Free fatty acids profile analysis of alcohol extract of Aconitum taipeicum Hand.-Mazz. with gas chromatography-mass spectrometry
Xuan-Feng
Yue
*abc,
Yan-Ni
Zhang
c,
Jin
Zhang
ab and
Zhi-Qi
Zhang
*ab
aCollege of Chemistry and Materials Science, Shaanxi Normal University, Xi'an, 710062, China. E-mail: xfyue@snnu.edu.cn; zqzhang@snnu.edu.cn; Fax: +86-29-85307774; Tel: +86-29-85308442
bKey Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062, China
cKey Laboratory of Ministry of Education for Medicinal Plant Resources and Natural Pharmaceutical Chemistry, Xi'an, 710062, China
First published on 30th April 2010
Abstract
Aconitum taipeicum Hand.-Mazz., widely used in traditional Chinese pharmaceuticals, has a dual effect on human health. Its alcohol extract is clinically controversial partly because its chemical components remain to be elucidated. In this paper, we report on an elaborately developed GC-MS method for the determination of free fatty acids (FFAs) in the alcohol extract of Aconitum taipeicum Hand.-Mazz., based on alcohol extraction and esterification with boron trifluoride-methanol. Six types of long chain FFAs were identified in the profile analysis, including two essential fatty acids to the human body. All the major six FFAs identified in the alcohol extract were quantified with nonadecanoic acid as an internal standard. The results showed that the alcohol extract was abundant in three types of FFAs, with unsaturated FFAs amounting to 67.42% of the total FFA content. Linoleic acid (46.24%, 13.89 ± 0.36 mg g−1) was the predominant fatty acid, followed by palmitic acid and oleic acid. These results indicate that the FFAs in the extract of Aconitum taipeicum Hand.-Mazz. contribute little to its adverse health effect.
1 Introduction
Aconitum taipeicum Hand.-Mazz. is a perennial herb found at an altitude of 2600–3400 meters in the Southern Shaanxi Province and Western Henan Province in China, addition of this plant to pharmaceuticals is a common practice in some areas to prevent heart disease. In addition to its beneficial health effect;1 it also has potential toxicity.2 This dual effect has resulted in a number of efforts to clarify the administration of this plant. However, clarification is not easy partly because detailed information on the chemical components of this plant remain unknown.2,3 As part of that effort, we have been funded by the National Natural Science Foundation of China to carry out a related project with the main aim of exploring the chemical components of Aconitum taipeicum Hand.-Mazz‥ The preliminary study on the volatile components of Aconitum taipeicum Hand.-Mazz. showed the presence of several free fatty acids (FFAs),4 which are seldom found free in plants.Fatty acids have been attracting increased interest in food nutrition evaluation,5–9 diagnosis of certain diseases and in pharmacology10 due to their biological and environmental importance.11,12 Some types of long-chain fatty acids (FAs) have proved beneficial for human health. Unsaturated fatty acids, including monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), have been found beneficial against inflammation, in reducing the risks of heart disease and enhancing the immune system.13 The intake of MUFAs lowers the risk of developing atherosclerosis and cholesterol accumulation in the blood,14,15 and PUFAs can improve the body's immunity16,17 and are beneficial in reducing coronary artery disease.18 However, some other long-chain fatty acids such as trans fatty acids (TFAs) are thought to have an increased risk of cardiovascular disease.19–21 FAs have also been reported to influence human immune function in a dose-dependent manner.13 Therefore, there is a need to evaluate the types of FAs, especially FFAs, as well as their contents in Aconitum taipeicum Hand.-Mazz., which may be helpful in elucidating the heath effects of this plant.
Many approaches have been developed for the determination of FFAs, including spectrophotometry, enzymic methods, high performance liquid chromatography (HPLC)22–24 and gas chromatography (GC).25 GC-MS is now the primary choice for fatty acids profile analysis if available, due to its speed, high resolution and sensitivity.26,27
The alcohol extract of Aconitum taipeicum Hand.-Mazz. has usually been used in Chinese traditional medicine. Described here is a cost-efficient GC-MS method for the profile analysis of FFAs in the alcohol extract of Aconitum taipeicum Hand.-Mazz., where the yields of two extraction methods were compared before the derivatization of FFAs in the extract.
