
- V. Smil, Annu. Rev. Energy Environ., 2000, 25, 53–88 CrossRef.
- B. Hawkins, S. Bosch, M. Odin and D. Wohlers, Toxicological reviews of cyanobacterial toxins: anatoxin-a. U.S.Environmental Protection Agency (USEPA), Washington, DC, EPA/600/R-06/137, NCEA-C-1743, 2006 Search PubMed.
- B. Hawkins, S. Bosch, M. Odin and D. Wohlers, Toxicological reviews of cyanobacterial toxins: cylindrospermopsin. USEPA, Washington, DC, EPA/600/R-06/138, NCEA-C-1763, 2006 Search PubMed.
- B. Hawkins, S. Bosch, M. Odin and D. Wohlers, Toxicological reviews of cyanobacterial toxins: microcystins LR, RR, YR and LA. USEPA, Washington, DC, EPA/600/R-06/139, NCEA-C-1765, 2006 Search PubMed.
- Testing Methods for Drinking Waters (tutorial edition). Japan Water Works Association (JWWA): Tokyo, 1993 Search PubMed.
- H. Nakamura and I. Karube, Review: Current research activities in biosensors, Anal. Bioanal. Chem., 2003, 377, 446–468 CrossRef CAS.
- D. Lelie, P. Corbisiera, W. Baeyensa, S. Wuertza, L. Dielsa and M. Mergeaya, Review: The use of biosensors for environmental monitoring, Res. Microbiol., 1994, 145, 67–74 CrossRef.
- M. Farré, R. Brix and D. Barceló, Review: Screening water for pollutants using biological techniques under European Union funding during the last 10 years, TrAC, Trends Anal. Chem., 2005, 24, 532–545 CrossRef CAS.
- K. R. Rogers, Review: Recent advances in biosensor techniques for environmental monitoring, Anal. Chim. Acta, 2006, 568, 222–231 CrossRef CAS.
- Algal growth potential (AGP) test. US Environmental Protection Agency (USEPA), USA, 1971 Search PubMed.
- AGP testing method in Testing Methods for Sewage Waters(Vol. 1). Japan Sewage Works Association (JSWA): Tokyo, 1997 Search PubMed.
- Eutrophication of Waters. Monitoring, Assessment, and Control. OECD: Paris, 1982, 154 pp Search PubMed.
- Testing Methods for Industrial Wastewater (JIS K 0102); edited by JIS Committee: Tokyo, 1964 (revised in 1993, 2008) Search PubMed.
- S. O. Engblom, Review: The phosphate sensor, Biosens. Bioelectron., 1998, 13, 981–994 CrossRef CAS.
- H. Nakamura, Phosphate ion determination in water for drinking using biosensors, Bunseki Kagaku, 2001, 50, 581–582 CrossRef CAS.
- J. M. Estela and V. Cerdà, Review: Flow analysis techniques for phosphorus: an overview, Talanta, 2006, 66, 307–331.
- M. Miró, J. M. Estela and V. Cerdà, Review: Application of flowing stream techniques to water analysis. Part I. Ionic species: dissolved inorganic carbon, nutrients and related compounds, Talanta, 2003, 60, 867–886 CrossRef CAS.
- M. M. Villalba, K. J. McKeegan, D. H. Vaughan, M. F. Cardosi and J. Davisa, Bioelectroanalytical determination of phosphate: A review, J. Mol. Catal. B: Enzym., 2009, 59, 1–8 CrossRef CAS.
- G. G. Guilbalt and M. Nanjo, A phosphate-selective electrode based on immobilized alkaline phosphatase and glucose oxidase, Anal. Chim. Acta, 1975, 78, 69–80 CrossRef.
- E. M. D'Urso and P. R. Coulet, Phosphate-sensitive enzyme electrode: a potential sensor for environment control, Anal. Chim. Acta, 1990, 239, 1–5 CrossRef CAS.
- U. Wollenberger, F. Schubert and W. F. Scheller, Biosensor for sensitive phosphate detection, Sens. Actuators, B, 1992, 7, 412–415 CrossRef.
