Differentiation of Bacillus endospore species from fatty acid methyl ester biomarkers
Tai V.
Truong
a,
Aaron N.
Nackos
b,
John R.
Williams
c,
Douglas N.
VanDerwerken
d,
Jon A.
Kimball
a,
Jacolin A.
Murray
a,
Jason E.
Hawkes†
a,
Donald J.
Harvey‡
c,
H. Dennis
Tolley
d,
Richard A.
Robison
c,
Calvin H.
Bartholomew
b and
Milton L.
Lee
*a
aDepartment of Chemistry and Biochemistry, Brigham Young University, Provo, UT 84602, USA. E-mail: milton_lee@byu.edu; Fax: +1-801-422-0157
bDepartment of Chemical Engineering, Brigham Young University, Provo, UT 84602, USA
cDepartment of Microbiology, Brigham Young University, Provo, UT 84602, USA
dDepartment of Statistics, Brigham Young University, Provo, UT 84602, USA
First published on 12th April 2010
Abstract
A simple method to detect and differentiate Bacillus anthracis (BA), Bacillus thuringiensis (BT), Bacillus atrophaeus (BG), and Bacillus cereus (BC) endospores using biomarker compounds, including dipicolinic acid methyl ester (DPAME) and fatty acid methyl esters (FAMEs), has been developed. The method is based on thermochemolysis methylation (TCM) of the endospores and gas chromatography-mass spectrometry (GC-MS) of the reaction products. A suspension of the sample mixed with sulfuric acid (H2SO4) and tetramethylammonium hydroxide (TMAH) in methanol (MeOH) at room temperature is sampled using a coiled wire filament (CWF) device, which consists of a tiny platinum helical wire coil attached to a retractable plunger that moves the coil in and out of a syringe needle housing. Sampling is accomplished by dipping the CWF in an endospore sample suspension, evaporating the suspension liquid, and then introducing the CWF into the injection port. While DPAME can be used for the general detection of endospores, specific saturated and unsaturated C15, C16, and C17 fatty acid methyl esters provide additional information for differentiating various Bacillus species grown at different temperatures and in different media. DPAME could be detected in samples containing as few as 6000 endospores, and the GC-MS peak area percent reproducibility for FAMEs varied from 3 to 13% (RSD). Better than 97% correct predictability of Bacillus species identity was obtained from a blind experiment consisting of 145 samples.
Introduction
Bacillus anthracis (BA) is a gram-positive, rod-shaped bacterium that is a member of the Bacillus cereus group in the genus Bacillus. It is of particular interest because it causes anthrax, a serious and often fatal disease of mammalian livestock and humans. Although more than 400 distinct strains of BA are known to exist, genetic variation of the species is represented by 89 strains,1 ranging from highly virulent (sometimes used for biological warfare and bioterrorism purposes) to benign (those used for vaccines). Vegetative cells of virulent strains produce three exotoxin proteins—the protective antigen, the edema factor, and the lethal factor—and surround themselves with a capsule comprised of poly-D-glutamic acid. BA is the only known bacterium with these virulence factors.2The Bacillus cereus group represents a highly homogenous subdivision that includes the three closely related species, BA, B. cereus (BC), and B. thuringiensis (BT), and the more distantly related B. mycoides and B. weihenstephanensis. BC is a food-borne agent responsible for some 5% of food poisonings in the United States and BT is a commercially available biological larvicide that is harmless to humans.2 Differentiation of these species using molecular assays has been problematic due to their high level of genetic similarity. However, the three closest species of this group each produce unique compounds related to their pathogenesis.
B. atrophaeus (BG) is not closely related to BA; however, it has been used for decades as an important model organism, especially as a nonpathogenic surrogate for BA in bio-weapons research.3,4 Historically, BG has been called different names including B. niger, B. globigii, and B. subtilis.3,4 It is a naturally occurring soil bacterium, capable of producing subtisin, a bacteriocin which kills closely related species or competitive BG strains.2 BA, BC, BT, and BG were selected for study in several recent reports regarding the differentiation of closely related Bacillus species.5,6 Differentiation of BA from its non-lethal relatives is essential for correctly identifying a bioterrorism event and controlling the outbreak of disease resulting therefrom.
