Development & validation of an in vitro dissolution method with HPLC analysis for misoprostol in formulated dosage form
Jigar
Mehta
*,
Kanhaiyalal
Patidar
,
Vipul
Patel
,
Nayan
Kshatri
and
Niranjan
Vyas
Analytical Research & Development Laboratory, Research & Development Unit – I, Cadila Pharmaceutical LTD., Survey No.: 1389, Trasad Road, Dholka, Ahmedabad, 387810, Gujarat, India. E-mail: jigar.mehta@cadilapharma.co.in; Fax: +91-02714-221481; Tel: +91-02714-221481, 221483, 221484
First published on 13th November 2009
Abstract
The intended purpose of this work is to develop and validate a dissolution test for misoprostol tablets containing 200 μg misoprostol [1% in hydroxypropyl methylcellulose {HPMC}] using a reverse-phase liquid chromatographic method. After testing sink conditions, dissolution medium and stability of the drug, the best conditions were: paddle at 50 rotations per minute (rpm) stirring speed, deaerated water dissolution medium with volume of 500 ml, as per very low label content of the drug substance & drug product. The method was validated to meet requirements for a global regulatory filing and this validation included specificity, precision, linearity and accuracy. Release of more than 85% of the label amount was achieved over 30 min in the medium through out the study. The dissolution test developed was adequate for its purpose and could be applied for quality control of misoprostol formulation dosage form.
1.0 Introduction
Dissolution test has emerged in the pharmaceutical field as a very important tool to characterize drug product performance.1 It provides measurements of the bioavailability of a drug as well as demonstrating bioequivalence from batch-to-batch. Besides, dissolution is a requirement for regulatory approval for product marketing and is a vital component of the overall quality control program.2–4Misoprostol is a synthetic prostaglandin E1 (PGE1) analogue and chemically known as methyl 7-((1R,2R,3R)-3-hydroxy-2-((S,E)-4-hydroxy-4-methyloct-1-enyl)-5-oxocyclopentyl)heptanoate.
 | ||
| Fig. 1 Chemical structure of misoprostol. | ||
Misoprostol is a drug that is used for the prevention of non-steroidal anti-inflammatory drug (NSAID)-induced gastric ulcers, for early abortion, and to treat missed miscarriage. Misoprostol is also commonly used to induce labor. It causes uterine contractions and the ripening (effacement or thinning) of the cervix.5 Misoprostol is more effective in starting labor than other drugs used for labor induction.6 It is also significantly less expensive than the other commonly used ripening agent, dinoprostone.7 Misoprostol is a highly unstable compound and its stability is significantly improved in a hydroxypropyl methylcellulose (HPMC) dispersion (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100).8 During method development, the API misoprostol is used as 1% dispersion of HPMC for formulation and determination of dissolution rate should be done for misoprostol only.
100).8 During method development, the API misoprostol is used as 1% dispersion of HPMC for formulation and determination of dissolution rate should be done for misoprostol only.