- I. Kubo, I. Inagawa, T. Sugawara, Y. Arikawa and I. Karube, Phosphate Sensor Composed from Immobilized Pyruvate Oxidase and an Oxygen Electrode, Anal. Lett., 1992, 24, 1711–1717.
- K. Ikebukuro, I. Kubo, M. Inagawa, T. Sugawara, H. Nakamura, Y. Arikawa, M. Suzuki, T. Takeuchi and I. Karube, Phosphate sensing system using pyruvate oxidase and chemiluminescence detection, Biosens. Bioelectron., 1996, 11, 959–965 CrossRef CAS.
- K. Ikebukuro, R. Nishida, H. Yamamoto, Y. Arikawa, H. Nakamura, M. Suzuki, I. Kubo, T. Takeuchi and I. Karube, A novel biosensory system for the determination of phosphate, J. Biotechnol., 1996, 48, 67–72 CrossRef CAS.
- H. Nakamura, H. Yamamoto, I. Kubo, M. Suzuki, K. Hayashi, S. J. McNiven, K. Ikebukuro and I. Karube, A chemiluminescent FIA biosensor for phosphate monitoring using pyruvate oxidase, Biosens. Bioelectron., 1997, 12, 959–966 CrossRef CAS.
- M. Suzuki, H. Kurata, Y. Inoue, H. Shin, I. Kubo, H. Nakamura, K. Ikebukuro and I. Karube, Reagentless phosphate ion sensor system for environmental monitoring, Denki Kagaku, 1998, 66, 579–583 Search PubMed.
- H. Nakamura, H. Tanaka, M. Hasegawa, Y. Masuda, Y. Arikawa, K. Ikebukuro and I. Karube, An automatic flow-injection analysis system for determining phosphate ion in river water using pyruvate oxidase G (from Aerococcus viridans), Talanta, 1999, 50, 799–807 CrossRef CAS.
- H. Nakamura, M. Hasegawa, Y. Nomura, Y. Arikawa, R. Matsukawa, K. Ikebukuro and I. Karube, Development of a highly sensitive CL-FIA sensor for phosphate ion detection using maltose phosphorylase, J. Biotechnol., 1999, 75, 127–133 CrossRef CAS.
- H. Nakamura, M. Hasegawa, Y. Arikawa, Y. Nomura, K. Ikebukuro and I. Karube, Improvement of a CL-FIA system using maltose phospharylase for the determination of phosphate-ion in freshwater, Anal. Lett., 2003, 36, 1805–1817 CrossRef CAS.
- K. Nakashima, Review: Analytical methods utilizing a chemiluminescence measurement, Bunseki Kagaku, 2000, 49, 135–159 CrossRef CAS (in Japanese).
- S. Kulmala and J. Suomi, Review: Current status of modern analytical luminescence methods, Anal. Chim. Acta, 2003, 500, 21–69 CrossRef CAS.
- A. Roda, P. Pasini, M. Mirasoli, E. Michelini and M. Guardigli, Review: Biotechnological applications of bioluminescence and chemiluminescence, Trends Biotechnol., 2004, 22, 295–303 CrossRef CAS.
- Z. Zhang, S. Zhang and X. Zhang, Review: Recent developments and applications of chemiluminescence sensors, Anal. Chim. Acta, 2005, 541, 37–46 CrossRef CAS.
- N. P. Evmiridis, A. G. Vlessidis and N. C. Thanasoulias, Review: Chemical analysis through CL-detection assisted by periodate oxidation, Bioinorg. Chem. Appl., 2007, 1–10 Search PubMed.
- J. Ružička and E. H. Hansen, Review: Flow injection analyses: Part I. A new concept of fast continuous flow analysis, Anal. Chim. Acta, 1975, 78, 145–157 CrossRef CAS.
- O. Chailapakul, P. Ngamukot, A. Yoosamran, W. Siangproh and N. Wangfuengkanagul, Review: Recent electrochemical and optical sensors in flow-based analysis, Sensors, 2006, 6, 1383–1410 CrossRef CAS.
- M. A. Feres, P. R. Fortes, E. A. G. Zagatto, J. M. L. Santos and J. L. F. C. Lima, Review: Multi-commutation in flow analysis: Recent developments and applications, Anal. Chim. Acta, 2008, 618, 1–17 CrossRef CAS.