Bacterial endospores are differentiated bodies formed in response to nutrient deprivation that are resistant to adverse environmental conditions, including radiation, heat, toxic chemicals, and pH extremes.7,8 Mature spores are almost completely metabolically inactive and have a highly ordered structure that provides the protection required for survival over long periods, even in the face of harsh environmental conditions.9 Endospores are comprised of an inner core (spore core) surrounded by a cytomembrane (core wall), a cortex of peptidoglycan, outer membrane, and an exterior spore coat.10,11 Spores of many bacterial species (including BA, BT, BC, and BG) are surrounded by a loosely attached exosporium.12 A typical desiccated bacterium generally consists of 70% protein, 10% RNA, 5% DNA, 6% lipid, and 5% polysaccharide.13 Any molecule or molecular fragment from these chemical classes that are unique to a particular bacterium can be considered to be a biomarker of that microorganism. DNA and dipicolinic acid (DPA) are contained in the spore core. DPA is present in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chelate with Ca2+ (Ca-DPA), which comprises 5–15% (dry weight) of Bacillus spores.14 Ca-DPA plays a major role in the maintenance of spore dormancy and environmental resistance.15 The Ca-DPA concentration in spores can vary with spore species/strain, cell growth medium, sporulation conditions, and size.14
1 chelate with Ca2+ (Ca-DPA), which comprises 5–15% (dry weight) of Bacillus spores.14 Ca-DPA plays a major role in the maintenance of spore dormancy and environmental resistance.15 The Ca-DPA concentration in spores can vary with spore species/strain, cell growth medium, sporulation conditions, and size.14
While a number of techniques have been reported for general detection of bacterial endospores, most are not applicable for both detection and differentiation at the species level in the field, which requires them to be simple, robust, and portable. Those that are applicable include nucleic acid-based methods such as polymerase chain reaction (PCR),16 various antibody-based methods,17,18 and chemical separation/detection with coupled systems such as gas chromatography-mass spectrometry (GC-MS).19 PCR requires expensive reagents (primers, enzymes, and conjugated fluorophores) and considerable sample processing prior to analysis, and immunoassays require specific antibodies for each desired species.17,18 GC and MS alone can indicate the presence of a biomarker compound in a sample, however, they cannot provide unambiguous detection without being coupled together.
GC-MS analysis of bacteria or bacterial endospores requires rather harsh sample pre-treatment, such as thermal reaction, hydrolysis, extraction, and/or derivatization before analysis. As early as 1990, pyrolysis coupled with GC-MS was employed to detect biomarkers in the field.20 Micro-volume Curie-point pyrolysis GC-MS allowed for characterization of various lipid moieties in microorganisms. Gram-positive bacilli and gram-negative species were discriminated by the pyrolysis patterns of their lipid components.21–23 A hand-portable system comprised of a micro-pyrolyzer, micro GC, and surface acoustic wave (SAW) array detector was recently described for rapidly identifying microorganisms based on detection of fatty acid methyl esters (FAMEs) produced from pyrolysis and tetramethylammonium hydroxide (TMAH) methylation.24 Unfortunately, the SAW detector could not provide conclusive structural identification. A prototype field-portable pyrolysis GC-ion mobility spectrometer was described by Snyder et al., which was more useful than pyrolysis-GC alone, but not as diagnostically useful as when coupled to an MS detector.25
Although a number of methods using GC and/or MS have been reported for the detection of Bacillus endospores, most are not convenient for field application because of instrumentation size, weight, and electrical power requirements, or the methods are not simple and reproducible. Pyrolysis methods suffer from poor reproducibility and harsh reaction conditions that destroy potentially useful biomarker compounds. In this paper, we report a successful approach for fast, simple detection and differentiation of biomarkers of four related Bacillus species based on thermochemolysis methylation (TCM) using a coiled wire filament (CWF) sample introduction device followed by GC-MS. Dipicolinic acid methyl ester (DPAME) and FAME profiles of specific strains of BA, BT, BC, and BG spores grown under various conditions were reproducibly generated and utilized to differentiate these microorganisms from each other.