Literature search revealed that as such there is a lack of method by which in vitro dissolution rate can be accurately quantified. Although there are methods available for determination of misoprostol in human plasma by tandem mass spectrometry,9 assay determination by HPLC,10 with different combinations,11 also the data of stabilizations are available.12 This study, describes the development of a fast, accurate and precise HPLC method with isocratic elution for determination of misoprostol in pharmaceutical formulations and in dissolution media for drug quality control purposes. The dissolution method was also developed and validated according to USP guidelines.13
2.0 Experimental
2.1 Materials & reagents
All experiments were performed using ‘A class’ volumetric glassware, and an in-house standard of pharmaceutical grade misoprostol (1% HPMC dispersion). Analytical reagent grade orthophosphoric acid (Spectrochem, India), HPLC grade acetonitrile (Finar chem., A'bad, India) and highly pure HPLC grade Milli Q water (Millipore, Bedford, MA, USA) were used in mobile phase preparation. Purified water was used as a dissolution medium. The mobile phase was filtered through a 0.45 μm membrane filter (millipore, Barcelona) and degassed under vacuum by filtering assembly, prior to use. The pharmaceutical preparation, declaring to contain misoprostol (200 μg) with other excipients (placebo mixture) was obtained from M/s Cadila Pharmaceuticals LTD., Gujarat, India for analysis.2.2 Dissolution (instrumentation & conditions)
For all dissolution experiments, ‘Electrolab TDT 8L & 6L’ (Electrolab, India), dissolution apparatus was used, while in the preparation of the mobile phase and sample & standard aliquots, analytical balance (Sartorius, CP225D) was used.Dissolution testing was performed in compliance with USP {711} using apparatus 2 with paddles. A dissolution media/agitation screen was performed for medium and paddle speed selection. A dissolution medium of deaerated purified water was chosen based on the solubility profiles obtained and its benefits compared to others. A paddle speed of 50 rpm was selected with media volume of 500 mL. The medium, which was vacuum degassed under degasser (electrolab), was maintained at 37 ± 0.5 °C. The 1-L glass dissolution vessels were covered to minimize evaporation. Samples were drawn at 30 min for early validation work. Manual sampling was performed using 10 mL aliquots. These solutions were immediately filtered using whatman filter No. 1. The first 5 mL of sample was discarded prior to collecting, and the sample prepared for analysis was collected.
2.3 Chromatographic method
An HPLC method with UV detection was selected for the method of analysis. The reversed-phase procedure utilized a Waters sperisorb ODS 1 (100 × 4.6 mm, 5 μm) and UV detection at 200 nm. This wavelength was selected because it is a UV maximum and provides enough sensitivity needed for quantitation of this very low drug concentration in the dissolution samples (about 0.4 μg mL−1). The column temperature was maintained at 40 °C. The mobile phase contained acetonitrile, purified water and 24.5 g l−1 solution of orthophosphoric acid (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 v/v/v, respectively). The flow rate was 0.75 mL min−1 for 20 min with an injection volume of 500 μL. A standard solution of active pharmaceutical ingredient (API) was prepared first in mobile phase, and subsequently diluted down to the appropriate concentration with dissolution medium. This standard solution contained 100% of the final assay concentration of drug (∼0.4 μg mL−1).
0.05 v/v/v, respectively). The flow rate was 0.75 mL min−1 for 20 min with an injection volume of 500 μL. A standard solution of active pharmaceutical ingredient (API) was prepared first in mobile phase, and subsequently diluted down to the appropriate concentration with dissolution medium. This standard solution contained 100% of the final assay concentration of drug (∼0.4 μg mL−1).
3.0 Results & discussion
3.1 Chromatographic method development
Drug solubility and solution stability are important properties to be considered when selecting the dissolution medium.14 In this study, the first approach was to compare buffers of different pH against the deaerated purified water, as commonly used for solid dosage forms. As per very low concentration of misoprostol into the formulation, sink conditions in water demonstrated the suitability of the dissolution medium and also the compatibility of HPMC dispersion with dissolution medium was checked. The stirring speed selection was done based on the range recommended (50–75 rpm) for apparatus 214,15 and the usual value for tablets. Since the results obtained using 50 rpm in the preliminary studies were satisfactory, no other speed was tested.After setting dissolution parameters, the chromatographic parameters were optimized. For some of the parameters the reference of related substances method from misoprostol API in British Pharmacopoeia16 was considered during development. During development the major problem observed is of the peak response and the peak shape. There were different trials taken for optimization of the HPLC column, the Waters spherisorb ODS 1 column is selected because of its larger surface area (220 m2 g−1) and medium carbon loading (6.2%), and as a result a good peak shape with tailing of about 1.2–1.3 was achieved, which was well within the acceptance criteria of <1.5 as per various pharmacopoeia. The concentration of sample solution was very low such as 0.0004 mg mL−1 (0.4 μg mL−1). During spectrum scanning the wavelength where maxima observed was 200 nm which is a magnifying wavelength, even though the desired response was not achieved, to overcome this difficulty the injection volume is increased, and an additional loop was attached to the Agilent HPLC system. Initially a few trials are taken with different injection volumes e.g. 100 μL etc. which has not satisfied the requirement of method by mean of peak response, and considering the probable very low concentration of initial time points during performance of dissolution profile the injection volume optimized is 500 μL, wavelength is 200 nm. After optimizing all the parameters, the method was checked for quality control purpose successfully.
