DOI:
10.1039/B9AY00183B
(Paper)
Anal. Methods, 2010,
2, 154-158
Received
22nd September 2009
, Accepted 23rd November 2009
First published on
7th December 2009
Abstract
A simple and sensitive indirect determination method for sulfide in water samples by anodic stripping voltammetry (ASV) using mercury-film electrode (MFE) has been developed, which is based on the determination of residual cadmium ion after reaction of Cd2+ with S2−. The linear range is adjustable depending on Cd2+ concentration, for example, the determination of S2− can be achieved in the range of 1.5–7.0 × 10−6 mol L−1 by selecting 3.0 × 10−6 mol L−1 Cd2+. The detection limit is 1.3 × 10−8 mol L−1 under optimum conditions, and the relative standard deviation (RSD, n = 10) for 2.0 × 10−6 mol L−1 S2− is 0.7%. Compared with other methods, this method has the following prominent advantages: with low detection limit, easy to operate and less interference. The proposed method has been successfully applied to the determination of S2− in synthetic wastewater, lake water, beverage, spring water and real wastewater samples.
Introduction
Sulfide is an anion found widely in both natural and waste waters, and it is one of the most important parameters to monitor in water due to its high toxicity for aquatic organisms.1,2 The toxicity of sulfide is attributed to the releasing of hydrogen sulfide (H2S). At a low concentration, H2S can produce personal distress, while at a higher concentration, it can result in loss of consciousness, permanent brain damage or even death through asphyxiation.3,4 In addition, hydrogen sulfide controls the bioavailability of heavy metals in anoxic environments.2,3 The sulfide concentrations are generally very low in real water samples, especially in sediment pore waters.5 Thus, it is very important to develop a highly sensitive and rapid method for sulfide determination.
Various methods have been developed for the determination of sulfide. They include spectrophotometric,6–8 fluorescence,9–11 chemiluminescence,12 inductively coupled plasma-atomic emission spectrometry (ICP-AES),13,14 atomic absorption spectrometry (AAS),15 flow injection analysis (FIA),16,17 ion chromatography,18 electrochemical methods19–45 and so on. However, the determination methods frequently used either have a relative high detection limit, low accuracy and precision, and poor selectivity or are time-consuming, cumbersome to operate, costly and need large-scale equipment. Classical spectrophotometric methods lack sensitivity.46 ICP-AES can be used for sulfide determination, but polyatomic oxygen ions greatly interfere with sulfur isotopes.5 Wardencki47 reviewed the problems encountered in the determination of sulfur compounds by gas chromatography. Methylene blue (MB) method plays a great role in the spectroscopic determination of sulfide,48,49 and the detection limit can be improved when combined with a smart multisyringe flow injection system.7 The nanomolar levels of sulfide can be measured when the MB generated was determined by a solid-phase extraction technique coupled with HPLC.50 Recently, our group9 presented a knotted reactor (KR) coupled with hydride generation atomic fluorescence spectrometry used for the indirect determination of sulfide, and the detection limit can be down to 1.6 × 10−9 mol L−1.
Compared with other methods, electrochemical methods possess some unique and distinct advantages: rapidity, cheap instrumentation, high sensitivity and a simple operation procedure. In particular, they have promising in situ applications, which is important for environmental monitoring. Generally, the determination of sulfide includes direct and indirect approaches. The direct determination methods involve cathodic stripping voltammetry (CSV) on mercury electrode,21–23 the electrocatalytic oxidation of sulfide on bare electrodes or modified electrodes,24–36 and so on. The indirect determination methods include several principles, such as electrochemically initiated reaction of sulfide with N,N-dimethylphenylene-1,4-diamine,37–39 and N,N-diphenyl-p-phenylenediamine,40 indirect determination of sulfide by measuring As(III) after reaction,41 and so on. Cadmium ion selective electrode can be used for sulfide determination,42 however, the sensitivity is low. Several inhibition biosensors,43,44 and carbon nanotube (CNT) modified glassy carbon electrodes45 for sulfide determination have been developed. Now, it remains a challenge for trace/ultra-trace sulfide determination in a complex matrix. Thus, it is very important to develop a more rapid and sensitive determination method for sulfide. Currently, the successfully used electrodes are MFE, which has various advantages such as high surface area/volume ratio, resulting in a higher concentration of amalgam during the deposition. In addition, MFE has a high mechanical resistance and remains stable under vigorous stirring or can be coupled with flow systems.51,52 In this paper, an indirect method for sulfide determination was developed based on the sensitive response of MFE to Cd2+, this method has been successfully applied to the determination of sulfide in synthetic wastewater, lake water, beverage, spring water and real wastewater samples.
