Functional analysis of G-protein coupled receptors using a new fluorescein lactone-based intracellular calcium indicator
Desuo
Yang
a,
Chunmei
Wei
b,
Jinfang
Liao
b and
Zhenjun
Diwu
*b
aDepartment of Chemistry & Chemical Engineering, Baoji University of Arts & Science, 44 Baoguang Road, Baoji, Shaanxi 721007, China
bABD Bioquest, Inc., 923 Thompson Place, Sunnyvale, CA 94085
First published on 7th January 2010
Abstract
Calcium flux assays are preferred methods in drug discovery for characterizing G-protein coupled receptors (GPCRs) and screening GPCR agonists and antagonists. A new fluorescent Ca2+ indicator has been developed to provide improved fluorescence-based assays for detecting intracellular Ca2+ mobilization of GPCR targets. This first fluorescein lactone-based Ca2+ indicator has >100 times fluorescence enhancement upon calcium binding. It has an intracellular Ca2+-induced fluorescence signal more than ∼2 fold brighter than Fluo-4 AM, and ∼4 times brighter than Fluo-3 AM, the two most popular fluorescent Ca2+ indicators.
G-protein coupled receptors (GPCRs) are well known as being the best characterized targets for developing new drugs. There are quite a few approaches for characterizing GPCRs and for screening GPCR agonists and antagonists.1,2 GPCR activations can be assayed by determining the extent of binding of radioactive GTP-γ-S following agonist stimulation.3 Such assays have seen recent improvements, but still lack sensitivity for Gs- and Gq-coupled receptors besides its other limitations such as safety, cost and limited shelf-life of radioactive isotopes.3 The transcriptional reporter assays for cAMP provide good signal-to-noise performance, but are indirect and often result in large numbers of false positives and false negatives.4 Functional assays for Gs- and Gi-coupled GPCRs can be performed using promiscuous Gα16 proteins and Gqi chimeras to redirect the signaling pathway to phospholipase C-mediated intracellular calcium release.5–7 Compared to other methods the functional calcium assay provides excellent signal-to-noise readout and greatest convenience for many Gi- and Gs-coupled receptors. The calcium assay approach enables the use of a FLIPR (fluorescence imaging plate reader) and equivalent fluorescence instruments in combination with fluorescent Ca2+ indicators to provide a “unified” calcium assay for Gq-, Gs- and Gi-coupled GPCRs.5–8
With the recent rapid advance in fluorescence instrumentation, calcium-based GPCR assays has become the gold standard for functional analysis of GPCR targets and high throughput screening of GPCR compound libraries.7,9 In recent years many GPCR targets and screenings have been performed on FLIPR and FDSS instruments using Fluo-3 AM or Fluo-4 AM. It is apparent that the success of a GPCR high throughput screening assay is now greatly dependent on the selection of a suitable calcium indicator, currently only either Fluo-3 AM or Fluo-4 AM.5,8,10 Although Fluo-3 AM and Fluo-4 AM have been widely used in a variety of calcium-based GPCR assays, their intracellular calcium-induced signal intensity is severely limited by their low fluorescence quantum yields (<0.2), the slow dye loading of their AM esters and rapid leakage of their esterase-hydrolyzed anionic fluorophores.
As shown in Scheme 1 we have synthesized a new fluorescein lactone-based Ca2+ indicator that overcomes these limitations associated with Fluo-3 AM and Fluo-4 AM. To our knowledge, cell-impermeable Compound 8 and cell-permeable Compound 9 are the first generation of fluorescein lactone-based Ca2+ indicators in which the BAPTA chelator is incorporated as a conjugated part of the fluorophore. It is impossible to convert Fluo-3 and Fluo-4 into a cell-permeable fluorescein lactone due to the lack of a 3-carboxy group in the structures of Fluo-3 and Fluo-4 (see Scheme 2). Among all the existing fluorescent calcium indicators Calcium Green AM esters contain a fluorescein lactone, but they lack sensitivity to calcium since the BAPTA chelator is not conjugated to their xanthone fluorophore. Both Calcium Green 1 and Calcium Green 2 are much less sensitive to Ca2+ than Fluo-3 and Fluo-4.