- M. Miŕo, J. M. Estela and V. Cerd', Review: Potentials of multisyringe flow injection analysis for chemiluminescence detection, Anal. Chim. Acta, 2005, 541, 57–68 CAS.
- N. Conrath, B. Grundig, K. Huwel and K. Cammann, A novel enzyme sensor for the determination of inorganic phosphate, Anal. Chim. Acta, 1995, 309, 47–52 CrossRef CAS.
- H. Nakamura, R. Yamazaki, T. Shirai, H. Sano, Y. Nakami, K. Ikebukuro, K. Yano, Y. Nomura, Y. Arikawa, Y. Hasebe, Y. Masuda and I. Karube, Development of an enzymatic flow-injection chemiluminescence system for determining inorganic pyrophosphate ion, Anal. Chim. Acta, 2004, 518, 45–49 CrossRef CAS.
- H. Nakamura, M. Gotoh and I. Karube, Japanese Patent Application Raid-Open Disclosure P2005/ 073399A. Japan Patent Office (JPO): Tokyo. 2005.
- R. C. H. Kwan, H. F. Leung, P. Y. H Hon, J. P. Barford and R. Renneberg, A screen-printed biosensor using pyruvate oxidase for rapid determination of phosphate in synthetic wastewater, Appl. Microbiol. Biotechnol., 2005, 66, 377–383 CrossRef CAS.
- C. Mousty, S. Cosnier, D. Shan and S. Mu, Trienzymatic biosensor for the determination of inorganic phosphate, Anal. Chim. Acta, 2001, 443, 1–8 CrossRef CAS.
- J. J. Fernández, J. R. Lopez, X. Correig and I. Katakis, Reagentless carbon paste phosphate biosensors: preliminary studies, Sens. Actuators, B, 1998, 47, 13–20 CrossRef.
- I. Kubo, Potentiometric phosphate-sensing system utilizing phosphate-binding protein, Anal. Bioanal. Chem., 2002, 372, 273–275 CrossRef CAS.
- L. L. E. Salins, S. K. Deo and S. Daunert, Phosphate binding protein as the biorecognition element in a biosensor for phosphate, Sens. Actuators, B, 2004, 97, 81–89 CrossRef.
- K. Wygladacz, Y. Qin, W. Wroblewski and E. Bakker, Phosphate-selective fluorescent sensing microspheres based on uranyl salophene ionophores, Anal. Chim. Acta, 2008, 614, 77–84 CrossRef CAS.
- Y. Umezawa, Review: Detection of phosphate ion and protein phosphorylation - crystal surfaces, ionophore monolayers, and protein interactions, J. Supramol. Chem., 2002, 2, 233–245 CrossRef CAS.
- P. P.-Y. Schreiter, O. Gillor, A. Post, S. Belkin, R. D. Schmid and T. T. Bachmann, Monitoring of phosphorus bioavailability in water by an immobilized luminescent cyanobacterial reporter strain, Biosens. Bioelectron., 2001, 16, 811–818 CrossRef CAS.
- M.-A. Dollard and P. Billard, Whole-cell bacterial sensors for the monitoring of phosphate bioavailability, J. Microbiol. Methods, 2003, 55, 221–229 CrossRef CAS.
- H. Nakamura, K. Taguchi, Y. Ito, N. Suka, J. Kanno, Y. Kita, K. Sudo, K. Hirabayashi, N. Yasue, T. Sakamaki, M. Taguchi and M. Ito, Investigations of eco-balance and water quality in a holding pond — organic pollution caused by eutrophication and its effects on the water quality —. Bulletin of Tokyo University of Technology, in press Search PubMed.
- Standard Methods for the Examination of Waters and Wastewater, American Public Health Association, Washington, DC, 16th Ed, 1986, pp. 525–531 Search PubMed.
- I. Karube, T. Matsunaga, S. Mitsuda and S. Suzuki, Microbial electrode BOD sensors, Biotechnol. Bioeng., 1977, 19, 1535–1547 CrossRef CAS.