Experimental
Chemicals and materials
HPLC grade methanol (MeOH) and dichloromethane (CH2Cl2) were obtained from EMD (San Diego, CA, USA). Tetramethylammonium hydroxide pentahydrate (TMAH, > 97%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). H2SO4 (98%) was from Mallinckrodt Chemical (Phillipsburg, NJ, USA). Pt–Ir (90–10) wires (90 μm) were from California Fine Wire (Grover Beach, CA, USA). The Pt–Ir wires were formed into coils by Motion Dynamics Corporation (Fruitport, MI, USA).Endospore growth
The development of a GC-MS methodology for discriminating between Bacillus endospore species consists of three steps: (1) identification of a list of candidate biomarkers, (2) reduction of the list to discriminatory biomarkers, and (3) confirmation that the discriminatory biomarkers are effective. For this process we used three groups of samples. For the first two sample groups (i.e., sample groups 1 and 2), four distinct Bacillus species were cultured: B. anthracis (BA, Sterne 1043), B. thuringiensis var. kurstaki (BT), B. cereus (BC, ATCC 14579), and B. atrophaeus (BG, ATCC 51189). Sample group 3 consisted of 25 samples each of BA and BT weaponized endospores, which were provided by Dugway Proving Ground (DPG), Dugway, UT. Except for the weaponized samples, all endospore suspensions were prepared in a biosafety level 2 facility located on the Brigham Young University campus.The first group included endospores grown at three different temperatures (24, 28 and 32 °C) on four different media (casamino acid, Columbia salt dextrose, Leighton-Doi, and semi-synthetic). Altogether, 600 samples constituted this group. The purpose of this sample group was to optimize the analytical methods as well as to identify potential biomarkers. Our initial TCM methods did not include sulfuric acid treatment. We learned early in this study (i.e., during the analysis of this first group of 600 samples) that addition of sulfuric acid greatly improved the yield of DPAME, while retaining the characteristic FAME profiles. Thereafter, the addition of sulfuric acid became part of the “standard” procedure.
The second sample group was designed to explicitly confirm the validity of the candidate biomarkers obtained from studies using the first sample group. The best sample preparation and analysis conditions for DPAME and FAMEs were selected for the analysis of endospores in this group using the results obtained from the analysis of sample group 1. In addition, this group was designed to examine if the candidate biomarkers were robust against changes in incubation temperature and/or growth media, neither of which would be known in the field. Samples of each spore type were prepared by incubation at either 32 or 37 °C on agar plates containing either Leighton-Doi or Columbia salt dextrose growth media (LD and Col, respectively). Altogether, there were 270 samples prepared for this group.
The purity of each organism was verified by isolation plating on Columbia agar plates followed by gram staining and inspection under a microscope by phase-contrast microscopy (Zeiss Axioskop 2 equipped with an AxioCam HRc digital camera, Göttingen, Germany). LD and Columbia salt dextrose plates were then inoculated with an isolated colony from the Columbia agar plates. Spore cultures were grown for approximately 10 days in an incubator at 32 or 37 °C. Additional microscope inspection ensured sufficient sporulation, that is, the spore to vegetative cell ratio was greater than 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, before the spores were harvested. Sporulation of BC was not very successful on Columbia media at 37 °C, so these samples were not analyzed. On the other hand, even though sporulation of BC on Columbia media at 32 °C was not as successful as for the other Bacillus species, it was deemed sufficient to include these spores in the study.
1, before the spores were harvested. Sporulation of BC was not very successful on Columbia media at 37 °C, so these samples were not analyzed. On the other hand, even though sporulation of BC on Columbia media at 32 °C was not as successful as for the other Bacillus species, it was deemed sufficient to include these spores in the study.
Spores were collected from culture plates and suspended in 10 mL of autoclaved HPLC water in 50-mL polypropylene centrifuge tubes. The spore suspensions were then placed in a 65 °C water bath (LAUDA-Brinkman RM20, Delran, NJ) for 45 min to kill any remaining vegetative cells. The endospores were separated from vegetative debris by centrifugation (Beckman GS-15R centrifuge, Brea, CA) at 3800 ×g for 10 min, followed by removal of the supernatant containing the vegetative debris. The remaining endospore pellet was then re-suspended in autoclaved HPLC water. This process (centrifugation and re-suspension, i.e., “washing”) was repeated daily for three days with suspensions being stored at 4 °C between washings. The endospore concentration was then determined by direct counting (Bright-Line hemacytometer, Horsham, PA). From the final spore suspensions, 1-mL samples were placed in 1.5-mL Eppendorf tubes and centrifuged at 16,000 ×g (Eppendorf 5415 C, Brinkman, Westbury, NY) for 4 min. The supernatant was removed and the Eppendorf tubes containing spore pellets were prepared for analysis.
Bacillus endospore sample preparation
In the final TCM procedure, centrifuged endospore pellets in polypropylene Eppendorf tubes (containing between 108 and 1010 spores) were re-suspended by adding 200 μL acidic MeOH (1% v/v H2SO4). The tubes were then vortexed or shaken well for 1 min to ensure total suspension. Then, 40 μL of 2.0 M TMAH (in MeOH) and 40 μL of internal standard (50 ppm chrysene in MeOH–CH2Cl2, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) were added. The solution/suspension was well mixed prior to sampling for GC-MS analysis. All sample preparation steps were performed at room temperature, and the volumes and concentrations of reagents were proportionally adjusted at times to accommodate some variation in the concentrations of samples. This procedure was used for the later studied samples in group 1 and all of the samples in group 2. The initial samples analyzed in group 1 and the weaponized samples comprising group 3 were analyzed before it was determined that adding sulfuric acid improved biomarker yields, especially DPAME. Otherwise, the methods were nearly the same, and produced similar distributions of biomarkers.
