Glycoprotein microarray for the fluorescence detection of antibodies produced as a result of erythropoietin (EPO) abuse
Sinéad M.
Hardy
a,
C. Jane
Roberts
b,
Pamela R.
Brown
c and
David A.
Russell
*a
aSchool of Chemistry, University of East Anglia, Norwich, Norfolk, UK NR4 7TJ. E-mail: d.russell@uea.ac.uk
bQuotient Bioresearch Ltd., Newmarket Road, Fordham, Cambridgeshire, UK CB7 5WW
cHFL Sport Science, Newmarket Road, Fordham, Cambridgeshire, UK CB7 5WW
First published on 2nd November 2009
Abstract
With the commercial availability of recombinant human erythropoietin (rHuEPO), there is significant scope for athletes, especially those competing in endurance sports, to illicitly enhance their performance by increasing their aerobic capacity through enhanced erythrocyte production and hence oxygen transport. While such abuse has been confirmed in a number of human sports, there is also the possibility that rHuEPO can be abused in animal based sports such as thoroughbred horseracing. The direct detection of rHuEPO abuse, using either urine or blood samples, is challenging as the recombinant glycoprotein is similar to that produced endogenously and typically can only be measured above background levels within 4 days of administration. However, it is known that an immune response occurs when horses are doped with rHuEPO. The production of a specific antibody in response to doping with rHuEPO provides a target analyte that is not only different to endogenous species but one which resides in the body for considerably longer than the glycoprotein itself, significantly extending the measurement window. Here we have developed a glycoprotein microarray which exploits the antibody–antigen interaction to provide a means of detecting rHuEPO abuse in animals through the measurement of erythropoietin (EPO) antibodies (anti-HuEPO antibodies). Three commercially available isoforms of rHuEPO (Eprex®, Aranesp® and NeoRecormon®) were arrayed onto the planar surface of a nitrocellulose-coated microarray slide to act as the capture molecule in the assay. The assay was achieved by incubation of the microarray with solutions containing the anti-HuEPO antibody, followed by incubation with a fluorescently tagged secondary antibody. This ‘sandwich’ based assay enabled the fluorescent based detection of anti-HuEPO antibodies using an array-scanner. The EPO glycoprotein microarray was shown to be specific for anti-HuEPO antibodies. To detect anti-HuEPO antibodies in spiked serum samples an optimal dilution of the serum with buffer of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was established. Using Eprex®-10,000 IU as the capture molecule, the lowest concentration of anti-HuEPO antibody which was detected using the microarray was 148 pM, suggesting that the developed microarray platform could be used as a screen of EPO abuse.
4 was established. Using Eprex®-10,000 IU as the capture molecule, the lowest concentration of anti-HuEPO antibody which was detected using the microarray was 148 pM, suggesting that the developed microarray platform could be used as a screen of EPO abuse.
Introduction
Oxygen is required for many processes of life at the cellular level. It is the erythrocytes (red blood cells) that transport oxygen around the body. Erythropoiesis, the production of erythrocytes from the blast cell, is dependent upon erythropoietin (EPO), a glycoprotein hormone. Generally, the synthesis of EPO is stimulated by low oxygen pressure, which forms new erythrocytes. The production of erythrocytes results in an increase in the oxygen carrying capacity of blood and elevates the value of haemoglobin and haematocrit in the blood stream.1 The production of recombinant human erythropoietin (rHuEPO) in commercial amounts, and of a high degree of purity, was made possible with genetic engineering techniques. In 1986, the amino acid sequence was determined2 and the gene coding for human EPO cloned.3,4 The corresponding protein was expressed in Chinese Hamster Ovary (CHO) cells and chromatographically purified. rHuEPO was commercially available by the late 1980s to treat anaemia resulting from a host of conditions, such as kidney failure, HIV, and patients receiving chemotherapy for cancer.5Recombinant human erythropoietin (rHuEPO) is a sialoglycoprotein hormone that appears to be immunologically and biologically equivalent to the endogenous compound. The recombinant product is structurally similar to native human erythropoietin.6 There are several commercially available recombinant products, including epoetin alfa (Eprex®), which varies from epoetin beta (Neorecormon®) in the degree of glycosylation. However, there is no reported difference in the clinical efficacy of these two isoforms of EPO.7 Darbepoetin (Aranesp®) is a further rHuEPO analogue that is more glycosylated than either the epoetin alfa or beta. The glycosylation slows clearance of this product by the liver, allowing a reduction in frequency of administration while maintaining efficacy.8,9
As EPO enhances the aerobic potential through increasing oxygen transport, there is a temptation for athletes, especially those competing in endurance sports, to illegally increase their erythrocyte concentration through the subcutaneous injection of commercially available recombinant EPO formulations. There are numerous examples where the abuse of rHuEPO by endurance athletes has been documented, with the ‘Tour de France’ cycling competition providing an obvious example.10–12
Rumours concerning the use of rHuEPO in thoroughbred horseracing have been circulating since the recombinant glycoprotein became commercially available.13 With large financial rewards available for some races, the pressure to excel has grown and as a result the desire to win has led to the possibility that some racehorse owners/trainers may resort to the use of performance enhancing drugs. Administration of rHuEPO to improve racing performance in the horse represents a form of ‘blood-doping’ with the misuse of rHuEPO having the potential to compromise both the integrity of racing and the health of equine athletes.14 The administration of rHuEPO produces an increase of the red blood cell mass, haemoglobin concentration and the maximal aerobic power, thereby potentially improving the exercise performance of a horse.15–17 A positive correlation between red-cell mass and maximal oxygen uptake has been demonstrated in both human athletes18 and horses.19 However, the potential to improve the sporting performance of racehorses is questionable since the horse has a splenic red cell reserve that can be released into circulation on exercise to increase the haematocrit by up to 33%.13 In addition, blood-doping is considered extremely dangerous as large increases in the blood viscosity can lead to death from clotting, stroke, or heart attack.20 There have also been reports that horses can develop non-regenerative anaemia after multiple injections of EPO over a period of several weeks.21 Detection of rHuEPO mis-use has been a problem. Analysis of haematological22 or biochemical23 parameters indicates only that erythropoiesis has been stimulated, but cannot confirm that drug administration is the cause.
