Disposable sensor for quantitative determination of hydrazine in water and biological sample
Abdolkarim
Abbaspour
*,
Ehsan
Mirahmadi
and
Abdolreza
Khajehzadeh
Chemistry Department, College of sciences, Shiraz University, Shiraz, Iran. E-mail: abbaspour@chem.susc.ac.ir; Fax: +98 711 228 0828; Tel: +98 711 2284822
First published on 9th February 2010
Abstract
A method for determination of hydrazine is described. The sensor was constructed by immobilizing a reagent on TLC (Thin Layer Chromatography) paper. This procedure is based on chromogenic reaction of hydrazine with p-(dimethylamino)benzaldehyde (reagent) and formation of red colour product followed by scanner-based detection. Changes in RGB values of colour spots on TLC strips create a pattern. The obtained pattern was analyzed by MATLAB software. The proposed sensor is linear in concentration range of 10–300 μg mL−1 of hydrazine and has practical detection limit of 0.1 μg mL−1. Parameters such as pH and concentration of p-(dimethylamino)benzaldehyde were optimized. The proposed sensor was successfully applied for the determination of hydrazine in bovine serum and tap water.
Introduction
The qualitative determination of materials in the form of spots on an absorbent paper or other inert support has been the subject of study for many years. Feigl and Anger have provided the basis for many such studies.1 Quantitative spot-test analysis; however, is not very usual. Attempts to obtain quantitative data directly from spot-test by reflectance spectrometry cannot yield the precision better than 10% as mentioned by Kealey2 and then quantitative results have often been considered unreliable. If transmittance spectroscopy is considered a quantitative and reproducible analysis, the reflectance spectroscopy is considered qualitative and non-reproducible due to the effect of the inhomogeneous media.3Our group have previously introduced paptode for speciation of iron(II) and iron(III), construction of a pH sensor4 and determination of ascorbic acid in Vitamin C Tablets.5 We have chosen the name of paptode because; this method is similar to optode in many features. In this method instead of immobilizing an ionophore on a hydrophobic or hydrophilic polymer as in optode, simply a paper or other ordinary porous material such as cotton or clay and even TLC can be used as a substrate support for reagent. In spite of conventional spot tests analysis which have used reflectance spectroscopy for quantification, in the developed method scanner was used as a detector.
It has been found that when a drop of solution of an analyte comes into contact with a reagent-impregnated inert support, coloured reaction products are produced on the surface of the support, producing distinct flecks or rings within a circle wetted by water. This local accumulation accompanying spot reaction on the inert support is very important because it enhances the discerniblity of coloured reaction products, especially if they are insoluble in aqueous media, or when coloured water-soluble reaction products are adsorbed on the support.1 Furthermore, degree of the colour of the spot was found to be proportional to the concentration of the tested analyte. We have used commercially available flatbed-scanners for obtaining the images of colour-spots. The obtained images have been transferred to computer for analyzing and determining the intensity of colour-spots.
Application of a CCD camera or flatbed scanner in this system makes it possible to use non-transparent supporting substrates. Unlike visible spectroscopic methods, in this method instead of measuring the transmittance or absorbance of light, the reflection properties of the strip are measured by using a flatbed scanner. Thus, turbid samples that are troublesome in transmittance or absorbance spectroscopy can be analyzed by this method and the interference species that produce precipitants with analyte or ligand do not interfere in this method.
It should be noted in the colour analysing programs, we are able to select a specific area for analysis and the number of pixels that can be indicated by this area was about 10000–300000, and this program can average these pixels. Therefore, the signal to noise ratio can be increased dramatically. Furthermore, the problem of inhomogeneous media, which was problematic in reflectance spectroscopy, is not important here due to averaging the intensity of each colour spot. Area of the spots, which were used to measure the colour intensity, was a square with 40000 dpi (200 × 200 dpi). The spots were not perfectly homogeneous; therefore, we have selected the central region of spots for analyzing the colour.
In the proposed method, not only is it possible to save experimental data, but it is also possible to save images of results (e.g. the colourful complex) in a computer, then they could be reviewed for applying more powerful software on it in the future. In addition, the porosity of support-based materials in these sensors causes shorter response times in these sensors compared with the typical response times in optodes. Other advantages, such as being portable and ease of reagent immobilization are considerable.