Experimental
Chemicals and instrumentation
An Autolab PGSTAT 302 (Metrohm China Ltd.) instrument was used for anodic stripping voltammetry (ASV). A three-electrode cell consisting of a bare glassy carbon disk working electrode (4 mm diameter) or a mercury-film glassy carbon working electrode, a saturated calomel reference electrode (SCE) and a platinum wire counter electrode was used for electrochemical measurements. ICP-AES J-A1100 (Jarrell-Ash, America) was used for the determination of heavy metals and other elements in beverage and different spiked water matrices. An ultraviolet spectrophotometer UV-3600 (Shimadzu, Japan) was used for methylene blue (MB) method determination. The pH values were measured with a PHSJ-4A pH meter (Shanghai, China). All potentials are reported vs. the SCE. The GPES software (Metrohm China Ltd.) was used to control the instrument and to perform preliminary data processing.
All chemicals were at least of analytical grade and were purchased from Shanghai Chemicals Co., Ltd. (Shanghai, China) unless otherwise stated. All aqueous solutions were prepared in doubly quartz deionized water (DDW). The stock standard solution of inorganic mercury (1000 mg L−1) was prepared with mercuric nitrate.53 The stock standard solution of cadmium ion (1000 mg L−1) was prepared with cadmium chloride.53 The stock standard solution of sulfide (1000 mg L−1) was prepared daily by dissolving the appropriate amount of crystal Na2S·9H2O and diluting it to volume with DDW.54 For the MB method,55 0.2% (m/v) N,N-dimethyl-p-phenylenediamine hydrochloride and 12.5% (m/v) NH4Fe(SO4)2 were prepared by dissolving the proper quantity in 20% (v/v) and 2.5% (v/v) H2SO4, respectively.
Preparation of the MFE
Prior to use, the glassy carbon electrode was polished under clean conditions using a series of alumina slurried (1.0, 0.3 and 0.05 μm) in water, following this, they were rinsed twice with distilled water and sonicated in a water bath for 10 min. The MFE was prepared under the condition of −1.3 V for 60 s in 0.1 mol L−1 hydrochloric acid containing 25 mg L−1 Hg2+ after deaerating this solution with a flow of nitrogen for 15 min.56 After this, the electrode was treated in 1 mol L−1 pH 4.5 sodium acetate by repetitive scanning in the potential range of −1.0 and −0.5 V for 90 cycles at a scan rate of 100 mV s−1.
Sample pretreatment
The synthetic wastewater samples54 were prepared to contain (mg L−1 in parentheses) phenol (500), CH3COONa (500), NaCl (500), KC1 (500), CaCl2 (500), KSCN (500), Na2CO3 (500), and (NH4)2SO4 (150), in addition to sulfide with the concentration of 1.0, 2.0 and 5.0 × 10−6 mol L−1. The beverage and spring water samples were purchased from the market. Lake water samples were collected locally. Wastewater sample was collected from a sewage treatment plant. After sampling, lake waters and wastewater samples were filtered through a 0.45 μm membrane immediately and determined at once.9 The basic water quality parameters are shown in Table S1 (Supporting Information).† For MB method determination,55 to a 50 mL flask containing water samples, 1 mL 0.2% (m/v) N,N-dimethyl-p-phenylenediamine hydrochloride and 0.5 mL 12.5% (m/v) NH4Fe(SO4)2 were added, shaken, equilibrated for 10 min, then diluted to 50 mL, and measured at 665 nm by a UV-Vis method.