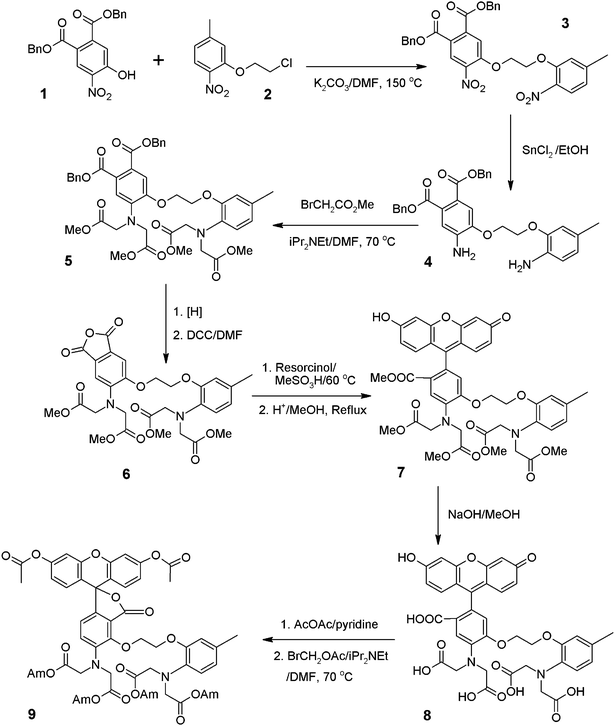 | ||
| Scheme 1 Synthesis of fluorescein-based Ca2+ indicators (Am = CH2OAc). | ||
 | ||
| Scheme 2 Structural comparison of Fluo-3 AM, Fluo-4 AM and Compound 9 (Am = CH2OAc). | ||
We reasoned that the low fluorescence quantum yields of Fluo-3 and Fluo-4 result from the lack of a 3-carboxy group compared to the extremely high fluorescence quantum yield of fluorescein (see Table 1 and Scheme 2). With the addition of 3-carboxy group, Compound 8 demonstrates a much higher fluorescence quantum yield than Fluo-3 and Fluo-4 as shown in Table 1. The addition of the 3-carboxy group makes it possible that Compound 8 can be converted to the non-fluorescent and cell-permeable Compound 9. Lactone 9 is readily loaded into live cells, and hydrolyzed by intracellular esterase to release Compound 8 that is used to monitor Ca2+ mobilization inside cells. Lactone 9 is colorless and non-fluorescent, minimizing the assay background. In contrast, Fluo-3 AM and Fluo-4 AM do not exist in a lactone form due to the lack of 3-carboxy group. Lactone 9 is not excited by the Argon laser (488 nm) due to the lack of a conjugation system while Fluo-3 and Fluo-4 AM (existing in the form of quinone methide) are readily excited by the 488 nm laser line, potentially causing high assay background (See Fig. 1 and 2).
| Ca2+ indicators | Excitation maximum | Emission maximum | Relative ΦFa | K d of Ca2+-binding |
|---|---|---|---|---|
| a In the Ca2+-saturated Tris buffer (pH 7.2). The fluorescence quantum yields were measured using fluorescein as the reference standard (ΦF = 0.88 in 0.1 M NaOH) followed by the protocol of R. A. Velapoldi and H. H. Tonnesen (J. Fluorescence, 2004, 14, 465–472). | ||||
| Fluo-3 | 506 nm | 525 nm | 0.15 | 325 nM |
| Fluo-4 | 493 nm | 515 nm | 0.16 | 350 nM |
| Compound 8 | 496 nm | 518 nm | 0.81 | 339 nM |
 | ||
| Fig. 1 Carbachol-induced calcium changes measured using Fluo-3 AM, Fluo-4 AM and Compound 9 in HEK-293 cells. HEK-293 cells were seeded overnight at 40,000 cells per 100 μl per well in a 96-well black wall/clear bottom costar plate. The growth medium was removed, and the cells were incubated with 100 μl of Compound 9 (A), Fluo-4 AM (B), or Fluo-3 AM (C) for 1 h at 37 °C. 30 μM of carbachol (50 μl/well) was added by NOVOstar (BMG Labtech). | ||
 | ||
| Fig. 2 ATP-induced calcium dose response of Fluo-3 AM, Fluo-4 AM and Compound 9 on HEK-293. HEK-293 cells were seeded overnight at 10,000 cells per 25 μl per well in a 384-well black wall/clear bottom costar plate. The growth medium was removed and one third of the plate wells were incubated with 25 μl of Compound 9, and the other two thirds of the plate wells were incubated with Fluo-3 AM and Fluo-4 AM respectively for 1 h at room temperature. ATP (12.5 μl/well) was added by FLIPR 384 (Molecular Devices) to achieve the final indicated concentration. The data were in triplicate. The EC50 is about 4 μM for Compound 9 (A), Fluo-4 AM (B) and Fluo-3 AM (C). | ||