3.2 Evaluation of validation data
Instrument precision (suitability of system). System suitability shall be checked for the conformance of suitability & reproducibility of chromatographic system for analysis. Systems suitability was checked by injecting six replicate injections of standard solution. For conformance of suitability, % RSD for standard peak shall not be more than 2.0. The results revealed that the % RSD was 1.0% and which proved the suitability of system.
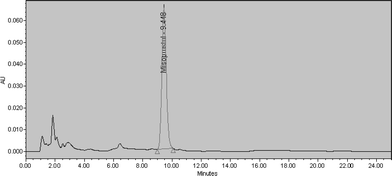 | ||
| Fig. 2 Standard chromatogram. | ||
Method precision. The purpose of this experiment is to prove the repeatability of the results obtained by this quantification methodology. To conform the repeatability six sets of sample solution were injected and % RSD of the results was observed. To comply this parameter the value of % RSD shall not exceed 6.0%, the resulting RSD was 2.2% and well within the acceptance limit and showed the method precise.
| Set number | Dissolution |
|---|---|
| Set 1 | 90.8% |
| Set 2 | 89.3% |
| Set 3 | 94.8% |
| Set 4 | 93.1% |
| Set 5 | 93.0% |
| Set 6 | 90.9% |
| Average | 92% |
| SD | 2.00 |
| RSD | 2.2% |
Intermediate precision (ruggedness). To demonstrate reliability of results obtained by the dissolution test method with day to day, analyst to analyst, system to system and column to column variability intermediate precision of dissolution test method was demonstrated by conducting method precision done by different analyst on different chromatographic system using different column (from same make of column but with different serial number), different dissolution system and on different day. For acceptance of results, % RSD of individual analyst shall not exceed 6.0% and difference between two analysis shall not be more than 5.0%. The experiment yielded the % RSD of 4.5% and 2.2% and the difference of %RSD was 2% which shown that the method is rugged.
| Linearity level | Concentration/μg ml−1 |
|---|---|
| 20.0% | 0.08 |
| 50.0% | 0.20 |
| 75.0% | 0.30 |
| 100.0% | 0.40 |
| 125.0% | 0.50 |
| 150.0% | 0.60 |
| Correlation coefficient (r) | 0.9998 |
| Square of correlation coefficient (r2) | 0.9996 |
| Slope of regression | 503.213 |
| RSD of response factor | 1.0% |
| Y-intercept | 0.932 |
| Y-intercept bias at 100.0% linearity level | 0.5% |
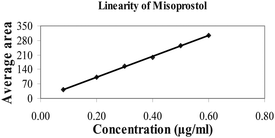 | ||
| Fig. 3 Linearity curve of misoprostol. | ||
| Accuracy level | Set no. | Theoretical concentration of analyte/μg ml−1 | Practical concentration of analyte/μg ml−1 | Recovery (%) | Average recovery (%) | RSD |
|---|---|---|---|---|---|---|
| 20.0% | Set 1 | 0.080 | 0.082 | 102.5 | 102.5 | 1.2% |
| Set 2 | 0.080 | 0.081 | 101.3 | |||
| Set 3 | 0.080 | 0.083 | 103.8 | |||
| 50.0% | Set 1 | 0.200 | 0.206 | 103.0 | 102.5 | 0.5% |
| Set 2 | 0.200 | 0.205 | 102.5 | |||
| Set 3 | 0.200 | 0.204 | 102.0 | |||
| 100.0% | Set 1 | 0.400 | 0.406 | 101.5 | 101.9 | 0.5% |
| Set 2 | 0.400 | 0.407 | 101.8 | |||
| Set 3 | 0.400 | 0.410 | 102.5 | |||
| 150.0% | Set 1 | 0.600 | 0.610 | 101.7 | 101.8 | 0.4% |
| Set 2 | 0.600 | 0.609 | 101.5 | |||
| Set 3 | 0.600 | 0.614 | 102.3 |
4.0 Conclusion