Procedure
25 mL 0.1 mol L−1 pH 4.0 NaAc-HAc were added into a electrolyte cell, and the linear sweep curve was recorded as blank after deposition 120 s under the condition of −1.3 V of preconditioning potential. Then a certain amount of Cd2+ was added into the cell and repeated the above procedure, and a linear sweep curve was recorded. Then a certain amount of S2− was added into the cell, and the corresponding linear sweep curve was recorded again after reacting for 2 min under stirring conditions. The concentration of S2− was calculated through the peak current difference of Cd2+ in the absence and presence of S2−. The data treatment was performed with Excel 2003 and Origin 6.0 software.
Results and discussion
The basic principle for indirect determination of sulfide by ASV
The basic principle for sulfide determination by ASV is based on the selective precipitation reaction between Cd2+ and S2− to form CdS. Cd2+ can be determined by ASV using the sensitive response of MFE to Cd2+. Therefore, S2− concentration can be calculated by determining the Cd2+. The theoretical formula is derived as follows:
The ASV formula57 is shown as eqn (1):
| | | ip = kn2Dox2/3ω1/2η−1/6AvtCox | (1) |
In which
n is number of electrons,
Dox is diffusion coefficient,
ω is stirring speed,
η is viscosity of solution,
A is
electrode area,
v is scan rate,
t is deposition time and
Cox is initial concentration.
Step I: Cd2+ response with absence of S2−. 25 mL 0.1 mol L−1 pH 4.0 NaAc-HAc containing certain amount of Cd2+ were added into a electrolyte cell, and the linear sweep curve was recorded after deposition 120 s under the condition of −1.3 V. Let K = kn2Dox2/3ω1/2η−1/6Avt, the peak current of Cd2+ can be expressed using eqn (2):
Step II: S2− determination. Then a certain amount of S2− was added into the above solution, after 2 min under stirring condition, the linear sweep curve was recorded again after depositing 2 min under the condition of −1.3 V. The concentration of S2− was calculated through the peak current difference of Cd2+ in the absence and presence of S2−, as shown in eqn (3)–(5). In 0.1 mol L−1 pH 4.0 NaAc-HAc, the reaction ratio between Cd2+ and S2− is not 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and stoichiometric ratio is less than 1, thus, a coefficient γ should be added to the equation. Due to S2− is not the unique form (There are three forms including S2−, HS− and H2S) in solution at pH 4.0, thus, C′Cd2+ is equal to γC*S2–, and S2− reacted with Cd2+ is γ times of total S2− (γ ≈ 0.4), which indicated that the added S2− does not all react with Cd2+, as shown in eqn (4) and (5). Under certain condition, ΔipCd2+ has linear relationship with C*S2–, which is consistent with the experimental results, as shown in Fig. 1.
1, and stoichiometric ratio is less than 1, thus, a coefficient γ should be added to the equation. Due to S2− is not the unique form (There are three forms including S2−, HS− and H2S) in solution at pH 4.0, thus, C′Cd2+ is equal to γC*S2–, and S2− reacted with Cd2+ is γ times of total S2− (γ ≈ 0.4), which indicated that the added S2− does not all react with Cd2+, as shown in eqn (4) and (5). Under certain condition, ΔipCd2+ has linear relationship with C*S2–, which is consistent with the experimental results, as shown in Fig. 1.
 |
| | Fig. 1 The ASV responses of Cd2+ reaction system changed with the addition of sulfide. 3.0 × 10−6 mol L−1 Cd2+. a → j: C*S2– = 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 7 × 10−6 mol L−1. T = 25 ± 1 °C, 0.10 mol L−1 pH 4.0 acetate buffer solution. Scan rate: 100 mV s−1. | |
i
p′Cd2+ is the electrochemical response of Cd2+ after reacting with S2−, and K is a constant.
| | | ip′Cd2+ = KC′Cd2+ = K[C*Cd2+ − γC*S2−] | (3) |
| |  | (4) |
The linear range for sulfide determination can be extended according to different determination requirements by selecting different concentrations of Cd2+, and the corresponding results are shown in Table 1.