The direct measurement of rHuEPO misuse in athletes has been previously described.24,25 This method is based on the analysis of isoelectric focusing, double blotting and chemiluminescent detection of the EPO present in urine and is currently the approved detection method. Both exogenous rHuEPO and endogenous EPO, although having identical amino acid sequences, have a different glycosylation pattern giving different isoelectric profiles. The isoelectric patterns of the two recombinant EPO-α and -β forms are similar (both have an isoelectric point, pI, in the range 4.42–5.11); although EPO- β has an extra basic band, both differ from natural, purified urinary EPO, which has more acidic bands (pI 3.92–4.42).24 Such differences in the urine analysis allow excreted EPO to be ascribed to a natural or recombinant origin. However, the application of this method is limited by time and workload required to conduct the assay. The major drawback of the urine-based tests is the disappearance of measurable levels of rHuEPO from the urine soon after administration,26 despite the athlete's ability to retain the physiological benefits associated with an elevated red blood cell mass for a longer time.27,28 EPO concentrations in urine return to base line values four days after the last subcutaneous rHuEPO administration;27 moreover, after seven days from the last subcutaneous rHuEPO administration, the isoelectric focusing method only detects rHuEPO in approximately one-half of the administrated subjects.28 In 2005, Lasne et al. proposed a method for the direct detection of rHuEPO administration to a horse based on screening of plasma or serum using an ELISA followed by confirmation, using a urine sample, through characterisation of the EPO isoelectric profile.29 Subsequently, a method for the confirmation of rHuEPO in equine plasma by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) has been reported.30 A year later, the same group reported the first method for the differentiation and identification of epoetins alfa and beta and darbepoetin in equine plasma by LC-MS/MS.31 While clearly important, these tests will be restricted by the short half-life of rHuEPO in the body.
With the difficulty of the direct detection of EPO another target analyte has been sought in order to prove EPO abuse. When rHuEPO is injected into an animal, such as a horse, an immune response occurs producing an antibody to the ‘foreign’ EPO formulation.13,14,21 The detection of this antibody has two advantages over the rHuEPO itself. The first advantage is that there should be no interfering endogenous species. Secondly, the antibody resides in the animal for a long period of time significantly increasing the measurement window over that of the rHuEPO. Various assays for the detection of anti-HuEPO antibodies have been described. These include a radioimmunoprecipitation assay (RIPA) using I125-labelled rHuEPO,32 ELISAs with6,33 or without34,35 a competitive displacement step and surface plasmon resonance (BIAcore) analysis.36 The RIPA and ELISA methods can be cumbersome, time-consuming and the RIPA technique requires the use of radiolabelled reagents. SPR can have insufficient sensitivity and specificity when analysing low analyte concentration in serum.37
Protein microarrays have rapidly become established as a powerful means to detect proteins and investigate protein interactions and functions. They are solid phase ligand binding assay systems using immobilised proteins on surfaces which include glass, membranes, beads and microtiter wells. The advantages of protein microarrays include being rapid and automatable, capable of high sensitivity, economical on reagents and giving an abundance of data from a single experiment.38,39
There are two main types of protein microarrays: functional and analytical. Functional protein microarrays are created by immobilising large numbers of purified, recombinant proteins on a solid surface. These arrays can be used to screen the interaction of the immobilised proteins with other proteins, with DNA, or the modification of the proteins by enzymes.40–42 Analytical microarrays are used to profile antibodies or proteins. Analytical microarrays used for antigen-antibody interactions can be divided into two categories based on the component bound on the array surface: (1) protein/antigen arrays and (2) antibody arrays. Antigen-antibody interactions on microarrays have enormous potential in medical diagnostics. Protein microarrays differing in surface materials, sensitivities and applications have now been established by several groups.38,39,43,44
There are different formats of protein microarrays, one of which is the three-dimensional microarray which has a gel-coated surface. This microarray includes fluorescence array surface technology (FAST™) slides, which are coated with a nitrocellulose-derived polymer.45–47 This method is based on non-specific/non-covalent immobilisation. Three-dimensional microarrays have a higher immobilisation capacity than microarray slides that have been coated with poly-L-lysine, aldehydes and epoxy derivatives for example.48 Nitrocellulose has a high protein-binding capacity and the protein is bound in a non-covalent but irreversible manner. The homogenous water environment minimises protein denaturation.