1 v/v) were added. The solution/suspension was well mixed prior to sampling for GC-MS analysis. All sample preparation steps were performed at room temperature, and the volumes and concentrations of reagents were proportionally adjusted at times to accommodate some variation in the concentrations of samples. This procedure was used for the later studied samples in group 1 and all of the samples in group 2. The initial samples analyzed in group 1 and the weaponized samples comprising group 3 were analyzed before it was determined that adding sulfuric acid improved biomarker yields, especially DPAME. Otherwise, the methods were nearly the same, and produced similar distributions of biomarkers.
Coiled wire filament sample introduction
A CWF (Fig. 1) was used both for sample introduction into the GC-MS system and for TCM reaction. Sampling was accomplished by dipping the CWF in the sample suspension. Details of the CWF and its use for TCM of microorganisms were recently published.26 A major advantage of this sampling device is that nonvolatile sample matrix components remained on the wire coil after the sample was heated, reducing the required injection port and liner cleaning frequency and lessening contamination of the head of the chromatographic column. The coil itself was easily cleaned between analyses by washing with a suitable solvent and/or burning off residues in a small flame.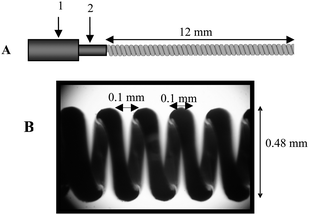 | ||
| Fig. 1 (A) Schematic drawing of a coiled wire filament. The wire filament is held in place by a wire filament socket (2), which may be extended from, or retracted inside, an SPME needle (1). (B) Photograph of an actual coiled wire filament produced by deflection coiling. | ||
The CWF helped to improve the reaction yield by concentrating the reagents and target compounds into a small volume on the filament. In addition, the use of a narrow i.d. liner (0.75 mm) also helped to improve the reaction efficiency as well as reaction velocity. Target compound vapors were transported to the column faster in a narrow i.d. liner compared to a conventional liner.
GC-MS
An Agilent 6890 gas chromatograph (Agilent, San Jose, CA) with split/splitless injector was fitted with a 0.75 mm i.d. Restek liner. The GC was coupled to an Agilent 5973 MS with electron ionization and quadrupole analyzer. Helium was used as carrier gas. Two fused silica capillary columns were used in this study: 30 m × 250 μm i.d. × 0.25 μm film thickness (column A, DB-5, J&W Scientific, Folsom, CA) and 10 m × 100 μm i.d. × 0.4 μm film thickness (column B, Rtx-5, Restek, Bellefonte, PA). Typical chromatographic operating parameters included 290 °C injector temperature, 270 °C transfer line temperature between the GC and MS, and column temperature programs and inlet pressures as indicated in the figure legends. Even though the endospores samples were basic (i.e., pH 9–10) after addition of TMAH, hundreds of runs could be performed without any noticeable column deterioration. Relative retention times of separated compounds were computed with reference to the internal standard, chrysene.The MS operating parameters for all experiments were: 230 °C quadrupole temperature, 150 °C source temperature, 30–500 m/z mass range, 1670 EM voltage, 35 μA emission current, and 70 eV ionizing voltage. Extracted ions m/z 74 and 137 were used to reconstruct the FAME and DPAME chromatograms, respectively. For SIM, m/z 74 and 87 ions were used for FAMEs, and m/z 137, 138 and 139 ions were used for DPAME.