We discovered that there is another surprising advantage from the addition of the 3-carboxy group. Compound 9 is loaded more readily into live cells in the form of the cell-permeable lactone. The esterase-cleaved anionic product (Compound 8) is retained inside cells in the form of quinone methide much longer than Fluo-3 and Fluo-4 due to its additional negative charge. This is further confirmed by the better retention of fluorescein diacetate (FDA) than Compound 11 and 12, the model compounds of Fluo-3 AM and Fluo-4 AM respectively (See Table 2 and Scheme 3).15 Compound 8 is selective to Ca2+ binding. As seen in Fig. 3, Compound 8 has a much larger fluorescence enhancement to Ca2+ than other physiological ions (such as Na+, K+, Cl− and Mg2+).
| Esterase substrates | Relative initial fluorescence intensityb | Relative remaining fluorescence intensityc |
|---|---|---|
| a Fluo-3 AM was used as the reference standard. (The initial arbitrary fluorescence intensity was set to be 1.0.) b U2OS cells were seeded overnight at 40,000 cells per 100 μL per well in a 96-well black wall/clear bottom costar plate. The growth medium was removed, and the cells were incubated with 100 μl of 4 μM of each dye in Hank's and Hepes Buffered Solution (HHBS) at 37 °C, 5% CO2 incubator for 1 h. The cells were washed twice with 200 μl HHBS and replaced with 100 μL HBSS. The cells were imaged with a fluorescence microscope (Olympus IX71) using FITC channel immediately after the last wash. c The same cells were imaged 30 min after the last wash under the same recording conditions. | ||
| Fluo-3 AM | 1.0 | 0.6 |
| Fluo-4 AM | 2.1 | 0.9 |
| Fluorescein diacetate (FDA) | 6.0 | 4.1 |
| Compound 9 | 4.6 | 3.6 |
| Compound 11 | 1.5 | 0.1 |
| Compound 12 | 2.1 | 0.1 |
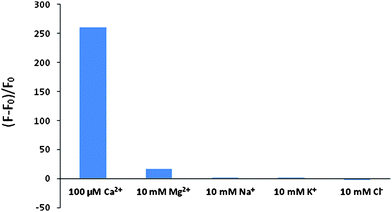 | ||
| Fig. 3 Ion responses to Compound 8. The maximum relative fluorescence intensity was measured for Compound 8 in the presence of different ions. The results were plotted as fluorescence changes relative to ion-free reference solution expressed as (F − F0)/F0 where F0 is the fluorescence intensity of ion-free solution while F is that of ion-containing solutions. Ion-free reference solution contains 10 mM EGTA. | ||
 | ||
| Scheme 3 Structures of FDA and Compounds 11 and 12, the model compounds of Fluo-3 AM, Fluo-4 AM (Am = CH2OAc). | ||
In summary, Compound 8, the cell-impermeable Ca2+ indicator, retains the essential spectral and calcium-binding properties of Fluo-3 and Fluo-4 with enhanced fluorescence signal strength. Compound 9, the cell-permeable version of Compound 8, demonstrates improved intracellular properties that might be used for characterizing some GPCR targets. Compound 9 demonstrates long wavelength, high sensitivity and >100 times fluorescence enhancement upon calcium binding and good cellular retention. These characteristics make this new fluorescein-based Ca2+ indicator a useful probe for monitoring intracellular calcium.