Many applications of protein microarrays use fluorescently-labelled detection methods, since they are stable, simple to manipulate and provide highly sensitive results with good resolution. In general, the assay involves capture of the antibody of interest in a complex mixture followed by washing of the surfaces to remove non-specifically bound species. Subsequently, a secondary antibody labelled with a fluorescent dye, capable of recognising exposed epitopes on the bound primary antibodies is added to quantify the results. Such ‘sandwich’ assays permit greater specificity than immunoassays based on single antibodies since duplicated recognition steps with two antibodies that bind two distinct epitopes successfully reduce cross-reactivity.49
We are interested to establish whether protein microarray technology could be used to detect antibodies produced as a result of blood-doping through EPO abuse. To achieve this goal, rHuEPO was immobilised onto the surface of nitrocellulose-coated (FAST™) microarray slides to act as the capture molecule in the assay. The assay was tested by incubation with solutions, including serum, spiked with the anti-HuEPO antibody. Subsequent incubation of the microarray with a fluorescently tagged secondary antibody fragment enabled the detection of the anti-HuEPO antibodies through fluorescence imaging.
Experimental
Reagents
The three brands of rHuEPO used, Eprex®, Aranesp® and NeoRecormon® were obtained from Janssen-Cilag, Amgen and Roche respectively. Anti-HuEPO antibody (purified rabbit IgG) was purchased from R&D Systems (Oxford, UK). Secondary antibodies, goat anti-rabbit (Fab)2 fragments, tagged with either Alexa Fluor 488 or Alexa Fluor 546 were purchased from Invitrogen (Paisley, UK). Rabbit serum was purchased from B&K Universal (Hull, UK). Human serum albumin (HSA), insulin, myoglobin and haemoglobin were purchased from Sigma-Aldrich (Gillingham, UK). All aqueous solutions were prepared using analytical reagent grade water. FAST™ slides (16-pad), a FAST™ frame and FAST™ PAK which included arraying buffer, blocking buffer, wash buffer and incubation chambers were purchased from Whatman (Kent, UK).Preparation of the rHuEPO microarray
Eprex®-1000 IU (50 μL, 0.53 μM) was mixed with an equal volume of supplied 2x protein arraying buffer. Four spots of the Eprex®-1000 IU were applied onto two of the nitrocellulose pads of a FAST™ slide (four spots on each pad) using a pin removed from a manual arraying device. Each arrayed spot contained the same rHuEPO concentration. This procedure was repeated with Aranesp® (1.08 μM) and NeoRecormon® (0.74 μM) and also with four control proteins, insulin (0.86 μM), myoglobin (0.86 μM), haemoglobin (0.74 μM) and human serum albumin (0.75 μM). The two pads in the middle of the slide were not arrayed with protein in order to separate the rHuEPO preparations from the control proteins. The slide was stored overnight at room temperature to optimise binding of the proteins to the nitrocellulose surface.Addition of anti-HuEPO antibody to the rHuEPO microarray
An incubation chamber was applied to the slide and pressed firmly to form a secure seal. The incubation chamber and slide were placed in the FAST™ frame to hold the pair together. Blocking buffer (80 μL) was added to each well and left for 45 min. After blocking, excess buffer was removed by inverting the FAST™ frame. Anti-HuEPO antibody (80 μL, 740 nM) was added to each well and left to incubate for 90 min. The antibody sample was removed and wash buffer (80 μL) was added to each well and the slide washed three times. A 1 μg mL−1 solution of the secondary antibody tagged with Alexa Fluor 488 (2 μL) in wash buffer (1278 μL) was prepared. A 1 μg mL−1 solution of the secondary antibody tagged with Alexa Fluor 546 was also prepared. 80 μL of Alexa Fluor 488-tagged secondary antibody was added to each chamber on the left hand side of the slide and 80 μL of Alexa Fluor 546-tagged secondary antibody was added to each chamber on the right hand side of the slide, as schematically shown in Fig. 1. The slide was covered with foil and left to incubate for 40 min. The residual secondary antibody solution was removed and the washing step repeated. Once completed, the slide was removed from the chamber, rinsed with a small amount of water and air dried until the membrane appeared white. The slide was imaged using the fluorescence function of a Zeiss SteREO Lumar V12 microscope (Welwyn Garden City, UK) and also a Perkin Elmer ScanArray GXPLUS. To image the Alexa Fluor 488 side of the slide, a Green Fluorescent Protein (GFP) filter (ex: 489 nm, em: 509 nm) was used. To image the Alexa Fluor 546 side of the slide, a Cy3 filter (ex: 549 nm, em: 562 nm) was used. | ||
| Fig. 1 Schematic diagram illustrating the ‘sandwich’ assay using the recombinant HuEPO microarray for the detection of anti-HuEPO antibodies. The slides used for the microarray contained 16 separate pads, on each of which four separate spots were arrayed (the figure only shows 12 of the 16 pads). | ||
Fluorescence intensity as a function of rHuEPO concentration
The effect of varying the spotted rHuEPO concentration was investigated by preparing and arraying four concentrations (0.1 μM to 0.4 μM) of Eprex® (1000 IU) and NeoRecormon® and six concentrations (0.1 μM to 0.6 μM) of Aranesp®. Anti-HuEPO antibody (740 nM) was added to each well followed by the fluorescently tagged Alexa Fluor 488 secondary antibody as described above.Variation of anti-HuEPO antibody concentrations
Anti-HuEPO antibody concentrations were varied to investigate the lowest concentration at which a signal from the bound fluorescently-tagged secondary antibody could be detected. Eprex® (1000 IU) was arrayed on a slide and eight concentrations of anti-HuEPO antibody, ranging from 148 pM to 370 nM, were incubated on two pads each of the slide. Alexa Fluor 488- and Alexa Fluor 564-tagged secondary antibody was then incubated on the left and right side of the microarray respectively. The experiment was repeated for Aranesp®, NeoRecormon® and a further Eprex® sample at a concentration of 10,000 IU (5.3 μM).rHuEPO protein–antibody specificity