Results and discussion
Repeatability and detection limits
The repeatability of the new TCM method was examined by repeated injections of a BT endospore (Col, 32 °C) sample. Data from 10 replicates were used to calculate the percentages of total peak areas for 9 representative FAMEs. The results indicate that the method is quantitatively repeatable (see Table 1), which can be attributed to: (1) well-controlled TCM reaction conditions, (2) consistent volume of sample taken up by the CWF due to capillary action, (3) constant drying time, and (4) use of an internal standard. The addition of methanolic H2SO4 prior to TMAH helped in the extraction of DPA from the Ca-DPA complex in the spores and/or hydrolysis of the spore structure.27 Furthermore, H2SO4 is a catalyst for methylation and a water-scavenging chemical that may help to release substances from the spores. The detection limit for DPAME was approximately 6000 endospores using selected-ion-monitoring (SIM, m/z 137, 138, and 139 ions). This is one to three orders of magnitude higher than the detection limits of PCR, which varies from ten to a few hundred endospores.28–31 The GC-MS detection limit depended on bacteria species, growth conditions (i.e., growth medium and temperature), state of endospore dehydration, and whether total-ion-current (TIC, m/z 137 extracted ion) or SIM was used.| FAME | Mean Area (%) | Std. Dev. |
|---|---|---|
| i14:0 | 7.5 | 0.4 |
| n14:0 | 6.7 | 0.3 |
| i15:0 | 33.8 | 1.5 |
| a15:0 | 9.1 | 0.3 |
| n15:0 | 9.7 | 0.3 |
| n16:1 | 3.7 | 0.2 |
| n16:0 | 8.1 | 1.1 |
| i17:0 | 14.4 | 0.5 |
| n18:0 | 2.8 | 0.2 |
Differentiation of Bacillus endospore species
DPA is unique to endospore-forming bacteria, the most familiar examples being Bacillus and Clostridium. The presence of DPAME indicates the likely presence of at least one of these microorganisms in the sample being analyzed. Unfortunately, DPA alone is not diagnostic beyond indicating the general presence of spores. Our approach relies on detection and differentiation based on the presence, absence, and intensities of both DPAME and FAMEs.Statistical methods such as principal component analysis (PCA), cluster analysis, or classification and regression trees (known as CART) have often been used to identify the most predictive sources of variance among different Bacillus species.6 Much of the variability in our samples could be attributed to different growth, sample preparation, and analysis conditions. Rather than using sophisticated empirical pattern recognition methods, which would necessitate adjustment for experimentally induced variation, we utilized expert elicitation coupled with a survey of the literature described below.32 While purely statistical methods use all of the empirical information in the sample, they do not readily incorporate known scientific relationships. Elicitation, on the other hand, allows for utilization of expertise, which implicitly incorporates previous experience as well as data obtained from the current sample. It is essential to confirm candidate biomarkers obtained by elicitation using an independent set of experiments.
Fig. 2 and Table 2 indicate the FAMEs selected for detection (i.e., DeFAMEs) and FAMEs selected for differentiation (i.e., DiFAMEs), of the four Bacillus species in sample group 2. Of these, i13:0 (i = iso), i15:0, i16:1, a17:1 (a = anteiso) FAMEs (absent from the NIST mass spectral library) were predicted based on their intensities, mass spectra, and elution orders, and from comparison of FAMEs detected from previously published papers. These identifications agreed with results obtained by different authors, such as Kaneda.33 The results indicate the general rule (due to biosynthetic mechanisms) that saturated fatty acid pairs differing in 2 carbons, such as i15:0 and i17:0, and a15:0 and a17:0, are always present in lipids of bacteria, although they may have different abundances in different species.33 Kaneda also confirmed the presence of i13:0, a13:0, and a17:1 in lipids of BC by GC analysis.34 Kämpfer35 and Väisänen and M. Salkinoja-Salonen36 identified lipids of many different Bacillus species and indicated the presence of almost all fatty acids shown in Table 2. Of these, i16:1 and a16:1 were found in BC, BG, and BT.
 | ||
| Fig. 2 Extracted ion (m/z 74) chromatograms of Bacillus species (BG, BA, BC, and BT) grown in LD medium at 32 °C. Numbered peaks are identified in Table 2. Conditions: column B, temperature programmed from 60 °C to 300 °C at 33 °C min−1, 75 psi He inlet pressure. | ||
In the Bacillus species, some fatty acids such as i15:0, a15:0, i17:0, and a17:0 have especially high intensities that can be used effectively for differentiation from non-Bacillus species. In other words, these fatty acids, similar to DPAME, can be used for detection of Bacillus species. Within Bacillus, various fatty acids were reported to be unique to certain organisms or groups of organisms. For example, Song et al. reported that fatty acid i17:1(Δ7) did not appear in BA but was present in BC, BT, and BG. These results were consistent for the several strains studied of each species.37 Conversely, Whittaker found that a17:1 fatty acid was present in BA, but was substantially less pronounced or non-existent in BC.38 In the same study, Whittaker confirmed the finding of Song et al. that fatty acid i17:1(Δ7) was present in BC but absent from BA.38 It should be noted here, however, that while individual studies tend to be internally consistent regarding intensities of individual FAMEs in the same species, and especially consistent for the same strains, there is generally little agreement between authors. These discrepancies can be at least partially attributed to the strong effect of growth conditions on fatty acid profiles and to the misidentification of unsaturated FAMEs (see ref. 33 for a detailed discussion).