The possibility to obtain with a dissolution test reliable results on the pharmaceutical to be tested, is essential to ensure the quality, safety and efficacy of the developed drug product. A dissolution method with HPLC analysis for misoprostol low dose tablets has been fully validated to meet global regulatory requirements. The methodology was evaluated for specificity, linearity, precision and accuracy in order to establish the suitability of the analytical method. The conditions that allowed the dissolution profile determination were deaerated water, paddle (USP apparatus 2) and 50 rpm stirring speed. The method was demonstrated to be adequate for quality control of misoprostol dosage form, since there is no official monograph in any of the international pharmacopoeia.5.0 Acknowledgement
All raw data from the validation work archived at Cadila Pharmaceutical LTD. All the validation work performed at Analytical Research Laboratory, Cadila Pharmaceutical LTD., Dholka, India. The author is thankful to M/s Cadila Pharmaceuticals LTD., Dholka, India for providing facilities and infrastructure for the study. The author greatly acknowledges Mr. Niranjan Vyas for his constant motivation and support.6.0 References
- S. Furlanetto, F. Maestrelli, S. Orlandini, S. Pinzauti and P. Mura, J. Pharm. Biomed. Anal., 2003, 32, 159–165 CrossRef CAS.
- S. M. A. Frost, Dissol. Tech., 2004, 19–21 Search PubMed.
- H. Ansel, L. Allen Jr., N. Popovich, Pharmaceutical Dosage Forms and Drug Delivery Systems, 7th ed., Lippincott Williams & Wilkins, Baltimore, 1999 Search PubMed.
- Cassia V. Garcia, Clesio S. Paim, Martin Steppe and Elfrides E. S. Schapoval, J. Pharm. Biomed. Anal., 2006, 41, 833–837 CrossRef.
- A. B. Goldberg, M. B. Greenberg and P. D. Darney, Misoprostol and pregnancy, N. Engl. J. Med., 2001, 344(1), 38–47 CrossRef CAS.
- Marsden Wagner, Born in the USA: how a broken maternity system must be fixed to put mothers and infants first, University of California Press,Berkeley, 2006, ISBN 0-520-24596–2 Search PubMed.
- L. Summers, Methods of cervical ripening and labor induction, J. Nurse Midwifery, 1997, 42(2), 71–85 CrossRef CAS.
- T. T. Kararli and T. Catalano, Pharm. Res., 1990, 7(11), 1186–1189 CrossRef CAS.
- Yu Zou, Xiaoyan Chen, Bo Song and Dafang Zhong, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 852(1–2), 122–127 CrossRef CAS.
- B. M. Rao, M. K. Srinivasu, B. S. S. Reddy, T. Sivaleela, P. R. Kumar, K. B. Chandrasekhar, M. H. Krishna, M. Shanker, E. V. V. V. Reddy and H. R. Mohan, Indian Drugs, 2005, 42(11), 744–748 Search PubMed.
- Iolanda M. Womack, Arthur S. Lee, Burde Kamath, K. C. Agrawal and Vimal Kishore, Prostaglandins, 1996, 52(4), 249–259 CrossRef.
- David Chen, Rong-Jer Tsay, Hue-In Lin, Huilan Chen, Shou-Chung Chao and Hao Ku, Int. J. Pharm., 2000, 203(1–2), 141–148 CrossRef CAS.
- The United States Pharmacopoeia, 32nd ed., United States Pharmacopoeial Convention, Rockville, 2009.
- The United States Pharmacopoeia, Pharmacopeial Forum, 2004, 30, 351–363 Search PubMed.
- FDA, Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms, Food and Drug Administration, Rockville, 1997 Search PubMed.
- British pharmacopoeia commission, BP/Ph.Eur., 2009, Volume II, Monograph 1731, Page No.: 1384.
| This journal is © The Royal Society of Chemistry 2010 |