Eprex® (1000 IU) was arrayed on a slide and incubated with a primary antibody of anti-BSA (raised in mouse) followed by an Alexa Fluor 488 fluorescently tagged secondary antibody (goat anti-mouse) (1 μg mL−1). Eprex® (1000 IU) was arrayed on a further slide. However on this occasion the pads were not incubated with a primary antibody but only with the fluorescently tagged secondary antibody fragments; Alexa Fluor 488 Goat Anti-Rabbit and Alexa Fluor 488 Goat Anti-Mouse. This was repeated for the Aranesp® and NeoRecormon® brands of rHuEPO.rHuEPO microarray for the detection of anti-HuEPO antibody in the presence of serum
Eprex® (10,000 IU) and Aranesp® were arrayed on slides. Initially, a range of dilutions of rabbit serum (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) were prepared with phosphate buffer prior to being spiked with anti-HuEPO antibody (200 nM). These dilutions were incubated for 90 min together with a control of undiluted rabbit serum also spiked with anti-HuEPO antibody (200 nM). The slides were then incubated with the fluorescently tagged Alexa Fluor 488 secondary antibody fragment for 40 min. The range of dilutions was then further narrowed following this initial experiment so that dilutions of 1
20) were prepared with phosphate buffer prior to being spiked with anti-HuEPO antibody (200 nM). These dilutions were incubated for 90 min together with a control of undiluted rabbit serum also spiked with anti-HuEPO antibody (200 nM). The slides were then incubated with the fluorescently tagged Alexa Fluor 488 secondary antibody fragment for 40 min. The range of dilutions was then further narrowed following this initial experiment so that dilutions of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 of the rabbit serum were prepared which were again spiked with anti-HuEPO antibody (200 nM).
7 of the rabbit serum were prepared which were again spiked with anti-HuEPO antibody (200 nM).
Results and discussion
Recombinant proteins can be immobilised in a microarray format to probe sera for the presence of specific antibodies as shown schematically in Fig. 1. Microarrays were constructed by spotting the rHuEPO proteins (Eprex®-1000 IU, Aranesp® and NeoRecormon®) as the antigen onto nitrocellulose-coated (FAST™) glass slides. Subsequently, the following solutions were added to the protein arrays: (a) Positive controls—where solutions contained the primary antibody (anti-HuEPO antibody); (b) Negative controls—where solutions did not contain anti-HuEPO antibody; or (c) Samples of serum that had been spiked with anti-HuEPO antibody. Addition of the detection probe, a fluorescently tagged secondary antibody fragment, completed the assay after which the presence of the anti-HuEPO antibody analyte was readily visualised using a fluorescence microscope and/or a microarray scanner.rHuEPO microarray for the detection of anti-HuEPO antibody
A microarray slide was arrayed with three pharmaceutical preparations of rHuEPO (Eprex®-1000 IU, Aranesp® and NeoRecormon®) and four ‘control’ proteins (insulin, myoglobin, haemoglobin and human serum albumin). After incubation with anti-HuEPO antibody (740 nM), followed by a fluorescently tagged secondary probe antibody fragment (Alexa Fluor 488 on the left-hand side and Alexa Fluor 546 on the right-hand side), the slide was imaged using an array scanner. The microarray slides were each scanned twice, first at a wavelength of 489 nm for the Alexa Fluor 488 and secondly at 549 nm for the detection of Alexa Fluor 546 tagged secondary antibodies. Once both scans had been processed, a composite image of the entire microarray slide was obtained. Typical results are shown in Fig. 2. Fluorescent signals, i.e., sets of 4 green or red spots were only detected on the FAST pads which had been arrayed with the three brands of rHuEPO. It should be noted that signal strength varied with rHuEPO concentration. The most intense signal was observed with Aranesp®, the preparation with the highest rHuEPO protein concentration. The least intense signal was observed on the pads arrayed with Eprex®-1000 IU, the preparation provided at the lowest concentration of rHuEPO. The fluorescent signal obtained from the microarray was due to the specific interaction between the arrayed rHuEPO and the anti-HuEPO antibody and the subsequent interaction between the anti-HuEPO antibody and the secondary detection antibody fragment tagged with either the green or red fluorophores. While 4 spots can be clearly seen using the Eprex®-1000 IU protein and the Alexa-Fluor 546 tagged secondary antibody fragment (Fig. 2b), only 3 spots are apparent when the Alexa-Fluor 488 tagged secondary antibody was used (Fig. 2a). This was likely to be due to a combination of the low concentration of the rHuEPO in the Eprex®-1000 IU and the less intense fluorescent signal obtained for the latter fluorophore. No fluorescence signal was observed on the FAST pads that had been arrayed with any of the control proteins as there was no interaction between these proteins and the anti-HuEPO antibody. | ||
| Fig. 2 ScanArray images showing the fluorescence detection of anti-HuEPO antibody using the three pharmaceutical preparations of rHuEPO and four control proteins deposited on a microarray. The images are of: (a) the left hand side of the microarray incubated with the Alexa Fluor 488-tagged secondary antibody fragment; (b) the right hand side of the microarray incubated with the Alexa Fluor 546-tagged secondary antibody fragment; and (c) the composite image of the whole microarray. | ||
It was found that the FAST™ slides used throughout these experiments displayed excellent stability both after arraying the rHuEPO and also following incubation with the fluorescently tagged secondary antibodies. An undiminished signal was detected on an array slide that had been constructed one month prior to antibody detection. Additionally, no obvious decrease in signal was observed when the slides were imaged using the ScanArray up to three months after original fluorescent detection, with the microarrays stored in the dark at room temperature in a non-humid environment.