2 Materials and methods
2.1 Chemicals and material
Individual fatty acids of a purity >99% were purchased from NU-CHEK, PREP, INC (Elysian, MN, USA). A stock solution of mixed fatty acids containing 120 μg mL−1 palmitoleic acid (C16:1, No. 373-49-9), palmitic acid (C16, No. 57-10-3), linoleic acid (C18:2, No. 60-33-3), oleic acid (C18:1, No. 112-80-1), linolenic acid (C18:3, No. 463-40-1) and stearic acid (C18, No. 57-11-4) was prepared by dissolving appropriate amounts of the fatty acids in methanol, and the stock solution was proportionally diluted with methanol in the following experiments when needed. 30 μg mL−1 of each type of fatty acid was prepared by dissolving it in appropriate amounts of methanol, respectively. A standard solution of 10 μg mL−1 of nonadecanoic acid (C19, No. 646-30-0) was prepared by dissolving appropriate amounts in methanol. The boron trifluoride–methanol complex (BF3-CH3OH) was purchased from Tianjin Sixth Chemical Reagent Factory (Tianjin, China) for esterification. 0.4 M KOH/CH3OH was freshly prepared by dissolving appropriate amounts of KOH in methanol.All water used was obtained from a purification system (Millipore, USA). All chemicals used were of analytical reagent grade or higher unless otherwise specified.
The Chinese traditional medicine Aconitum taipeicum Hand.-Mazz. (Fig.1) was collected in July 2007 from Mountain Taibai in the Shaanxi Province of China. The plant was authenticated and the voucher specimen has been deposited in Key Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, China. The samples were dried in a shady ventilated area.
 | ||
| Fig. 1 The picture of Aconitum taipeicum Hand.-Mazz. | ||
2.2 Preparation of the alcohol extract
Immersion extraction was carried out as follows: 200 g of Aconitum taipeicum Hand.-Mazz. was ground using a grinder (FW- 200, high speed versatile grinder, Beijing Zhongxin Weiye Instruments Company) into a powder then filtered through a sieve of diameter 0.28 mm. 40 g of the powder was immersed into a 100 mL mixture of methanol and ethanol (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 15 °C for 12 h with a stirring speed of 830 rpm. The immersion extraction repeated another two times using fresh solvent each time. Anhydrous sodium sulfate was added to a total 300 mL of the resulting extraction solution to remove any possible moisture; and the solvent was removed in a rotary vacuum evaporator at 50 °C. Finally, the alcohol extract obtained was estimated by a gravimetric method.
1) at 15 °C for 12 h with a stirring speed of 830 rpm. The immersion extraction repeated another two times using fresh solvent each time. Anhydrous sodium sulfate was added to a total 300 mL of the resulting extraction solution to remove any possible moisture; and the solvent was removed in a rotary vacuum evaporator at 50 °C. Finally, the alcohol extract obtained was estimated by a gravimetric method.
2.3 Esterification of standard FFAs
The BF3-CH3OH method28 was used to methyl esterify FFAs in the extract. 1.00 mL of mixed standards and 1.00 mL of the internal standard (nonadecanoic acid) were added to 3.00 mL of boron trifluoride–methanol, and the reaction mixture was then kept at 55 °C for 10 min to enhance the esterification reaction of the fatty acids. After 2 mL of saturated sodium chloride was added to the reaction mixture, the produced fatty acid methyl esters (FAMEs) were extracted into a total volume of 9 mL hexane using 3.00 mL each time. The hexane phase was then concentrated to 1.00 mL under vacuum pending GC-MS analysis.2.4 Establishment of quantification method
A series of standard solutions of a mixture of six fatty acids were prepared through diluting the stock solution 2, 4, 8, 16, 32, 64 and 128 times with methanol, and then the esterification procedure was conducted as described above.All chromatographic measurements were performed with a GC-MS system on a QP2010 gas chromatograph (Japan, Shimadzu), which was fitted with a split injector for capillary columns and a quadrupole mass spectrometer with an electron impact (EI) mode of 70 eV as the ionization source. The operating GC-MS conditions were as follows: Helium (purity 99.999%) was used as the carrier gas with a flow rate of 1.20 mL min−1. 2 μL of solution was injected into an RTX-5MS fused-silica capillary column (30 m length, 0.25 mm diameter, 0.25 μm film thickness, coated with 5% diphenyl and 95% dimethylpolysiloxane) with a split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and injector port temperature was set at 250 °C. The column temperature was programmed to increase from 183 °C to 189 °C at 0.2 °C min−1 and then kept for 2 min. The temperature of the ion source and interface were 200 °C and 250 °C, respectively. Solvent cut time was set at 3.5 min. EI mass spectra were recorded in full scan mode with monitoring mass ranging from 20 to 800 amu.