Table 1 The characteristics of linear regression equations
| Cd2+ content (μM) |
Regression equation (CS2–/μM) |
Linear range (μM) |
| 0.08 |
ΔipCd2+ = 6.47CS2– − 0.05 |
0.02–0.22 |
| 0.3 |
ΔipCd2+ = 0.615CS2– − 0.013 |
0.3–0.7 |
| 0.8 |
ΔipCd2+ = 2.36CS2– + 0.014 |
0.7–1.5 |
| 3.0 |
ΔipCd2+ = 9.77CS2– − 9.32 |
1.5–7.0 |
Optimization of experimental parameters
The main parameters affecting the system containing the pH of the buffer and reaction time tR were investigated. (a) The pH of the buffer: the acidity of the solution was controlled by adjusting the pH of buffer solution.56 The experiments results showed that ΔipCd2+ reached the highest value when the pH of buffer solution is 4.0. Since the reaction could not progress entirely under these pH conditions due to the reaction kinetic reasons, only a part of sulfide participated in the CdS precipitation. Reaction coefficient γ explaining the kinetic principle of the reaction in the system was 0.4 by summarizing a number of results (n = 20). (b) Reaction time tR: the reaction between Cd2+ and S2− needs a period of time, so that tR was controlled to achieve the best efficiency. With Cd2+ = 3 × 10−6 mol L−1 and S2− = 2 × 10−6 mol L−1, the results (Fig. 2) showed that 120 s was the most proper value for tR.
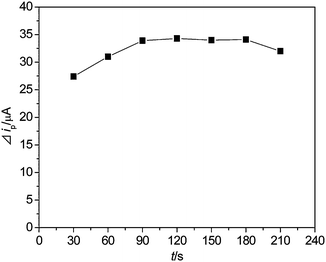 |
| | Fig. 2 Effect of tR on the ΔipCd2+. 3 × 10−6 mol L−1 Cd2+ and 3 × 10−6 mol L−1 S2−. Other conditions are shown in Fig. 1. | |
Precision and detection limit
In order to inspect the reproducibility of MFE, the precision experiment was carried out. Under the optimum conditions, the RSD (n = 10) for 2 × 10−6 mol L−1 S2− (at 3 × 10−6 mol L−1 Cd2+) is 0.7%, which indicates that the MFE showed good reproducibility. Under the optimum conditions, the detection limit of this method for S2− is 1.3 × 10−8 mol L−1 (at 8.0 × 10−8 mol L−1 Cd2+).
Interference study
The influences of potential coexisting ions on the signal of 2 × 10−6 mol L−1 sulfide were investigated. The tolerance ratios (Cion/Csulfide) when interference concentration varying the analyte signal by 10% are presented as follows: PO43−, CO32−, Na+, CH3COO− and F− (5000); SO42− (3800); Al3+ (3000); SO32− (2700); NO3−, Cl−, NO2−, Ca2+, Mg2+ and K+ (2000); SCN− (1600); NH4+ (500); Br− (450); I− (320); Fe3+ (90); Fe2+ (50); and S2O32− (15). One fold of Cu2+, Zn2+, Pb2+, Ag+, and Ni2+ interfere the determination, therefore, this method does not suit those water samples containing high concentration of heavy metals. Generally, the levels of heavy metals in natural waters are very low.58,59 Heavy metals can also react with S2− to form MS precipitate. Thus, they can not coexist in real environmental water samples. Thus, this method can be used for the determination of sulfide in environmental water samples.
Practical application
In natural water samples, the concentrations of heavty metals are very low.58,59 Thus, this proposed method can be used for the determination of S2− in natural water samples. In real water samples containing low levels of heavy metals, this method can be used for the determination of free S2− because heavy metals can react with S2− to form MS precipitate. However, if the concentrations of heavy metals in real water samples are too high, this method will be not suitable. The proposed method has been applied to the determination of S2− in synthetic wastewater, lake water, beverage, spring water and real wastewater samples. (Table S1 in Supporting Information gives the basic water quality parameters.)† Compared with the classical MB method, the results listed in Table 2 demonstrate the validity of the developed method. The analytical results in Table 2 showed that sulfide contents in these water samples were very low.