Protein/antibody specificity
To further establish the rHuEPO protein/antibody specificity, anti-BSA (from mouse) was incubated followed by the subsequent incubation of its fluorescently-tagged species-specific corresponding antibody (Alexa Fluor 488, Goat Anti-Mouse) on a microarray spotted with the three isoforms of rHuEPO. The anti-BSA antibody is not specific for, and therefore, should not bind to rHuEPO. As expected, no fluorescent signal was detected with any of the rHuEPO preparations (data not shown).The second part of this investigation was to determine whether there was any non-specific binding occurring between the arrayed proteins and the secondary detection antibody. For this study, incubation with a primary antibody was omitted. Instead, after arraying with the three brands of rHuEPO, one side of the array was incubated with Alexa Fluor 488 Goat Anti-Rabbit secondary antibody and on the other side with Alexa Fluor 488 Goat Anti-Mouse secondary antibody. No fluorescent signal was detected on any of the FAST™ pads demonstrating that non-specific binding of the secondary detection antibody did not occur (images not shown).
Effect of varying the arrayed concentration of rHuEPO
A range of concentrations of Aranesp® (0.1 μM to 0.6 μM) were arrayed to determine the effect of the rHuEPO concentration on the detection of anti-HuEPO antibody (740 nM). As expected, by decreasing the concentration of the Aranesp®, a progressively reduced intensity of fluorescence signal was obtained. Indeed, when the as supplied concentration of Aranesp® (1.08 μM) was used to detect the anti-HuEPO antibody via the fluorescently tagged secondary antibody, a more intense fluorescent signal was obtained when compared to the diluted (0.1 μM to 0.6 μM) rHuEPO protein samples. Similar results were found using Eprex®-1000 IU and NeoRecormon®. While the anti-HuEPO antibody was detected using all concentrations (0.1 μM to 0.4 μM) of spotted Eprex®-1000 IU and NeoRecormon®, the fluorescence intensity was the most intense at the higher rHuEPO concentrations. Consequently, all further studies were carried out using the rHuEPO preparations without dilution.Effect of varying anti-HuEPO antibody concentration
The lowest concentration of the anti-HuEPO antibody that could be detected based on the fluorescence sandwich assay was investigated. When the microarray, based on Eprex®-1000 IU, was imaged using the fluorescence microscope, the lowest concentration of anti-HuEPO antibody at which a fluorescent signal was detected was 74 nM. At this concentration of anti-HuEPO antibody (74 nM), the fluorescent signal detected was extremely weak. However, when the microarray was imaged using the sensitive ScanArray instrument, the fluorescent signal was more intense enabling the detection of significantly lower concentrations of anti-HuEPO antibody. As shown in Fig. 3, using the Array scanner instrument for the imaging of the Eprex®-1000 IU microarrays, the lowest concentration of anti-HuEPO antibody which could be detected was 14.8 nM. | ||
| Fig. 3 (Left) ScanArray images of an Eprex®-1,000 IU microarray following incubation with varying concentrations of anti-HuEPO antibody. (Right) An enlargement of the pad incubated with 14.8 nM anti-HuEPO antibody with the fluorescent spots circled. | ||
As described above, the intensity of the fluorescent signal from the sandwich assay declined as the concentration of the rHuEPO capture molecule decreased. To enhance the limit of detection a rHuEPO preparation of Eprex®-10,000 IU (5.3 μM) was used in the fabrication of a microarray. This 10-fold more concentrated Eprex® sample enabled the detection of significantly lower concentrations of the anti-HuEPO antibody. When imaged with the Array Scanner, the sensitivity of the assay improved 100-fold, the lowest concentration of anti-HuEPO antibody detected was 148 pM as shown in Fig. 4. Results using both fluorescently tagged secondary antibody fragments (i.e. Alexa Fluor 488 and Alexa Fluor 546) have been reproduced to show the clarity of the images. However, at this low anti-HuEPO concentration, the images with optimum clarity were obtained using the secondary antibody fragment tagged with Alexa Fluor 546.