In our research, discriminating biomarkers were found by studying selected-ion chromatograms of m/z 74 for FAMEs and m/z 137 for DPAME. Observations from GC-MS analyses of the presence, absence, and intensities of FAME peaks for the variety of samples analyzed in sample group 1 include: (1) DPAME is an indicator of either Bacillus or Clostridium endospores; (2) n16:1(Δ9) and i16:1 FAMEs are from BA, BC, and BT, but not from BG endospores; (3) a17:1 FAME is from all Bacillus endospores; (4) i16:1 and i17:1 FAMEs are from BC, BG, and BT, but not from BA endospores; (5) i15:0 > a15:0 FAMEs (referring to peak areas) in BA, BC, and BT endospores—the reverse being true for BG; (6) i17:0 > a17:0 FAMEs (referring to peak areas) in BA, BC and BT endospores—the reverse being true for BG; (7) i15:0, a15:0, i17:0, and a17:0 FAMEs are the main components (highest intensity peaks) of all Bacillus species; and (8) 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 FAMEs > 14
0 FAMEs > 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 FAMEs (referring to peak areas) in all Bacillus endospores. From these data, a flow chart for organism differentiation was constructed (Fig. 3) to simplify the discrimination procedure.
0 FAMEs (referring to peak areas) in all Bacillus endospores. From these data, a flow chart for organism differentiation was constructed (Fig. 3) to simplify the discrimination procedure.
 | ||
| Fig. 3 Differentiation flow chart for Bacillus and Clostridium endospores using DPAME and FAMEs. | ||
Generally, BG can be recognized based on the absence of n16:1(Δ9) FAME which exists only in BA, BC, and BT spores. BA is differentiated from BT and BC by the absence of i17:1 FAME. Peak areas of these and other FAMEs, as well as potential sugar biomarkers, are currently being investigated in order to more easily distinguish BC from BT.
One would expect that if such methods allow differentiation between very closely related species, they should also allow differentiation between Bacillus and non-Bacillus species. Clostridium is differentiated from Bacillus by very low intensity (or absence) of i15:0, a15:0, i17:0 and a17:0 FAME peaks compared to the 14:0 and 16:0 FAME groups, although a high intensity DPAME peak is detected in both (data not presented here). We expect similar differentiation ability between Bacillus and other non-Bacillus species.
Confirmation of the biomarkers used to differentiate BA, BG, and BC/BT endospores was accomplished in two ways using the 270 endospore samples from sample group 2: (1) using an automated statistical decision-making model and (2) manual inspection by an expert. The automated differentiation algorithm has relevance to potential field applications. The basis for the algorithm is the decision tree shown in Fig. 3, which utilized DiFAMEs to differentiate between BA, BG, and BC/BT. As indicated by Fig. 3, detection of an additional biomarker is required for reliable differentiation between BC and BT.
The parameters of the decision tree (Fig. 3) were estimated using 125 random samples of BA, BG, BC, and BT taken from the 270 samples of group 2 previously described, accounting for within-species variation in peak areas and relative retention times. The algorithm was then tested using the remaining 145 samples. Only two of the 145 samples were misidentified, for an overall success rate of 98.6%, and a 97.4% success rate for BA. These results (Table 3) suggest that automated differentiation may be sufficiently reliable for field applications.
| Species | Total predicted | Total correct | Percentage correct |
|---|---|---|---|
| BG | 41 | 40 | 97.6 |
| BA | 38 | 37 | 97.4 |
| BC or BT | 66 | 66 | 100 |
In the manual confirmation step, which in part was used to validate the automated step, we were able to correctly detect and differentiate all but one of the 270 BA, BT/BC, and BG endospore samples grown in Col and LD media at 32 °C and 37 °C (see Table 4, rows 1–4). Upon further investigation, it was discovered that the single misclassification was a case in which an extremely high peak left so much residue in the column that the next chromatogram was affected. This emphasizes the need for a blank run between each test run. Importantly, this observation was one of the two missed by the automated procedure.