5, and injector port temperature was set at 250 °C. The column temperature was programmed to increase from 183 °C to 189 °C at 0.2 °C min−1 and then kept for 2 min. The temperature of the ion source and interface were 200 °C and 250 °C, respectively. Solvent cut time was set at 3.5 min. EI mass spectra were recorded in full scan mode with monitoring mass ranging from 20 to 800 amu.
The GC-MS Labsolution software package was used for regression analysis (linear model) and statistical analysis of the data.
2.5 Identification and quantitation of FAAs in Aconitum taipeicum Hand.-Mazz
0.05 g of alcohol extract was dissolved in 10.0 mL methanol, which was filtered after stirring for 30 min, and then 1.0 mL of the filtrate was esterified using BF3-CH3OH method and determined on GC-MS as described above, where only FFAs were assumed to be esterified. As a confirmative comparison, 1.0 mL of the filtrate was also esterified according to the KOH, BF3/MeOH method,29 where both esterified fatty acids (EFAs) and FFAs could be esterified through esterifying them with BF3/MeOH following saponifying the extract with 0.5 mol L−1 KOH in CH3OH.A recovery test was carried out to investigate the accuracy of the quantification method by spiking a given amount of each fatty acid into the extract.
Identification of the fatty acids was carried out by comparing their fragmentation pattern in EI mass spectra with those in the Mass Spectral database (NIST05 and NIST05s). They were further confirmed by comparing their retention times with those of authentic compounds available in our laboratory. Relative percentages of individual FAMEs were calculated based on GC peak areas without response factors correction. Absolute contents were obtained from the total ion current (Tic) peak areas and expressed as the mass (mg) per 1.00 g extract.
3 Results and discussion
3.1 Investigation of extraction methods
The choice of extraction method primarily depended on the type of matrix analyzed and the possible preliminary information. According to the common practice of the administration of this traditional Chinese medicine, a mixed alcohol of methanol and ethanol was used as the extraction solvent, and immersion extraction was compared with refluxing method. The extraction yields for the two extraction methods were compared on the main MS signal of the corresponding extracts.In immersion extraction, the sample was consecutively extracted three times as described above, each time leaving a fraction of the dry extract (0.05 g) to investigate the extent of extraction completeness, and this fraction was dissolved in 1.00 mL of ether. After excluding the insoluble residue by filtration, the filtrate was stored at 4 °C pending GC-MS analysis. In the refluxing method, 40 g of Aconitum taipeicum Hand.-Mazz. powder was refluxed with 100 mL of the same extraction solvent at 74 °C for 6 h, and the extraction was also carried out three times, each time leaving a fraction of the dry extract (0.05 g), the fractions were also used for the investigation of extraction efficiency.
Some GC-MS conditions were different from those in the quantification section on the split ratio and column temperature program, the split ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and the column temperature was programmed to increase from 80 °C to 250 °C at 5 °C min−1 and then kept for 2 min.
20, and the column temperature was programmed to increase from 80 °C to 250 °C at 5 °C min−1 and then kept for 2 min.
Tic chromatograms of GC-MS for both immersion and refluxing methods are shown in Fig. 2. No appreciable difference in the composition was observed between the two methods with the exception of total extraction yields. The total extracts obtained by the immersion and refluxing methods were 5.35 g and 5.08 g, respectively. Based on the main Tic peak areas, the percentages of the fraction obtained each time are listed in Table 1. The results suggested that the immersion method with a three-time extraction was a better choice.