Hydrazine and its derivatives are widely used in industry, agriculture, explosives, blowing agents for the plastics industry, and other fields. Besides being reactive and explosive, hydrazine is highly toxic. It may cause skin irritation and systemic poisoning.6 Hydrazine is a suspected carcinogen.7 Because of these considerable toxicological effects and its industrial significance, the determination of hydrazine in different samples is of interest.
Several methods have been described in the literature for the determination of hydrazine using different analytical techniques such as voltammetry,8–10 coulometry,11 gas chromatography,12 spectrofluorimetry,13,14 spectrophotometry,15–20 and titrimetry.21 Many methods have been suggested for the determination of hydrazine based on its basic character or reducing property.18,22 Spectrophotometric methods are more useful for the determination of hydrazine at low concentration levels,14,19 but these methods suffer from poor linear dynamic ranges and some of them require expensive instruments.
The present paper describes the use of paptode for analysis of hydrazine. In this proposed sensor, p-(dimethylamino)benzaldehyde (DAB) was immobilized on TLC strip as a chromogenic reagent. After injection of hydrazine containing solution, a red product was formed on the TLC strip. After the strip was dried, an image of each strip was recorded using a scanner. The colour values of the spots were analyzed with special software written in Visual Basic 6 (VB 6) media.
Experimental
Apparatus and software
The scanner (Canoscan-4200F) was used for scanning the TLC strip. Resolution of the scanner was regulated at 150 dpi. For analyzing colour values in RGB (red, green, blue) system, the software, which was written in Visual Basic media, was used. An eppendorf micropipette was used for injecting samples on TLC strips. The pH values of buffer solutions were measured by a Metrohm (780) pH meter.Chemicals and reagents
All solutions were prepared using analytical-reagent grade substances and deionized water. Hydrazinium dichloride and p-(dimethylamino)benzaldehyde (DAB) were purchased from Merck.A stock solution of 1000 μg mL−1 hydrazine was prepared daily by dissolving 32.8 mg of hydrazinium dichloride in 10 mL deionized water. Buffer solutions were prepared by using potassium hydrogen phthalate (Fluka) and hydrochloric acid with appropriate concentration. For preparation of each solution, proper volumes of stock solution were added to a 10 ml volumetric flask and diluted to the mark with buffer solution of desired pH. Solutions of DAB were prepared by dissolving an appropriate amount of reagent in ethanol (Fluka) and diluting it to the mark in a 5 ml volumetric flask.
Procedure
Hydrazine forms a highly red colour product with DAB. To construct the sensor strip for hydrazine, strips of TLC were immersed into known concentration of the DAB solution for few seconds and then dried at ambient temperature. Aliquots of 10 μl of hydrazine solutions were injected on these TLC strips. After formation of the red spots (product of the reaction between hydrazine and immobilized DAB), the strips were scanned with scanner and images of spots were analyzed by the written computer software for finding their R, G and B values. The RGB colour model is an additive colour model in which red, green, and blue light are added together in various ways to produce a broad array of colours. Any colour can be analyzed to obtain its corresponding R, G and B value. Effective intensity for any colour values of colour spot was calculated as follow:| Ar = −Log (Rs/Rb) | (1) |
| Ag = −Log (Gs/Gb) | (2) |
| Ab = −Log (Bs/Bb) | (3) |
Where, for red, green and blue colour effective intensities are shown as Ar, Ag and Ab, respectively. Rs, Gs, Bs and Rb, Gb and Bb refer to R, G and B values of sample and blank, respectively. To obtain calibration curves, effective intensities of R, G and B values were plotted vs. analyte concentrations.
Results and discussion
The condensation reactions of aromatic aldehydes with hydrazine derivatives produce coloured products. The condensation reaction of DAB with hydrazine produces azine according to the stoichiometric equation which is shown in Fig. 1. | ||
| Fig. 1 Condensation reaction of DAB with hydrazine. | ||
It is reported that, this colour formation reaction is being forced to completion with a large excess of reagent but since the colour of DAB reagent is originally light yellow the immobilization of this reagent on the strip changes the colour of the background and reduces the DL. Therefore, it is useful to use as little reagent as possible.
The effect of pH on the reaction is complex.23 Protonation of hydrazine and benzaldazine pushes the reaction to the desired way whereas protonation of DAB reverses the main reaction. In the kinetic point of view, H+ catalyzes the reaction but it is considered that only unprotonated hydrazine can participate in acid catalyzed nucleophilic attack on DAB. Thus there are two conflicting kinetic effects. For this reason, the optimization of the pH of the system is very important.