| Sample group | Growth temp./°C | Growth media | Species examined | Number of observations | Correctly predicted percentages | ||||
|---|---|---|---|---|---|---|---|---|---|
| BA | BG | BC/BT | BA | BG | BC/BT | ||||
| 2 | 32 | Col | BA, BG, BC/BT | 16 | 16 | 32 | 100 | 93.8 | 100 |
| 2 | 32 | LD | BA, BG, BC/BT | 19 | 19 | 38 | 100 | 100 | 100 |
| 2 | 37 | Col | BA, BG, BT | 18 | 18 | 18 | 100 | 100 | 100 |
| 2 | 37 | LD | BA, BG, BC/BT | 19 | 19 | 38 | 100 | 100 | 100 |
| 3 | unknown | unknown | BA, BT | 25 | 0 | 25 | 100 | NA | 100 |
As a final illustrative example, chromatograms displayed in Fig. 4 were produced from weaponized BA and BT endospores (sample group 3) provided by Dugway Proving Ground. It should be emphasized that these samples were prepared by methods entirely unknown to us in a different laboratory by different personnel. Furthermore, they were analyzed using our earlier TCM procedure that did not include the addition of sulfuric acid. Even so, straightforward differentiation between these organisms was possible based on DiFAMES, especially i16:1 (peak 9) and i17:1 (peak 15) FAME peaks (see Table 4, row 5 for differentiation results). Both were correctly classified.
 | ||
| Fig. 4 Extracted ion chromatogram (m/z 74) of FAMEs from weaponized BA and BT samples. Conditions: column A, temperature programmed from 60 °C to 300 °C at 14 °C min−1, 12.5 psi He inlet pressure. | ||
Conclusions
Conventional methods for fatty acid characterization of endospores and vegetative cells are laborious and time-consuming, usually taking more than 1 h.37 In comparison, TCM with CWF sample introduction is fast and simple, making it easily adaptable to field analysis. By screening the TCM products for DPAME and specific FAMEs, BA can be easily detected and differentiated from other microorganisms, including closely-related Bacillus species, when cultured under well-defined conditions. We plan to expand this approach to include analyses of these and other biological threat agents in more complex matrices, including real environmental samples, with the ultimate objective of reliable field detection using portable GC-MS.Acknowledgements
We thank Dugway Proving Ground and Torion Technologies for financial and technical contributions, and Supelco® for kindly providing empty SPME assemblies for housing the coiled wire filaments. This work was supported by the Mission & Installation Contracting Command, Dugway Proving Ground Directorate of Contracting, under Contract No. W911S6-09-C-0001.References
- P. Keim, L. B. Price, A. M. Klevytska, K. L. Smith, J. M. Schupp, R. Okinaka, P. J. Jackson and M. E. Hugh-Jones, J. Bacteriol., 2000, 182, 2928–2936 CrossRef CAS
.
-
Brock Biology of Microorganisms, ed. M. Madigan and J. Martinko, Prentice Hall, 11th edn, 2005 Search PubMed
.
- S. A. Burke, J. D. Wright, M. K. Robinson, B. V. Bronk and R. L. Warren, Appl. Environ. Microbiol., 2004, 70, 2786–2790 CrossRef CAS
.
- D. Fritze and R. Pukall, Intl. J. of System. and Evol. Microbiol., 2001, 51, 35–37 Search PubMed
.
- A. Fox, G. C. Stewart, L. N. Waller, K. F. Fox, W. M. Harley and R. L. Price, J. Microbiol. Methods, 2003, 54, 143–152 CrossRef CAS
.
- W. Zhong, Y. Shou, T. M. Yoshida and B. L. Marrone, Appl. Environ. Microbiol., 2007, 73, 3446–3449 CrossRef CAS
.
- M. Plomp, T. J. Leighton, K. E. Wheeler, M. E. Pitesky and A. Malkin, Langmuir, 2005, 21, 10710–10716 CrossRef CAS
.
- A. Driks, Microbiol. Mol. Biol. Rev., 1999, 63, 1–20 CAS
.
- H. Liu, J. Bacteriol., 2004, 186, 164–178 CrossRef CAS
.
-
A. Driks and P. Setlow, in Prokaryotic Development, Y. V. Brun and L. J. Shimkets, ed., Washington, DC, 2000, pp. 191–218 Search PubMed
.
- A. Driks, Phytopathology, 2004, 94, 1249–1251 CrossRef CAS
.
- J. Poerschmann, Z. Parsi, T. Gorecki and J. Augustin, J. Chromatogr., A, 2005, 1071, 99–109 CrossRef CAS
.
-
T. G. Tournabene, Development of a Biochemical Database for Bacillus Anthracis, Brucellar Melitensis, Yersenia Pestis, Franciscella Tularensis, Vibrio Cholerae, and Coxiella Burnetti, Chemical and Biological Defense Information Analysis Center, Aberdeen Proving Ground, MD, 1995, pp. 1–88 Search PubMed
.
-
A. Driks, in Bacillus subtilis and its Closest Relatives, J. A. Hoch and R. Losik, ed., Washington, DC, 2002, pp. 527–535 Search PubMed
.