 | ||
| Fig. 4 ScanArray images of an Eprex® 10,000 IU microarray following incubation with varying concentrations of anti-HuEPO antibody. The green and red fluorescent images are the pads incubated with Alexa Fluor 488 and Alexa Fluor 546 –tagged secondary antibody fragments respectively. The pads incubated with 148 pM anti-HuEPO antibody have been enlarged and the fluorescent spots have been circled. | ||
Serial dilution of serum spiked with anti-HuEPO antibody
Background fluorescence from endogenous proteins could potentially be a significant problem when attempting to detect rHuEPO antibodies in serum samples. As the rHuEPO antibody used in this study was originally raised in rabbits, samples of rabbit serum were serially diluted with buffer prior to being spiked with anti-HuEPO antibodies (200 nM). In preliminary studies, a wide range of dilutions of rabbit serum (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 to 1
20 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used. These studies were carried out using Eprex® (10,000 IU) and Aranesp® microarrays as these rHuEPO preparations had provided the optimum fluorescence signal at low anti-HuEPO antibody concentrations. Fluorescent images of microarrays spotted with Eprex® (10,000 IU) are shown in Fig. 5. Fluorescent spots were observed on all of the pads arrayed with the recombinant EPO protein, confirming the successful detection of the anti-HuEPO antibodies (Fig. 5 a–h). However, whilst background fluorescence did appear on pads incubated with 1
1) was used. These studies were carried out using Eprex® (10,000 IU) and Aranesp® microarrays as these rHuEPO preparations had provided the optimum fluorescence signal at low anti-HuEPO antibody concentrations. Fluorescent images of microarrays spotted with Eprex® (10,000 IU) are shown in Fig. 5. Fluorescent spots were observed on all of the pads arrayed with the recombinant EPO protein, confirming the successful detection of the anti-HuEPO antibodies (Fig. 5 a–h). However, whilst background fluorescence did appear on pads incubated with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diluted and undiluted rabbit serum, fluorescent spots where the anti-HuEPO antibodies had bound to the rHuEPO were still clearly visible against this background on the 1
2 diluted and undiluted rabbit serum, fluorescent spots where the anti-HuEPO antibodies had bound to the rHuEPO were still clearly visible against this background on the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diluted sample (Fig. 5g).
2 diluted sample (Fig. 5g).
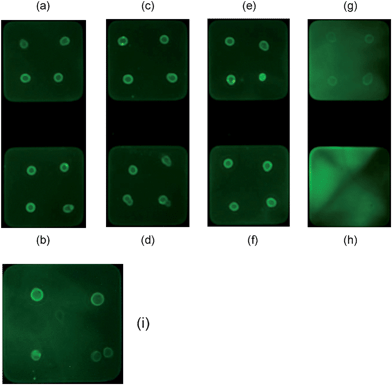 | ||
Fig. 5 Fluorescent images of Eprex® (10,000 IU)-microarray to assess the effect of serial dilution of rabbit serum for the detection of anti-HuEPO antibody. Dilutions are: (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; (b) 1 20; (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15; (c) 1 15; (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12; (d) 1 12; (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; (e) 1 10; (e) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8; (f) 1 8; (f) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; (g) 1 5; (g) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (h) undiluted; and (i) 1 2; (h) undiluted; and (i) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. 4. | ||
The range of serum dilution was further reduced (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 1
7 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to determine the dilution at which no background fluorescence was observed but at which anti-HuEPO antibodies could be readily detected. It was found that 1
2) to determine the dilution at which no background fluorescence was observed but at which anti-HuEPO antibodies could be readily detected. It was found that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was the optimum dilution of the rabbit serum as there was no background fluorescence but fluorescent spots showing the detection of anti-HuEPO antibodies could clearly be seen (Fig. 5i). There does not appear to be any background fluorescence in a 1
4 was the optimum dilution of the rabbit serum as there was no background fluorescence but fluorescent spots showing the detection of anti-HuEPO antibodies could clearly be seen (Fig. 5i). There does not appear to be any background fluorescence in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 dilution sample (not shown) but the fluorescent signal detecting anti-HuEPO antibodies appeared weak. Therefore, 1
3 dilution sample (not shown) but the fluorescent signal detecting anti-HuEPO antibodies appeared weak. Therefore, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was determined to be the optimal dilution for detection of the anti-HuEPO antibody in serum.
4 was determined to be the optimal dilution for detection of the anti-HuEPO antibody in serum.
Conclusions
A glycoprotein microarray has been developed for the detection of anti-HuEPO antibodies which are produced as a result of rHuEPO abuse. Preliminary investigations of anti-HuEPO antibody-protein interactions demonstrated that non-specific interactions between anti-HuEPO antibody and four control proteins (insulin, myoglobin, haemoglobin and HSA) did not occur when used at concentrations similar to those of the rHuEPO preparations. However, a fluorescent signal was observed when rHuEPO preparations, Eprex®, Aranesp® and NeoRecormon®, were arrayed as the capture molecules demonstrating a specific interaction between the anti-HuEPO antibody and the recombinant EPO glycoprotein. Investigations into protein/antibody specificity with anti-BSA demonstrated that non-specific interactions between rHuEPO and a control antibody did not occur. rHuEPO was also incubated with fluorescently tagged secondary antibodies without a primary antibody to establish whether non-specific binding occurred between the proteins and detection antibodies. As no fluorescent signal was observed these studies confirmed that the rHuEPO microarrays are capable of specifically detecting the presence of anti-HuEPO antibody. Investigations involving the serial dilution of rabbit serum were undertaken to overcome the appearance of background fluorescence from endogenous proteins with detection of the anti-HuEPO antibodies in a biological matrix. Background fluorescence was only observed in samples of undiluted rabbit serum and serum that had been 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diluted, although in the latter instance the fluorescent signals indicating the presence of anti-HuEPO antibody were clearly visible despite the background. It was determined that a 1
2 diluted, although in the latter instance the fluorescent signals indicating the presence of anti-HuEPO antibody were clearly visible despite the background. It was determined that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio was the optimum dilution level for the detection of anti-HuEPO antibodies in rabbit serum. By spotting the highest concentration of commercially available rHuEPO (Eprex®-10,000 IU) on the microarray, a concentration of anti-HuEPO antibody at 148 pM was fluorecently detected using the array scanner. However, the ultimate sensitivity of this method can only be determined when the microarrays are produced using automated techniques rather than manual spotting. This study suggests that the developed glycoprotein microarray could be applied for the detection of antibodies produced in response to illicit doping with recombinant human EPO preparations.