Optimization of the conditions
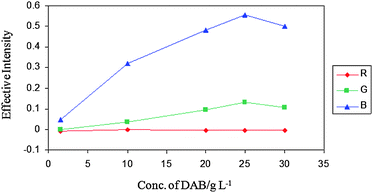 | ||
| Fig. 2 Effect of DAB concentration on effective intensities of spots in fixed hydrazine concentration (CHydrazine = 100 μg mL−1). | ||
 | ||
| Fig. 3 Effect of pH on collar intensities of spots in fixed hydrazine and DAB concentrations (CHydrazine = 100 μg mL−1 and CDAB = 25 g L−1). | ||
Drying methods
After injection of reagent onto the TLC strip, the strip needs to be dried. Some methods such as drying at room temperature, oven and hot air were applied and no change in signal was observed. However, for increasing the speed of analysis, using an oven or hot air is recommended.Calibration curves
Fig. 4 shows the calibration curves of hydrazine under optimum conditions. This figure shows a linear relationship between effective intensities of RGB colours with concentration, and the linear range was 10 μg mL−1 to 300 μg mL−1 of hydrazine. | ||
| Fig. 4 Calibration graph of hydrazine at optimum conditions (CDAB = 25 g L−1 and pH = 3). | ||
Fig. 4 indicates that more than one response (ARed, AGreen and ABlue) could be used for determination of hydrazine concentration. As it is clear from the linear equations for each colour coordinate, the B value is more sensitive than G value and the R value is relatively ineffective.
Reproducibility, response time, stability and detection limit of the system
Reproducibility of the system was investigated at eight different sensors under optimum conditions for various concentrations of hydrazine. The Table 1 shows that the proposed method is reproducible.The response time of the system was evaluated under optimum experimental conditions for 80 μg mL−1 of hydrazine. It was calculated by measuring the time required to achieve a 90% value of steady colour intensity. The response time of two min was achieved.
To study the stability of colour spot, 80 μg mL−1 of hydrazine was injected under optimum experimental conditions on the sensor. Scanning of the strip was done in the time period of 2, 60, 120, 180, 240, 300, 360, 420, 480 and 540 min. No change in colour intensity was observed for a period of 360 min. Therefore, the sensor is stable for 6 h after injection of sample.
In order to study the stability of the sensor, after immobilizing DAB on the TLC strip, it was used periodically each day. The signals did not show any significant changes within thirty days of experiments. This reveals that the prepared strip is stable at least for thirty days.
For each RGB factor there is one DL.4 Theoretical DLs of this method were 1.75 and 0.48 μg mL−1 for G and B values respectively. As the R value does not vary considerably by changing the concentration of hydrazine, we calculated DL only for G and B values. We can also determine the detection limit by practical experiment. Practical DL is the lowest concentration that would give a colour on the TLC strip.1 Practical DL was about 0.1 μg mL−1.
Interference studies
To study the selectivity of the proposed method, the effect of various species on the determination of 100 μg mL−1 hydrazine was tested under optimum experimental conditions. The tolerance limit was defined as the concentration of added species that causes less than ±5% relative error. The results indicated that more than 10000 μg mL−1 F−, Cl− SO42−, CO32−Ca2+, Na+, K+, urea and citrate had no significant interferences and 10000 μg mL−1 I−, Br−, NH4+, Ag+, Ba2+, Pb2+, Al3+, acetate, 5000 μg mL−1 Mg2+, Cd2+, Zn2+, Ni2+, 1000 μg mL−1 Cu2+, semicarbazide and 500 μg mL−1 phenyl hydrazine did not interfere in the determination of hydrazine. It is notable that phenyl hydrazine also can react with DAB as do hydrazine but the rate of its reaction on TLC is low. Therefore, it does not interfere with the response time of hydrazine.Analysis of real samples
Using the calibration curve for B coordinate, the concentrations of hydrazine in a tap water and bovine serum that were spiked with hydrazine were calculated. The results for these determinations are given in Table 2, which confirm that hydrazine can be determined quantitatively in the region assessed.Conclusions
The described method has many advantages: it does not need expensive apparatus, it is simple and rapid. As Table 3 shows, the linear range of proposed method is wide relative to the methods based on chromogenic reactions that have been applied for determination of hydrazine and also proposed sensors have shorter response times. Immobilization of the reagent is very simple. In addition, the porosity of support based materials cause short response times and because we measure the reflectance, non-transparent substrate can be used. Finally, the sensor can be used by emergency responding teams for testing of hydrazine spill or contamination in the various samples.| Reagent | Technique | Linear range/μg mL−1 | Detection limit/μg mL−1 | Response time/min | Ref. |
|---|---|---|---|---|---|
| Para-dimethylaminobenzaldehyde | Spectrophotometry | 0.06–0.47 | — | — | 24 |
| Salicylaldehyde | Spectrophotometry | 0.29–1.25 | — | 15 | 25 |
| 2-hydroxy-1-naphthaldehyd | Extraction - Spectrophotometry | 0.035–0.7 | — | 20 | 26 |
| Para-dimethylaminobenzaldehyde | FIA - Spectrophotometry | 2.0–40.0 | 1.0 | — | 19 |
| Para-dimethylaminobenzaldehyde | Spectrophotometry | 0.020–0.50 | 0.01 | — | 18 |
| Para-dimethylaminobenzaldehyde | Proposed method | 10–300 | 0.1 | 2 | This work |
Notes and references
- F. Feigl and V. Anger, Spot Test in Inorganic Analysis, Elsevier, Amsterdam, 6th ed., 1972 Search PubMed.