Table 2 Analytical results for beverage and different spiked water samplesa
| Samples |
Added (μM) |
Found (μM) |
Recovery (%) |
|
ND means not detected. The sulfide concentrations for lake waters, beverage, nongfu spring water and wastewater samples are also ND by MB method.
|
| Synthetic waste water 1 |
1.00 |
0.93 ± 0.03 |
93 |
| Synthetic waste water 2 |
2.00 |
2.00 ± 0.01 |
100 |
| Synthetic waste water 3 |
5.00 |
5.10 ± 0.04 |
102 |
| Lake water 1 |
|
ND |
|
| Lake water 2 |
|
ND |
|
| Lake water 3 |
|
ND |
|
| Maidong beverage |
0 |
ND |
|
| 1.00 |
1.03 ± 0.02 |
103 |
| 2.00 |
2.00 ± 0.04 |
100 |
| Nongfu spring water |
0 |
ND |
|
| 1.00 |
0.99 ± 0.04 |
99 |
| 2.00 |
2.03 ± 0.05 |
102 |
| Wastewater |
0 |
ND |
|
| 1.00 |
1.04 ± 0.02 |
104 |
| 2.00 |
2.02 ± 0.02 |
101 |
Conclusions
A rapid, sensitive and simple electrochemical method for the indirect determination of trace amount of sulfide was developed. Table 3 is a comparison of this method with other methods reported in recent five years. Compared with other methods, this proposed method has the following prominent advantages: (1) Sensitive, the detection limit of this method for S2− can be reduced to 1.3 × 10−8 mol L−1. From Table 3 we can see that only the limit of detection (LOD) of Ref. 12 and 30 is lower than that of this method, but Ref. 30 needs to be combined with flow system. This proposed method is simple and does not require a flow system; (2) Less interference, it does not suffer from interference by the common cations and anions in water samples. Generally, ECL is easy to suffer from interference; (3) Easy to operate, the MFE is easy to prepare and has good reproducibility. Coupled with FIA-KR technique, the LOD can be reduced and we anticipate the development of some simple and portable electrochemical sensors for detecting sulfide in environmental water samples.
Table 3 Comparison of this method with other methodsa
Acknowledgements
This project is supported by the NSFC (20575025 & NFFTBS-J0630425), State Key Laboratory of Electrochemistry of China, Changchun Applied Chemistry Institute (2008008), Analytical Center of Nanjing University, and Postdoctoral Research Funding of Nanjing University (0205003069).
References
- M. O. Andreae, Science, 2007, 315, 50 CrossRef CAS.
- L. Ferrer, G. de Armas, M. Miro, J. M. Estela and V. Cerda, Analyst, 2005, 130, 644 RSC.
- W. S. Yao and F. J. Millero, Mar. Chem., 1996, 52, 1 CrossRef CAS.
- M. Leslie, Science, 2008, 320, 1155 CrossRef CAS.
- M. Colon, M. Iglesias, M. Hidalgo and J. L. Todoli, J. Anal. At. Spectrom., 2008, 23, 416 RSC.
- Y. J. An and T. B. Xin, The Administration and Technique of Environmental Monitoring, 2008, 20, 64 Search PubMed.
- L. Ferrer, J. M. Estela and V. Cerda, Anal. Chim. Acta, 2006, 573–574, 391 CrossRef.
- L. Ferrer, M. Miro, J. M. Estela and V. Cerda, Trends Anal. Chem., 2007, 26, 413 CrossRef CAS.
- Y. Jin, H. Wu, Y. Tian, L. H. Chen, J. J. Cheng and S. P. Bi, Anal. Chem., 2007, 79, 7176 CrossRef CAS.
- C. Fu, Y. Cao, K. Wang, S. S. Liang, L. Xiao, S. M. Ding and X. Yang, Asian J. Ecotoxicol., 2008, 3, 488 Search PubMed.
- J. Shi, J. J. Li and L. B. Qu, Spectrosc. Spectral Anal., 2002, 22, 871 CAS.
- R. F. Huang, X. W. Zheng and Y. J. Qu, Anal. Chim. Acta, 2007, 582, 267 CrossRef CAS.
- M. Colon, J. L. Todoli, M. Hidalgo and M. Iglesias, Anal. Chim. Acta., 2008, 609, 160 CrossRef CAS.
- Y. Wang, W. L. Kang, Y. Wu, D. Zhao and J. J. Zhang, Chin. J. Anal. Chem., 2008, 36, 1575 CAS.
- A. Afkhami and L. Khalafi, Microchim. Acta, 2005, 150, 43 CrossRef CAS.