- M. Hikuma, H. Suzuki, T. Yasuda, I. Karube and S. Suzuki, Amperometric estimation of BOD by using living immobilized yeasts, Eur. J. Appl. Microbiol. Biotechnol., 1979, 8, 289–297 CrossRef CAS.
- H. Nakamura and I. Karube, In Encyclopedia of Sensors(Vol. 6); Craig A. Grimes, Elizabeth C. Dickey, Michael V. Pishko, ed.; American Scientific Publishers: California, 2005, pp. 87–126 Search PubMed.
- H. Nakamura, M. Shimomura-Shimizu and I. Karube, Review: Development of microbial sensors and their application. In Biosensing for the 21st Century(Vol. 109); Reinhard Renneberg, Fred Lisdat, ed.; Springer: Berlin/Heidelberg, 2008, pp. 351–394 Search PubMed.
- Apparatus for the Estimation of Biochemical Oxygen Consumption (BODs) with Microbial Sensor (JIS K 3602); JIS Committee: Tokyo, 1990 Search PubMed.
- L. Bousse, Review: Whole cell biosensors, Sens. Actuators, B, 1996, 34, 270–275 CrossRef.
- S. F. D'Souza, Review: Microbial biosensors, Biosens. Bioelectron., 2001, 16, 337–353 CrossRef CAS.
- K. H. R. Baronian, Review: The use of yeast and moulds as sensing elements in biosensors, Biosens. Bioelectron., 2004, 19, 953–962 CrossRef CAS.
- Y. Lei, W. Chen and A. Mulchandani, Review: Microbial biosensors, Anal. Chim. Acta, 2006, 568, 200–210 CrossRef CAS.
- V. Lazarova and J. Manem, Review: Biofilm characterization and activity analysis in water and wastewater treatment, Water Res., 1995, 29, 2227–2245 CrossRef CAS.
- S. Belkin, Review: Whole-cell sensing systems of environmental pollutants, Curr. Opin. Microbiol., 2003, 6, 206–212 CrossRef CAS.
- J. Liu and B. Mattiasson, Review: Microbial BOD sensors for wastewater analysis, Water Res., 2002, 36, 3786–3802 CrossRef CAS.
- W. Bourgeois, J. E. Burgess and R. M. Stuetz, On-line monitoring of wastewater quality: A review, J. Chem. Technol. Biotechnol., 2001, 76, 337–348 CrossRef CAS.
- N. Pasco, K. H. R. Baronian, C. Jeffries and J. Hay, Biochemical mediator demand - a novel rapid alternative for measuring biochemical oxygen demand, Appl. Microbiol. Biotechnol., 2000, 53, 613–618 CrossRef CAS.
- N. Yoshida, K. Yano, T. Morita, S. J. McNiven, H. Nakamura and I. Karube, A mediator-type biosensor as a new approach to biochemical oxygen demand estimation, Analyst, 2000, 125, 2280–2284 RSC.
- N. Yoshida, J. Hoashi, T. Morita, S. J. McNiven, H. Nakamura and I. Karube, Improvement of a mediator-type biochemical oxygen demand sensor for on-site measurement, J. Biotechnol., 2001, 88, 269–275 CrossRef CAS.
- N. Yoshida, J. Hoashi, T. Morita, S. J. McNiven, K. Yano, A. Yoshida, H. Nakamura and I. Karube, Monitoring of composting process using a mediator-type biochemical oxygen demand sensor, Analyst, 2001, 126, 1751–1755 RSC.
- H. Nakamura, M. Gotoh and I. Karube, Japanese Patent Application Raid-Open Disclosure P2008/96415A. Japan, JPO: Tokyo. 2008.
- H. Nakamura, K. Suzuki, H. Ishikuro, S. Kinoshita, R. Koizumi, S. Okuma, M. Gotoh and I. Karube, A new BOD estimation method employing a double mediator system by ferricyanide and menadione using the eukaryote Saccharomyces cerevisiae, Talanta, 2007, 72, 210–216 CrossRef CAS.
- H. Nakamura, K. Suzuki, S. Okuma, M. Yataka, Y. Mogi and I. Karube, Improvement of double mediator system for BOD determination, Res. Rev. ElectroChem., 2008, 1, 21–25 Search PubMed.