 | ||
| Fig. 2 Tic chromatograms of GC-MS for both immersion and refluxing methods. | ||
| Method | Extraction order | ||
|---|---|---|---|
| First time | Second time | Third time | |
| Immersion | 92% | 7.0% | 1.0% |
| Fluxing | 93% | 6.0% | 1.0% |
3.2 Choice of derivatization method
For long-chain FFAs, derivatization procedures are usually required to decrease their polarity and increase volatility, as well as thermal stability before GC analysis30,31 FAMEs are the most commonly prepared derivatives due to their high volatilities.32 The methods for transesterification of EFAs and methylation of FFA have been reviewed27,33 Derivatization methods that esterify both EFAs and FFAs, such as direct esterification (H2SO4/MeOH) or saponification–esterification (KOH, BF3/MeOH)29 are still widely used. An improved method has been developed for direct preparation and extraction of FAMEs from matrices without prior separation, which is less time consuming and less costly.34 In this work FFAs were esterified with BF3-CH3OH as described above,28 where EFAs do not undergo transesterification.The effect of the amount of derivatization reagent BF3-CH3OH (ranging from 1 to 10 mL) and reaction time (ranging from 5 to 30 min) were investigated. The results showed that the best sensitivity for determination was obtained when 3.0 mL of BF3-CH3OH and a derivatization time of 10 min were chosen. The effect of the time on determining some FFAs is shown in Fig. 3.
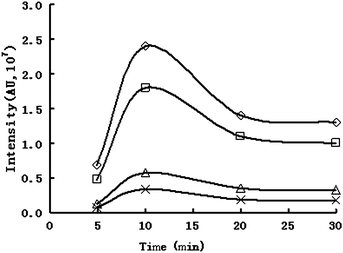 | ||
| Fig. 3 The effect of the time on derivatisation (○ palmitic acid, □ linoleic acid, △ oleic acid, × linolenic acid). | ||
3.3 FFAs profile in Aconitum taipeicum Hand.-Mazz
Six main types of fatty acids were identified based on their retention times and mass spectra fragmentation. They included palmitoleic acid, palmitic acid, linoleic acid, oleic acid, linolenic acid and stearic acid.3.4 Features of the quantification method for FFAs
In this work the GC-MS quantification method for determining FFAs could be set up in three ways: changing split ratios while keeping the concentration of the analyte constant, changing injection volumes of analyte while maintaining other conditions, or changing the concentration of the analyte while keeping the injection volume and split ratio constant. In the first two methods, they allow mere external standard calibrations, which are known to have worse reproducibility and linearity than internal standard calibrations. Therefore, the third method was chosen. Among Tic and Mic (Multiple ion current) signals of MS, the Tic signal was chosen, which is popular for regular analysis with the simple data process. The Mic signal is better for complex matrices and low concentrations due to its superior signal to noise ratio and selectivity.The quantification method for the six main fatty acids identified was developed with nonadecanoic acid (C19:0) as the internal standard (IS), a typical chromatogram of the mixed standards is shown in Fig. 4. A regression equation was obtained by plotting the area ratio Y (analyte to IS) versus the concentration ratio X (analyte to IS).
 | ||
| Fig. 4 The Tic chromatogram of standards 1, palmitoleic acid methyl ester; 2, palmitic acid methyl ester; 3, linoleic acid methyl ester; 4, oleic acid methyl ester; 5, linolenic acid methyl ester; 6, stearic acid methyl ester; 7, nonadecanoic acid methyl ester (IS). | ||
The limit of detection (LOD) for each acid was expressed as the amount of analyte which gave a signal of 3 times the standard deviation of the blank signal, the LODs ranged from 0.40 to 0.76 ng. Repeatability of the method was calculated through six reduplicate analyses of the analyte and expressed as relative standard deviations (RSD). RSDs for all fatty acids were less than 5%, and acceptable recovery results for the extract of Aconitum taipeicum Hand.-Mazz. were obtained, which were between 96% and 104%. All the features of the quantification method for individual fatty acids are shown in Table 2.
| Fatty acid | Regression equation | Detection limit/ng | Regression coefficient | Linear range/ng | Repeatability RSD(%) | Recovery (%) |
|---|---|---|---|---|---|---|
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
y = 2.019x + 0.015 | 0.53 | 0.998 | 1.6–472 | 2.1 | 104 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
y = 1.399x + 0.028 | 0.76 | 0.998 | 2.3–682 | 1.8 | 99 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
y = 2.065x − 0.018 | 0.51 | 0.998 | 1.5–462 | 2.3 | 101 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
y = 1.855x − 0.021 | 0.57 | 0.998 | 1.7–514 | 3.5 | 97 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
y = 2.652x − 0.016 | 0.40 | 0.997 | 1.2–360 | 3.2 | 96 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