- J. C. C. Santos, E. B. G. N. Santos and M. Korn, Microchem. J., 2008, 90, 1 CrossRef CAS.
- F. Maya, J. M. Estela and V. Cerda, Anal. Chim. Acta, 2007, 601, 87 CrossRef CAS.
- C. Giuriati, S. Cavalli, A. Gorni, D. Badocco and P. Pastore, J. Chromatogr., A, 2004, 1023, 105 CrossRef CAS.
- D. Giovanelli, N. S. Lawrence, L. Jiang, T. G. J. Jones and R. G. Compton, Analyst, 2003, 128, 173 RSC.
- A. Salimi, S. Pourbeyram and M. K. Amini, Analyst, 2002, 127, 1649 RSC.
- A. Manova, M. Strelec, F. Cacho, J. Lehotay and E. Beinrohr, Anal. Chim. Acta, 2007, 588, 16 CrossRef CAS.
- H. M. Carapuça, M. Valega, E. Pereira and A. C. Duarte, Anal. Chim. Acta, 2004, 524, 127 CrossRef CAS.
- R. Al-Farawati and C. M. G. van den Berg, Mar. Chem., 1997, 57, 277 CrossRef CAS.
- B. Yang, S. Q. Wang, S. B. Tian and L. Z. Liu, Electrochem. Commun., 2009, 11, 1230 CrossRef CAS.
- N. S. Lawrence, Electroanalysis, 2006, 18, 1658 CrossRef CAS.
- D. Giovanelli, N. S. Lawrence, L. Jiang, T. G. J. Jones and R. G. Compton, Sens. Actuators, B, 2003, 88, 320 CrossRef.
- N.
S. Lawrence, M. Thompson, C. Prado, L. Jiang, T. G. J. Jones and R. G. Compton, Electroanalysis, 2002, 14, 499 CrossRef CAS.
- D. Giovanelli, N. S. Lawrence, S. J. Wilkins, L. Jiang, T. G. J. Jones and R. G. Compton, Talanta, 2003, 61, 211 CrossRef CAS.
- A. Salimi, M. Roushani and R. Hallaj, Electrochim. Acta, 2006, 51, 1952 CrossRef CAS.
- D. M. Tsai, A. S. Kumar and J. M. Zen, Anal. Chim. Acta, 2006, 556, 145 CrossRef CAS.
- J. Lawrence, K. L. Robinson and N. S. Lawrence, Anal. Sci., 2007, 23, 673 CrossRef CAS.
- B. Spilker, J. Randhahn, H. Grabow, H. Beikirch and P. Jeroschewski, J. Electroanal. Chem., 2008, 612, 121 CrossRef CAS.
- N. S. Lawrence, G. J. Tustin, M. Faulkner and T. G. J. Jones, Electrochim. Acta, 2006, 52, 499 CrossRef CAS.
- J. M. Zen, J. L. Chang, P. Y. Chen, R. Ohara and K. C. Pan, Electroanalysis, 2005, 17, 739 CrossRef CAS.
- C. E. Banks, A. S. Yashina, G. J. Tustin, V. G. H. Lafitte, T. G. J. Jones and N. S. Lawrence, Electroanalysis, 2007, 19, 2518 CrossRef CAS.
- J. J. Chen, K. Wang, L. Hartman and W. L. Zhou, J. Phys. Chem. C, 2008, 112, 16017 CrossRef CAS.
- N. S. Lawrence, J. Davis, L. Jiang, T. G. J. Jones, S. N. Davies and R. G. Compton, Electroanalysis, 2001, 13, 432 CrossRef CAS.
- N. S. Lawrence, J. Davis, L. Jiang, T. G. J. Jones, S. N. Davies and R. G. Compton, Electroanalysis, 2000, 12, 1453 CrossRef CAS.
- N. S. Lawrence, L. Jiang, T. G. J. Jones and R. G. Compton, Anal. Chem., 2003, 75, 2054 CrossRef CAS.
- M. Pandurangappa, N. S. Lawrence, L. Jiang, T. G. J. Jones and R. G. Compton, Analyst, 2003, 128, 473 RSC.
- Y. S. Song, G. Muthuraman and J. M. Zen, Electrochem. Commun., 2006, 8, 1369 CrossRef CAS.