- J. D. Rabinowitz, J. F. Vacchino, C. Beeson and H. M. McConnell, Potentiometric Measurement of Intracellular Redox Activity, J. Am. Chem. Soc., 1998, 120, 2464–2473 CrossRef CAS.
- K. H. R. Baronian, A. J. Downard, R. K. Lowen and N. Pasco, Detection of two distinct substrate-dependent catabolic responses in yeast cells using a mediated electrochemical method, Appl. Microbiol. Biotechnol., 2002, 60, 108–113 CrossRef CAS.
- A. Heiskanen, J. Yakovleva, C. Spégel, R. Taboryski, M. Koudelka-Hep, J. Emnéus and T. Ruzgas, Amperometric monitoring of redox activity in living yeast cells: comparison of menadione and menadione sodium bisulfite as electron transfer mediators, Electrochem. Commun., 2004, 6, 219–224 CrossRef CAS.
- H. Nakamura, M. Gotoh and I. Karube, Japanese Patent Application Raid-Open Disclosure P2006/242933A. Japan, JPO: Tokyo. 2006.
- S. Kaimori, T. Kitamura, M. Ichino, T. Hosoya, F. Kurusu, T. Ishikawa, H. Nakamura, M. Gotoh and I. Karube, Structural development of a minimally invasive sensor chip for blood glucose monitoring, Anal. Chim. Acta, 2006, 573–574, 104–109 CrossRef.
- T. Kitamura, S. Kaimori, A. Harada, T. Ishikawa, T. Fujimura, H. Nakamura, M. Gotoh and I. Karube, Development of blood glucose monitoring sensor strip that uses small blood sample, SEI Tech. Rev., 2006, No. 63, 19–21 Search PubMed.
- H. Nakamura, S. Shinohara, M. Gotoh and I. Karube, United Stets Patent Application, US/20050247573. United Stets Patent and Trademark Office (USPTO): USA. 2005 Search PubMed.
- H. Nakamura, K. Tohyama, M. Tanaka, S. Shinohara, Y. Tokunaga, F. Kurusu, S. Koide, M. Gotoh and I. Karube, Development of a package-free transparent disposable biosensor chip for simultaneous measurements of blood constituents and investigation of its storage stability, Biosens. Bioelectron., 2007, 23, 621–626 CrossRef CAS.
- H. Nakamura, R. Tanaka, K. Suzuki, M. Yataka and Y. Mogi, A direct determination method for ethanol concentrations in alcoholic beverages employing a eukaryote double-mediator system, Food Chem., 2009, 117, 509–513 CrossRef CAS.
- Y. Hashimoto, H. Nakamura, K. Asaga and I. Karube, A new diagnosis method for soil-borne disease by a microbial biosensor, Microbes Environ., 2008, 23, 35–39 Search PubMed.
- N. Yoshida, S. J. McNiven, T. Morita, H. Nakamura and I. Karube, A simple, multiple simultaneous spectrophotometric method for biochemical oxygen demand determination using DCIP as the electron acceptor, Anal. Lett., 2002, 35, 1541–1549 CrossRef CAS.
- N. Yoshida, S. J. McNiven, A. Yoshida, T. Morita, H. Nakamura and I. Karube, A compact optical system for multi-determination of Biochemical Oxygen Demand using disposable strips, Field Anal. Chem. Technol., 2001, 5, 222–227 CrossRef CAS.
- H. Nakamura, S. Kobayashi, Y. Hirata, K. Suzuki, Y. Mogi and I. Karube, A spectrophotometric biochemical oxygen demand determination method using DCIP as the redox color indicator and eukaryote Saccharomyces cerevisiae, Anal. Biochem., 2007, 369, 168–174 CrossRef CAS.
- H. Nakamura, Y. Mogi, H. Hattori, Y. Kita, D. Hattori, A. Yoshimura and I. Karube, Absorption-based highly sensitive and reproducible biochemical oxygen demand measurement method for seawater using salt-tolerant yeast Saccharomyces cerevisiae ARIF KD-003, Anal. Chim. Acta, 2008, 620, 127–133 CrossRef CAS.