y = 1.575x + 0.026 | 0.67 | 0.999 | 2.0–606 | 2.6 | 102 |
3.5 Quantitative analyses of FFAs in the extract of Aconitum taipeicum Hand.-Mazz.
No corresponding FAMEs were detected without prior esterification of the extract of Aconitum taipeicum Hand.-Mazz., which suggested that no FAMEs existed in the extract. The MS signal intensities of FAMEs obtained from the two derivatization methods (described in section 2.5) are listed in Table 3, where no significant difference was observed between the methods, this indicated that there were little if any EFAs, and that FFAs in the extract were responsible for the FAMEs signal when the esterified extract was detected. The relative and absolute contents of individual FAAs in the extract of Aconitum taipeicum Hand.-Mazz. are listed in Table 4, and a Tic chromatogram of the sample is shown in Fig. 5.| Fatty acids | BF3-CH3OH method | KOH-CH3OH method |
|---|---|---|
| a Mean of six determinations, expressed in arbitrary units. | ||
| Palmitoleic acid | 1.24 × 107 | 1.29 × 107 |
| Palmitic acid | 0.86 × 107 | 0.82 × 107 |
| Linoleic acid | 1.27 × 107 | 1.32 × 107 |
| Oleic acid | 1.14 × 107 | 1.18 × 107 |
| Linolenic acid | 1.63 × 107 | 1.69 × 107 |
| Stearic acid | 0.97 × 107 | 0.92 × 107 |
| Fatty acid | Relative content (%) | Absolute content/mg g−1a |
|---|---|---|
| a Mean of six determinations, expressed as average ± SD. | ||
| Palmitoleic acid | 2.38 | 0.73 ± 0.02 |
| Palmitic acid | 28.24 | 12.47 ± 0.32 |
| Linoleic acid | 46.24 | 13.89 ± 0.36 |
| Oleic acid | 15.93 | 5.34 ± 0.12 |
| Linolenic acid | 2.87 | 0.67 ± 0.02 |
| Stearic acid | 4.35 | 1.67 ± 0.04 |
 | ||
| Fig. 5 Tic chromatogram of sample. The numbers on the peaks stand for the same as in Fig. 4. | ||
4 Conclusion
This study provides a complete FFAs profile of Aconitum taipeicum Hand.-Mazz., which is abundant in three types of FFAs. Linoleic acid (46.24%, 13.89 ± 0.36 mg g−1) was the predominant FFA, followed by palmitic acid (28.24%, 12.47 ± 0.32 mg g−1) and oleic acid (15.93%, 5.34 ± 0.12 mg g−1). Linoleic acid and another identified linolenic acid are well known among the few essential fatty acids for human health, and their prime importance to human health has been reviewed.17,35 Linoleic acid is closely related to the metabolism of lipids and cholesterol, and plays an important role in reducing cholesterol, decreasing arteriosclerosis, and in cancer prevention. There were no appreciable TFAs detected in the alcohol extract, which are known to be responsible for certain adverse health effects,19–21 therefore, the types of FAs in this alcohol extract would have little adverse health effects, and this conclusion does not conflict with the dose-dependent rule in FFAs intake. Therefore, other components in the extract such as alkaloids are likely to be responsible for the penitential toxicity of Aconitum taipeicum Hand.-Mazz‥2,3 A comprehensive study of the other components in Aconitum taipeicum Hand.-Mazz. is still necessary.Acknowledgements
The research was supported by the Chinese Traditional Medicine Modernization Foundation of Shaanxi Province, China (No. 2008K16-07, 2008K08-04) and the Doctor Innovation Foundation of Shaanxi Normal University of China.References
- Y. Xu, Z. J. Gao and L. Tan, Res. Prac. Chin. Med., 2008, 22, 38–41 Search PubMed.
- Y. L. Lu and F. Wang, Chin. Folk Med., 1999, 6, 25–25 Search PubMed.
- Y. Q. He, Z. Y. Ma and Q. Yang, et al, Acta Pharm. Sin., 2008, 43, 934–937 Search PubMed.
- Y. Xu, Z. J. Gao and L. Tan, et al, Chin. Med. Mater., 2008, 31, 1569–1571 Search PubMed.
- R. M. Tomaino, J. D. Parker and D. K. Larick, J. Agric. Food Chem., 2001, 49, 3993–3998 CrossRef CAS.