4 ratio was the optimum dilution level for the detection of anti-HuEPO antibodies in rabbit serum. By spotting the highest concentration of commercially available rHuEPO (Eprex®-10,000 IU) on the microarray, a concentration of anti-HuEPO antibody at 148 pM was fluorecently detected using the array scanner. However, the ultimate sensitivity of this method can only be determined when the microarrays are produced using automated techniques rather than manual spotting. This study suggests that the developed glycoprotein microarray could be applied for the detection of antibodies produced in response to illicit doping with recombinant human EPO preparations.
Acknowledgements
The authors are grateful to the BBSRC for a CASE studentship for SMH and to the British Horseracing Authority (BHA) for financially supporting this research. Additionally, the authors would like to thank Professor Ten Feizi (Imperial College, London, UK) for access to her laboratory facilities and specifically her ScanArray instrument.References
- D. Choi, M. Kim and J. Park, J. Chromatogr., B: Biomed. Sci. Appl., 1996, 687, 189–199 CrossRef CAS.
- P. Lai, R. Everett, F. Wang, T. Arakawa and E. Goldwasser, J. Biol. Chem., 1986, 261, 3116–3121 CAS.
- K. Jacobs, C. Shoemaker, R. Rudersdorf, S. D. Neill, R. J. Kaufman, A. Mufson, J. Seehra, S. S. Jones, R. Hewick, E. F. Fritsch, M. Kawakita, T. Shimizu and T. Miyake, Nature, 1985, 313, 806–810 CAS.
- F. K. Lin, S. Suggs, C. H. Lin, J. K. Browne, R. Smalling, J. C. Egrie, K. K. Chen, G. M. Fox, F. Martin, Z. Stabinsky, S. M. Badrawi, P. H. Lai and E. Goldwasser, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 7580–7584 CrossRef CAS.
- H. M. E. Azzazy, M. M. H. Mansour and R. H. Christenson, Clin. Biochem., 2005, 38, 959–965 CrossRef CAS.
- J. M. Urra, M. de la Torre, R. Alcazar and R. Peces, Clin. Chem., 1997, 43, 848–849 CAS.
- C. Breymann, Best Pract. Res. Clin. Endocrinol. Metab., 2000, 14, 135–145 CrossRef CAS.
- F. Locatelli, L. Del Vecchio and P. Marai, Contrib. Nephrol., 2002, 137, 403–407 Search PubMed.
- I. C. Macdougall, Kidney Int., 2002, 61, S55–S61.
- G. Zorpette, Scientific American, 2000, 282, 20 CAS.
- J. J. M. Marx and P. C. J. Vergouwen, Lancet, 1998, 352, 451 CrossRef CAS.
- C. A. N. Jarvis, Br. J. Sports Medicine, 1999, 33, 142–143 Search PubMed.
- J. Roberts, Proc. 14th Intl. Conf. Racing Analysts and Vets, 1999, 234–242 Search PubMed.
- K. H. McKeever, Proc. 11th Intl. Conf. Racing Analysts and Vets, 1996, 79–84 Search PubMed.
- B. Berglund and B. Ekblom, J. Intern. Med., 1991, 229, 125–130 CrossRef CAS.
- B. Ekblom, Drug Abuse Handbook, CRC Press, Florida, 1998, pp 710–720 Search PubMed.
- M. Audran, R. Gareau, S. Matecki, F. Durand, C. Chenard, M. T. Sicart, B. Marion and F. Bressolle, Med. Sci. Sports Exercise, 1999, 31, 639–645 Search PubMed.
- B. Ekblom and B. Berglund, Scand. J. Med. Sci. Sports, 1991, 1, 88–93.
- S. Persson, Acta Veterinaria Scandinavica, 1967, S19, 1–189 Search PubMed.
- J. W. Adamson and D. Vapnek, New Eng. J. Med., 1991, 324, 698–699 CAS.
- P. R. Woods, G. Campbell and R. L. Cowell, Eq. Vet. J., 1997, 29, 326–328 Search PubMed.
- I. Casoni, G. Ricci, E. Ballarin, C. Borsetto, G. Grazzi, C. Guglielmini, F. Manfredini, G. Mazzoni, M. Patracchini, E. D. Vitali, F. Rigolin, S. Bartalotta, G. P. Franze, M. Masotti and F. Conconi, Int. J. Sports Med., 1993, 14, 307–311 CrossRef CAS.