- A. C. Calokerinos, M. Timotheou-Potamia, E. Sarantonis and T. P. Hadjiioannou, Anal. Chim. Acta, 1983, 151, 85 CrossRef CAS.
- Y. H. Yang, M. H. Yang, H. Wang, J. H. Jiang, G. L. Shen and R. Q. Yu, Sens. Actuators, B, 2004, 102, 162 CrossRef.
- L. J. Liu, Z. C. Chen, S. M. Yang, X. Jin and X. F. Lin, Sens. Actuators, B, 2008, 129, 218 CrossRef.
- N. S. Lawrence, R. P. Deo and J. Wang, Anal. Chim. Acta, 2004, 517, 131 CrossRef CAS.
- N. S. Lawrence, J. Davis and R. G. Compton, Talanta, 2000, 52, 771 CrossRef CAS.
- W. Wardencki, J. Chromatogr., A, 1998, 793, 1 CrossRef CAS.
- M. S. P. Silva, I. S. da Silva, G. Abate and J. C. Masini, Talanta, 2001, 53, 843 CrossRef CAS.
- M. S. P. Silva, C. X. Galhardo and J. C. Masini, Talanta, 2003, 60, 45 CrossRef CAS.
- D. G. Tang and P. H. Santschi, J. Chromatogr., A, 2000, 883, 305 CrossRef CAS.
- D. M. Serafim and N. R. Stradiotto, Fuel, 2008, 87, 1007 CrossRef CAS.
- R. Possari, R. F. Carvalhal, R. K. Mendes and L. T. Kubota, Anal. Chim. Acta, 2006, 575, 172 CrossRef CAS.
-
Analytical Chemistry Faculty Working Office of Central-South Institute of Mining and Metallurgy, Chemical Analysis Handbook, Science Press. Beijing. 1997 Search PubMed.
- V. Kuban, P. K. Dasgupta and J. N. Marx, Anal. Chem., 1992, 64, 36 CrossRef CAS.
-
State Environmental Protection Administration, Methylene Blue Spectrophotometric Method for Sulfide Determination of the Water Quality (GB/T16489-1996), Beijing: Chinese Standard Publishing House, 1997 Search PubMed.
- L. M. de Carvalho, P. C. do Nasciniento, A. Koschinsky, M. Bau, R. F. Stefanello, C. Spengler, D. Bohrer and C. Jost, Electroanalysis, 2007, 19, 1719 CrossRef CAS.
-
Q. L. Li, Electroanalytical Chemistry, Beiing Normal University Press, Beijing. 1997, p358 Search PubMed.
- J. K. Kiptoo, J. C. Ngila and N. D. Silavwe, Microchim. Acta, 2008, 160, 211 CrossRef CAS.
- E. Alonso, A. Santos, M. Callejon and J. C. Jimenez, Chemosphere, 2004, 56, 561 CrossRef CAS.
- T. J. Waite, C. Kraiya, R. E. Trouwborst, S. F. Ma and G. W. Luther, Electroanal., 2006, 18, 1167 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1: The basic water quality parameters in various water samples. See DOI: 10.1039/b9ay00183b |
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and stoichiometric ratio is less than 1, thus, a coefficient γ should be added to the equation. Due to S2− is not the unique form (There are three forms including S2−, HS− and H2S) in solution at pH 4.0, thus, C′Cd2+ is equal to γC*S2–, and S2− reacted with Cd2+ is γ times of total S2− (γ ≈ 0.4), which indicated that the added S2− does not all react with Cd2+, as shown in eqn (4) and (5). Under certain condition, ΔipCd2+ has linear relationship with C*S2–, which is consistent with the experimental results, as shown in Fig. 1.
1, and stoichiometric ratio is less than 1, thus, a coefficient γ should be added to the equation. Due to S2− is not the unique form (There are three forms including S2−, HS− and H2S) in solution at pH 4.0, thus, C′Cd2+ is equal to γC*S2–, and S2− reacted with Cd2+ is γ times of total S2− (γ ≈ 0.4), which indicated that the added S2− does not all react with Cd2+, as shown in eqn (4) and (5). Under certain condition, ΔipCd2+ has linear relationship with C*S2–, which is consistent with the experimental results, as shown in Fig. 1.