- D. Kealey, Talanta, 1972, 19, 1563 CrossRef CAS.
- R. Narayanaswamy and F. Sevilla, Mikrochim. Acta, 1989, 97, 293 CrossRef.
- A. Abbaspour, M. A. Mehrgardi, A. Noori, M. A. Kamyabi, A. Khalafi-Nezhad and M. N. Soltani Rad, Sens. Actuators, B, 2006, 113, 857 CrossRef.
- A. Abbaspour, A. Khajezadeh and A. Noori, Anal. Sci., 2008, 24, 721 CrossRef CAS.
- I. Sax, Dangerous Properties of Industrial Materials, van Nostrand-Reinhold, New York, 4th ed., 1980, pp. 814 Search PubMed.
- H. W. Schiessl, Encyclopedia of Chemical Technology, Othmer Wiley, New York, 1980, vol. 12 K, pp. 734 Search PubMed.
- N. Maleki, A. Safavi, E. Farjami and F. Tajabadi, Anal. Chim. Acta, 2008, 611, 151 CrossRef CAS.
- A. Salimi, L. Miranzadeh and R. Hallaj, Talanta, 2008, 75, 147 CAS.
- M. S. M. Quintino, K. Araki, H. E. Toma and L. Angnes, Talanta, 2008, 74, 730 CrossRef CAS.
- A. G. Fogg, A. Y. Chamsi, A. A. Barros and J. O. Cabral, Analyst, 1984, 109, 901 RSC.
- Y. Y. Liu, I. Schmeltz and D. Hoffmann, Anal. Chem., 1974, 46, 885 CrossRef CAS.
- M. Roth and J. Rieder, Anal. Chim. Acta, 1962, 27, 20 CrossRef.
- A. A. Ensafi and B. Rezaei, Talanta, 1998, 47, 645 CrossRef CAS.
- A. Afkhami and M. Bahram, Talanta, 2006, 68, 1148 CrossRef CAS.
- A. Afkhami and A. Afshar-E-Asl, Anal. Chim. Acta, 2000, 419, 101 CrossRef CAS.
- M. George, K. S. Nagaraja and N. Balasubramanian, Talanta, 2008, 75, 27 CrossRef CAS.
- A. Afkhami and A. R. Zarei, Talanta, 2004, 62, 559 CrossRef CAS.
- A. A. Ensafi and B. Naderi, Microchem. J., 1997, 56, 269 CrossRef CAS.
- Ali Reza Zarei, Anal. Biochem., 2007, 369, 161 CrossRef CAS.
- K. K. Verma, A. Srivastava, J. Ahmed and S. Bose, Talanta, 1978, 25, 469 CrossRef CAS.
- S. Dadfarnia and K. Dehghan, Bull. Korean Chem. Soc., 2004, 25, 213.
- D. S. Gamble and I. Hoffman, Can. J. Chem., 1967, 45, 2813 CrossRef CAS.
- G. W. Watt and J. D. Chrisp, Anal. Chem., 1952, 24, 2006 CrossRef CAS.
- L. C. Bailey and T. Medwick, Anal. Chim. Acta, 1966, 35, 330 CrossRef CAS.
- S. Amlathe and V. K. Gupta, Analyst, 1988, 113, 1481 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