- C. Chan, M. Lehmann, K. Tag, M. Lung, G. Kunze, K. Riedel, B. Gruendig and R. Renneberg, Measurement of biodegradable substances using the salt-tolerant yeast Arxula adeninivorans for a microbial sensor immobilized with poly(carbamoyl) sulfonate (PCS) part I: construction and characterization of the microbial sensor, Biosens. Bioelectron., 1999, 14, 131–138 CrossRef CAS.
- M. Lehmann, C. Chan, A. Lo, M. Lung, K. Tag, G. Kunze, K. Riedel, B. Gruendig and R. Renneberg, Measurement of biodegradable substances using the salt-tolerant yeast Arxula adeninivorans for a microbial sensor immobilized with poly(carbamoyl)sulfonate (PCS): Part II: application of the novel biosensor to real samples from coastal and island regions, Biosens. Bioelectron., 1999, 14, 295–302 CrossRef CAS.
- K. Tag, M. Lehmann, C. Chan, R. Renneberg, K. Riedel and G. Kunze, Measurement of biodegradable substances with a mycelia-sensor based on the salt tolerant yeast Arxula adeninivorans LS3, Sens. Actuators, B, 2000, 67, 142–148 CrossRef.
- L. Lin, L. L. Xiao, S. Huang, L. Zhao, J. S. Cui, X. H. Wang and X. Chen, Novel BOD optical fiber biosensor based on co-immobilized microorganisms in ormosils matrix, Biosens. Bioelectron., 2006, 21, 1703–1709 CrossRef CAS.
- Y. Jiang, L. L. Xiao, L. Zhao, X. Chen, X. Wang and K. Y. Wong, Optical biosensor for the determination of BOD in seawater, Talanta, 2006, 70, 97–103 CrossRef CAS.
- L. Xin, X. Wang, G. Guo, X. Wang and X. Chen, An optical biosensing film for biochemical oxygen demand determination in seawater with an automatic flow sampling system, Meas. Sci. Technol., 2007, 18, 2878–2884 CrossRef CAS.
- G.-J. Chee, Y. Nomura, K. Ikebukuro and I. Karube, Optical fiber biosensor for the determination of low biochemical oxygen demand, Biosens. Bioelectron., 2000, 15, 371–376 CrossRef CAS.
- H.-L. Pang, N.-Y. Kwok, P.-H. Chan, C.-H. Yeung, W. Lo and K.-Y. Wong, High-throughput determination of biochemical oxygen demand (BOD) by a microplate-based biosensor, Environ. Sci. Technol., 2007, 41, 4038–4044 CrossRef CAS.
- C. K. Hyun, N. Inoue, E. Tamiya, T. Takeuchi and I. Karube, A novel BOD sensor based on bacterial luminescence, Biotechnol. Bioeng., 1993, 41, 1107–1111 CrossRef CAS.
- H. Nakamura, Y. Abe, R. Koizumi, K. Suzuki, Y. Mogi, T. Hirayama and I. Karube, A chemiluminescence biochemical oxygen demand measuring method, Anal. Chim. Acta, 2007, 602, 94–100 CrossRef CAS.
- Water quality - algal growth inhibition (AGI) test. ISO 8692, ISO, 2004 (revised version of 1993).
- I. E. Tothill and A. P. F. Turner, Developments in bioassay methods for toxicity testing in water treatment, TrAC, Trends Anal. Chem., 1996, 15, 178–188 CrossRef CAS.
- C. Blaise, Microbiotesting: An expanding field in aquatic toxicology, Ecotoxicol. Environ. Saf., 1998, 40, 115–119 CrossRef CAS.
- K. Ikebukuro, H. Nakamura and I. Karube, In Nollet L. M. L. (Ed.), Handbook of Water Analysis (Chap. 19)., Marcel Dekker, Inc.: New York, 2000, pp. 367–385 Search PubMed.
- P. Van Beelen, A review on the application of microbial toxicity tests for deriving sediment quality guidelines, Chemosphere, 2003, 53, 795–808 CrossRef CAS.
- M. Farré, E. Martínez and D. Barceló, Review: Validation of interlaboratory studies on toxicity in water samples, TrAC, Trends Anal. Chem., 2007, 26, 283–292 CrossRef CAS.