- R. Gareau, M. Audran, R. D. Baynes, C. H. Flowers, A. Duvallet, L. Senecal and G. R. Brisson, Nature, 1996, 380, 113–113 CrossRef CAS.
- F. Lasne and J. de Ceaurriz, Nature, 2000, 405, 635–635 CrossRef CAS.
- F. Lasne, L. Martin, N. Crepin and J. de Ceaurriz, Anal. Biochem., 2002, 311, 119–126 CrossRef CAS.
- L. Wide, C. Bengtsson, B. Berglund and B. Ekblom, Med. Sci. Sports Exerc., 1995, 27, 1569–1576 Search PubMed.
- A. Souillard, M. Audran, F. Bressolle, R. Gareau, A. Duvallet and J. L. Chanal, Br. J. Clin. Pharmacol., 1996, 42, 355–364 CrossRef CAS.
- A. Breidbach, D. H. Catlin, G. A. Green, I. Tregub, H. Truong and J. Gorzek, Clin. Chem., 2003, 49, 901–907 CrossRef CAS.
- F. O. Lasne, M. A. Popot, E. Varlet-Marie, L. Martin, J. A. Martin, Y. Bonnaire, M. Audran and J. De Ceaurriz, J. Anal. Toxicol., 2005, 29, 835–837 CAS.
- F. Guan, C. E. Uboh, L. R. Soma, E. Birks, J. Chen, J. Mitchell, Y. You, J. Rudy, F. Xu, X. Li and G. Mbuy, Anal. Chem., 2007, 79, 4627–4635 CrossRef CAS.
- F. Guan, C. E. Uboh, L. R. Soma, E. Birks, J. Chen, Y. You, J. Rudy and X. Li, Anal. Chem., 2008, 80, 3811–3817 CrossRef CAS.
- R. Tacey, A. Greway, J. Smiell, D. Power, A. Kromminga, M. Daha, N. Casadevall and M. Kelley, J. Immunol. Methods, 2003, 283, 317–329 CrossRef CAS.
- R. Kientsch-Engel, K. Hallermayer and A. Dessauer, Contrib. to Neph., 1989, 76, 100–101 Search PubMed.
- N. V. Sipsas, S. I. Kokori, J. P. A. Ioannidis, D. Kyriaki, A. Tzioufas and T. Kordossis, J. Infect. Dis., 1999, 180, 2044–2047 CrossRef CAS.
- G. Castelli, A. Famularo, C. Semino, A. M. Machi, A. Ceci, G. Cannella and G. Melioli, Pharmacol. Res., 2000, 41, 313–318 CrossRef CAS.
- S. J. Swanson, J. Ferbas, P. Mayeux and N. Casadevall, Nephron Clin. Pract., 2004, 96, 88–95.
- W. Hoesel, J. Gross, R. Moller, B. Kanne, A. Wessner, G. Muller, A. Muller, E. Gromnica-Ihle, A. Fromme, S. Bischoff and A. Haselbeck, J. Immunol. Methods, 2004, 294, 101–110 CrossRef CAS.
- M. F. Lopez and M. G. Pluskal, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2003, 787, 19–27 CrossRef CAS.
- S. Y. Seong and C. Y. Choi, Proteomics, 2003, 3, 2176–2189 CrossRef CAS.
- H. Zhu, M. Bilgin, R. Bangham, D. Hall, A. Casamayor, P. Bertone, N. Lan, R. Jansen, S. Bidlingmaier, T. Houfek, T. Mitchell, P. Miller, R. A. Dean, M. Gerstein and M. Snyder, Science, 2001, 293, 2101–2105 CrossRef CAS.
- G. MacBeath and S. L. Schreiber, Science, 2000, 289, 1760–1763 CAS.
- M. L. Bulyk, E. Gentalen, D. J. Lockhart and G. M. Church, Nat. Biotechnol., 1999, 17, 573–577 CrossRef CAS.
- M. F. Templin, D. Stoll, J. M. Schwenk, O. Potz, S. Kramer and T. O. Joos, Proteomics, 2003, 3, 2155–2166 CrossRef CAS.
- H. T. Zhu, C. Y. Yu and H. P. Zhang, Proteomics, 2003, 3, 1673–1677 CrossRef CAS.
- P. Angenendt, J. Glokler, J. Sobek, H. Lehrach and D. J. Cahill, J. Chromatogr., A, 2003, 1009, 97–104 CrossRef CAS.
- B. Kersten, T. Feilner, A. Kramer, S. Wehrmeyer, A. Possling, I. Witt, M. I. Zanor, R. Stracke, A. Lueking, J. Kreutzberger, H. Lehrach and D. J. Cahill, Plant Mol. Biol., 2003, 52, 999–1010 CrossRef CAS.
- T. Kukar, S. Eckenrode, Y. R. Gu, W. Lian, M. Megginson, J. X. She and D. H. Wu, Anal. Biochem., 2002, 306, 50–54 CrossRef CAS.
- T. Feilner, Curr. Proteomics, 2004, 1, 293–295 Search PubMed.
- K.-Y. Tomizaki, K. Usui and H. Mihara, ChemBioChem, 2005, 6, 782–799 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