- D. I. Skonberg and B. L. Perkins, Food Chem., 2002, 77, 401–404 CrossRef CAS.
- C. A. Martin, R. Carapelli and J. V. Visantainer, et al, Food Chem., 2005, 93, 445–448 CrossRef CAS.
- M. Sajid, O. Ilkay and A. Zaheer, et al, Food Chem., 2008, 109, 855–859 CrossRef.
- A. Phillip, S. Mark and B. Matt, et al, Atmos. Environ., 2008, 42, 6417–6424 CrossRef.
- A. L. Stoddart, N. J. Smith and G. Milligan, Pharmacol. Rev., 2008, 60, 405–417 CrossRef.
- F. A. Wallace, S. J. Neely and E. A. Miles, et al, Immunol. Cell Biol., 2000, 78, 40–48 CrossRef CAS.
- S. Cherif, F. Frikhaa and Y. Gargouria, et al, Food Chem., 2008, 111, 930–933 CrossRef CAS.
- P. C. Calder, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2008, 79, 101–108 CrossRef CAS.
- M. Hamberg and G. Hamberg, Phytochemistry, 1996, 42, 729–732 CrossRef CAS.
- R. L. Hargrove, T. D. Etherton and T. A. Pearson, et al, J. Nutr., 2001, 131, 1758–1763 CAS.
- P. J. Yaqoob, Eur. J. Clin. Nutr., 2002, 56, S9–S13 CrossRef CAS.
- B. Villa, L. Calbresi and G. Chiesa, et al, Pharmacol. Res., 2002, 45, 475–478 CrossRef CAS.
- D. S. Siscovick, T. E. Raghunathan and I. King, et al, JAMA, J. Am. Med. Assoc., 1995, 274, 1363–1367 Search PubMed.
- Cardiovascular Review Group, Committee on Medical Aspects of Food Policy. Nutritional aspects of cardiovascular disease, Report on Health and Social Subjects, Department of Health, London, 1994 Search PubMed.
- G. P. Zaloga, K. A. Harvey and W. Stillwell, et al, Nutr. Clin. Pract., 2006, 21, 505–512 Search PubMed.
- M. C. Craig-Schmidt, B. M. Holzer, C. K. Chow, Fatty Acids in Foods and Their Health Implications, 2nd Edition, Marcel Dekker, New York, 2000, 307 Search PubMed.
- A. L. Bailey and S. Southon, Anal. Chem., 1998, 70, 415–419 CrossRef CAS.
- J. Zhao, S. P. Li and F. Q. Yang, et al, J. Chromatogr., A, 2006, 1108, 188–194 CrossRef CAS.
- L. Romanowicz, Z. Galewska and T. Gogiel, et al, J. Biochem. Biophys. Methods, 2008, 70, 973–977 CrossRef CAS.
- M. Petrović, N. Kezića, V. Bolančaa, DOI:10.1016/j.foodchem. 2010.02.018.
- F. Destaillats and C. H. Cristina, J. Chromatogr., A, 2007, 1169, 175–178 CrossRef CAS.
- L. Z. Yi, J. He, Y. Z. Liang, D. Yuan, H. Gao and H. Zhou, Chem. Phys. Lipids, 2007, 150, 204–216 CrossRef CAS.
- P. Banerjee, G. Dawson and A. Dasgupta, Biochim. Biophys. Acta, Biomembr., 1992, 1110, 65–74 CrossRef CAS.
- P. W. Park and R. E. Goins, J. Food Sci., 1994, 59, 1262–1266 CrossRef CAS.
- K. Blau, J. Halket, Handbook of Derivatives for Chromatography, 2nd ed., John Wiley and Sons, London, 1993 Search PubMed.
- J. M. Rosenfeld, Anal. Chim. Acta, 2002, 465, 93–100 CrossRef CAS.
- J. Dron, R. Linke, E. Rosenberg and M. Schreiner, J. Chromatogr., A, 2004, 1047, 111–116 CrossRef CAS.
- N. C. Shantha and G. E. Napolitano, J. Chromatogr., A, 1992, 624, 37–51 CrossRef CAS.
- H. van.der Tempel, J. E. Tulleken and P. C. Limburg, et al, Ann. Rheum. Dis., 1990, 49, 76–80 CrossRef CAS.
- P. C. Calder, Lipids, 1999, 34, S137–S140 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