- Z. Kong, P. A. Vanrolleghem and W. Verstraete, An activated sludge-based biosensor for rapid IC50 estimation and on-line toxicity monitoring, Biosens. Bioelectron., 1993, 8, 49–58 CrossRef CAS.
- A. Tizzard, J. Webber, R. Gooneratne, R. John, J. Hay and N. Pasco, MICREDOX: application for rapid biotoxicity assessment, Anal. Chim. Acta, 2004, 522, 197–205 CrossRef CAS.
- A. Bentley, A. Atkinson, J. Jezek and D. M. Rawson, Review: Whole cell biosensors - electrochemical and optical approaches to ecotoxicity testing, Toxicol. in Vitro, 2001, 15, 469–475 CrossRef CAS.
- A. A. Bulich, In Aquatic Toxicology; Markings, L. L.; Kimerle, R. A. ed.; American Society for Testing and Materials: USA, 1979, pp. 98–106 Search PubMed.
- H. Nakamura, Y. Hirata, Y. Mogi, S. Kobayashi, K. Suzuki, T. Hirayama and I. Karube, A simple and highly repeatable colorimetric toxicity assay method using DCIP as the redox color indicator and whole eukaryote cells, Anal. Bioanal. Chem., 2007, 389, 835–840 CrossRef CAS.
- H. Nakamura and M. Suzuki, New concept for a toxicity assay based on multiple indexes from the wave shape of damped metabolic oscillation induced in living yeast cells - Part I: Characterization of the phenomenon -, Anal. Bioanal. Chem., 2007, 389, 1225–1232 CrossRef CAS.
- H. Nakamura and M. Suzuki, New concept for a toxicity assay based on multiple indexes from the wave shape of damped metabolic oscillation induced in living yeast cells - Part II: Application to analytical toxicology -, Anal. Bioanal. Chem., 2007, 389, 1233–1241 CrossRef CAS.
- L. N. M. Duysens and J. Amesz, Fluorescence spectrophotometry of reduced phosphopyridine nucleotide in intact cells in the near-ultraviolet and visible region, Biochim. Biophys. Acta, 1957, 24, 19–26 CAS.
- M. Campàs, B. Prieto-Simón and J. L. Marty, A review of the use of genetically engineered enzymes in electrochemical biosensors, Semin. Cell Dev. Biol., 2009, 20, 3–9 CrossRef CAS.
- B. Prieto-Simón, M. Campàs, S. Andreescu and J. L. Marty, Review: Trends in flow-based biosensing systems for pesticide assessment, Sensors, 2006, 6, 1161–1186 CrossRef.
- S. Andreescua and J. M. Marty, Review: Twenty years research in cholinesterase biosensors: From basic research to practical applications, Biomol. Eng., 2006, 23, 1–15 CrossRef CAS.
- C. P. Chan, Y. Cheung and R. Renneberg, Review: New trends in immunoassays, Adv. Biochem. Engin. Biotech., 2008, 109, 123–154 Search PubMed.
- B. Byrne, E. Stack, N. Gilmartin and R. O'Kennedy, Review: Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins, Sensors, 2009, 9, 4407–4445 CrossRef CAS.
- S. Hu, H. Mao and Y. Wang, Optical sensing of heavy metal ions in anionic micellar solution using a Pd porphyrin phosphorescent probe, J. Photochem. Photobiol., A, 2009, 204, 1–6 CrossRef CAS.
- S. Tombelli, M. Minunni and M. Mascini, Review: Analytical applications of aptamers, Biosens. Bioelectron., 2005, 20, 2424–2434 CrossRef CAS.
- I. Palchetti and M. Mascini, Review: Nucleic acid biosensors for environmental pollution monitoring, Analyst, 2008, 133, 846–854 RSC.
- K. Mosbach and K. Haupt, Review: Some new developments and challenges in non-covalent molecular imprinting technology, J. Mol. Recognit., 1998, 11, 62–68 CrossRef CAS.
- K. Haupt and K. Mosbach, Review: Molecularly imprinted polymers and their use in biomimetic sensors, Chem. Rev., 2000, 100, 2495–2504 CrossRef CAS.