Notes and references
- R. Heilker, L. Zemanova, M. J. Valler and G. U. Nienhaus, Curr. Med. Chem., 2005, 12, 2551–9 CrossRef CAS.
- S. J. Hill, Br. J. Pharmacol., 2006, 147(s1), S27–37 CrossRef CAS.
- E. N. Johnson, X. Shi, J. Cassaday, M. Ferrer, B. Strulovici and P. Kunapuli, Assay Drug Dev. Technol., 2008, 6, 327–37 CrossRef CAS.
- R. Golla and R. Seethala, J. Biomol. Screening, 2002, 7, 515–25 CrossRef CAS.
- K. J. Cassutt, M. J. Orsini, M. Abousleiman, D. Colone and W. Tang, J. Biomol. Screening, 2007, 12, 285–7 CrossRef CAS.
- M. U. Kassack, B. Hofgen, J. Lehmann, N. Eckstein, J. M. Quillan and W. Sadee, J. Biomol. Screening, 2002, 7, 233–246 CAS.
- S. Siehler and D. Guerini, J. Recept. Signal Transduction, 2006, 26, 549–75 Search PubMed.
- P. Hodder, R. Mull, J. Cassaday, K. Berry and B. Strulovici, J. Biomol. Screening, 2004, 9, 417–26 CrossRef CAS.
- T. Zhu, L. Y. Fang and X. Xie, Acta Pharmacol. Sin., 2008, 29, 507–16 Search PubMed.
- M. A. Gilchrist, 2nd, A. Cacace and D. G. Harden, J. Biomol. Screening, 2008, 13, 486–93 CrossRef.
- F. D. Bellamy and K. Ou, Tetrahedron Lett., 1984, 25, 839–842 CrossRef CAS.
- A. Minta, J. P. Kao and R. Y. Tsien, J. Biol. Chem., 1989, 264, 8171–8178 CAS.
- W. Sun, K. R. Gee, D. H. Klaubert and R. P. Hauland, J. Org. Chem., 1997, 62, 6469–6475 CrossRef CAS.
- Preparation of Compound 9. The alkylation of Compound 1 by Compound 2 gave Compound 3 that is readily reduced by SnCl2 to give the diamino Compound 4.11 Aniline 4 was readily alkylated by methyl bromoacetate to give Compound 5 as reported.12 Benzyl Ester 5 was hydrogenated and cyclized to give the desired Anhydride 6 that was condensed with resorcinol to give Compound 7 and its isomer.13 The crude Compound 7 was fully methylated with anhydrous methanol in the presence of sulfuric acid, and the resulted Methyl Ester 7 was further purified on two continuous silica gel columns to give pure Isomer 7. Fluorescein 7 was readily hydrolyzed to give the cell-impermeable Ca2+ Indicator 8 that was readily acetylated by acetic anhydride followed by the alkylation of bromomethyl acetate to give the cell-permeable Ca2+ Indicator 9.14 Specifically Compound 8 (50 mg, 67 μmole) is heated at 80 °C with Ac2O (3 mL) and pyridine (50 μL) until Compound 8 is completely consumed. The solution is cooled to room temperature. The reaction mixture is poured into ice water, and carefully neutralized with Na2CO3 (pH = 4–5). The aqueous mixture was filtered to collect the precipitate. The resulted solid is first air-dried, and further dried in a desiccator with P2O5 for 12 h to yield the crude fluorescein diacetate. The crude fluorescein diacetate (50 mg, 60 μmole) is dissolved in anhydrous DMF (2 mL) at RT. To the DMF solution of fluorescein diacetate BrCH2OAc (70 μL, Aldrich) in anhydrous DMF (2 mL) is slowly added while stirring in a water bath. To the resulted mixture iPr2Net (130 μL) is added slowly. The resulted mixture is stirred for 24–36 h. The reaction mixture is poured into ice/water. The suspension is filtered to collect the solid that is washed with water. The dried solid is purified on a silica gel column to give Compound 9 using a gradient of chloroform/ethyl acetate. Both Compounds 8 and 9 were well characterized for intracellular Ca2+ detection.
| This journal is © The Royal Society of Chemistry 2010 |