- S. S. Huang, D. Chen, P. L. Pelczar, V. R. Vepachedu, P. Setlow and Y. Li, J. Bacteriol., 2007, 189, 4681–4687 CrossRef CAS
.
- M. L. Perdue, J. Karns, H. Jim, and J. A. V. Kessel, Detection and Fate of Bacillus, Environmental Microbial Safety Laboratory, Animal and Natural Resources Institute, USDA-ARS, Beltsville, MD, pp. 1–18 Search PubMed.
- X. Zhang, M. A. Young, O. Lyandres and R. P. V. Duyne, J. Am. Chem. Soc., 2005, 127, 4484–4489 CrossRef CAS
.
- T. B. Tims and D. V. Lim, J. Microbiol. Methods, 2004, 59, 127–130 CrossRef CAS
.
- A. Fox, G. E. Black, K. Fox and S. Rostovtseva, J. Clinical Microbiol., 1993, 887–894 Search PubMed
.
- A. P. Snyder, W. H. McClennen, J. P. Dworzanski and H. L. C. Meuzelaar, J. Anal. Chem., 1990, 62, 2566–2573
.
- V. Ryzhov, Y. Hathout and C. Fenselau, Appl. Environ. Microbiol., 2000, 66, 3828–3834 CrossRef CAS
.
- M. B. Beverly, Rapid Commun. Mass Spectrom., 1996, 10, 455–458 CrossRef CAS
.
- F. Basile, M. B. Beverly and K. Voorhees, Anal. Chem., 1998, 17, 95–109 CAS
.
-
C. D. Mowry, C. H. Morgan, G. C. Frye-Mason, L. A. Theisen, D. E. Trudell, Q. J. Baca, W. C. Chambers, and J. I. Martinez, Sandia National Laboratories, SAND report 2003-0168, Jan., 2003
.
- A. P. Snyder, S. N. Thornton, J. P. Dworzanski and H. L. C. Meuzelaar, Field Anal. Chem. Technol., 1996, 1, 49–58 CrossRef CAS
.
- T. V. Truong, A. N. Nackos, J. A. Murray, J. A. Kimball, J. E. Hawks, D. J. Harvey, H. D. Tolley, R. A. Robison, C. H. Bartholomew and M. L. Lee, J. Chromatogr., A, 2009, 1216, 6852–6857 CrossRef CAS
.
- J. P. Dworzanski, L. Berwald, W. H. McClennen and H. L. C. Meuzelaar, J. Anal. Appl. Pyrolysis, 1991, 21, 221–232 CrossRef CAS
.
- A. S. Stedt, U. Eriksson, V. Ramisse and H. Garrigue, FEMS Microbiol. Ecol., 1997, 23, 159–168 CrossRef CAS
.
- C. A. Bell, J. R. Uhl, T. L. Hadfield, J. C. David, R. F. Meyer, T. F. Smith and F. R. Cockerill, J. Clin. Microbiol., 2002, 40, 2897–2902 CrossRef CAS
.
- E. Bode, W. Hurtle and D. Norwood, J. Clin. Microbiol., 2004, 42, 5825–5831 CrossRef CAS
.
- A. B. Herzog, S. D. McLennan, A. K. Pandey, C. P. Gerba, C. N. Haas, J. B. Rose and S. A. Hashsham, Appl. Environ. Microbiol., 2009, 75, 6331–6339 CrossRef CAS
.
-
Eliciting and Analyzing Expert Judgment: A Practical Guide, M. A. Meyer and J. M. Booker, ASA-SIAM, 2001 Search PubMed
.
- T. Kaneda, Microbiol. Rev., 1991, 55, 288–302 CAS
.
- T. Kaneda, Bacteriol. Rev., 1977, 41, 391–418 CAS
.
- P. Kämpfer, System. Appl. Microbiol., 1994, 17, 86–98 Search PubMed
.
- O. Väisänen and M. Salkinoja-Salonen, System Appl. Microbiol., 1989, 12, 103–111 Search PubMed
.
- Y. Song, R. Yang, G. Zhaobiao, M. Zhang, X. Wang and F. Zhou, J. Microbiol. Methods, 2000, 39, 225–241 CrossRef CAS
.
- P. Whittaker, J. Agric. Food Chem., 2005, 53, 3735–3742 CrossRef CAS
.
Footnotes |
| † Current address: University of Utah School of Medicine, Salt Lake City, UT, USA |
| ‡ Current address: Case Western Reserve University School of Medicine, Cleveland, OH, USA |
| This journal is © The Royal Society of Chemistry 2010 |