- K. Mosbach, Review: Toward the next generation of molecular imprinting with emphasis on the formation, by direct molding, of compounds with biological activity (biomimetics), Anal. Chim. Acta, 2001, 435, 3–8 CrossRef CAS.
- C. A. Alexander, H. S. Andersson, L. I. Andersson, R. J. Ansell, N. Kirsch, I. A. Nicholls, J. O'Mahony and M. J. Whitcombe, Review: Molecular imprinting science and technology: a survey of the literature for the years up to and including 2003, J. Mol. Recognit., 2006, 19, 106–180 CrossRef.
- B. Prieto-Simón, M. Campàs, S. Andreescu and J. L. Marty, Review: Trends in flow-based biosensing systems for pesticide assessment, Sensors, 2006, 6, 1161–1186 CrossRef.
- M. Campàs, D. Szydlowska and J. L. Marty, Procedure 21. Protein phosphatase inhibition-based biosensor for amperometric microcystin detection in cyanobacterial cells, Compr. Anal. Chem., 2007, 49, e151–e156 Search PubMed.
- M. Campàs and J. L. Marty, Chapter 16. Amperometric enzyme sensors for the detection of cyanobacterial toxins in environmental samples, Compr. Anal. Chem., 2007, 49, 331–355 Search PubMed.
- C. D. Broeckling and J. M. Vivanco, A selective, sensitive, and rapid in-field assay for soil catechin, an allelochemical of Centaurea maculosa, Soil Biol. Biochem., 2008, 40, 1189–1196 CrossRef CAS.
- H. S. Ji, S. J. McNiven, K. Yano, K. Ikebukuro, U. T. Bornscheuer, R. D. Schmid and I. Karube, Highly sensitive trilayer piezoelectric odor sensor, Anal. Chim. Acta, 1999, 387, 39–45 CrossRef CAS.
- M. Farre, L. Kantiani and D. Barcelo, Review: Advances in immunochemical technologies for analysis of organic pollutants in the environment, TrAC, Trends Anal. Chem., 2007, 26, 1100–1112 CrossRef CAS.
- J. P. Hart, A. Crew, E. Crouch, K. C. Honeychurch and R. M. Pemberton, Review: Some recent designs and developments of screen-printed carbon electrochemical sensors/biosensors for biomedical, environmental, and industrial analyses, Anal. Lett., 2005, 37, 789–830 CrossRef.
- O. Domínguez Renedo, M. A. Alonso-Lomillo and M. J. Arcos Martínez, Review: Recent developments in the field of screen-printed electrodes and their related applications, Talanta, 2007, 73, 202–219 CrossRef.
- H. Nakamura, M. Gotoh and I. Karube, Japanese Patent 3890417. JPO: Tokyo, 2006.
- H. Nakamura, M. Suda, Y. Murakami, K. Yokoyama, E. Tamiya, S. Uchiyama and I. Karube, A compactly integrated flow cell with a chemiluminescent FIA system for determining lactate concentration in serum, Anal. Chem., 2001, 73, 373–378 CrossRef CAS.
- P. S. Dittrich, K. Tachikawa and A. Manz, Review: Micro total analysis systems. latest advancements and trends, Anal. Chem., 2006, 78, 3887–3907 CrossRef CAS.
- G. A. Urban, Review: Micro- and nanobiosensors - state of the art and trends, Meas. Sci. Technol., 2009, 20, 18 pp.
- A. G. Crevillén, M. Hervás, M. A. López, M. C. González and A. Escarpa, Review: Real sample analysis on microfluidic devices, Talanta, 2007, 74, 342–357 CrossRef.
- M. T. Fernández-Abedul and A. Costa-García, Review: Carbon nanotubes (CNTs)-based electroanalysis, Anal. Bioanal. Chem., 2008, 390, 293–298 CrossRef CAS.
- R. Zenobi, Review: Analytical tools for the nano world, Anal. Bioanal. Chem., 2008, 390, 215–221 CrossRef CAS.
- U. Yogeswaran and S. M. Chen, A review on the electrochemical sensors and biosensors composed of nanowires as sensing material, Sensors, 2008, 8, 290–313 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
