DOI:
10.1039/C0AN00447B
(Paper)
Analyst, 2011,
136, 184-190
Core-shell nanostructured molecular imprinting fluorescent chemosensor for selective detection of atrazine herbicide
Received
26th June 2010
, Accepted 12th August 2010
First published on 1st October 2010
Abstract
To convert the binding events on molecularly imprinted polymers (MIPs) into physically detectable signals and to extract the templates completely are the great challenges in developing MIP-based sensors. In this paper, a core-shell nanostructure was employed in constructing the MIP chemosensor for the improvements of template extraction efficiency and imprinted sites accessibility. Vinyl-substituted zinc(II) protoporphyrin (ZnPP) was used as both fluorescent reporter and functional monomer to synthesize atrazine-imprinted polymer shell at silica nanoparticle cores. The template atrazine coordinates with the Lewis acid binding site Zn of ZnPP to form a complex for the molecular imprinting polymerization. These imprinted sites are located in polymer matrix of the thin shells (∼8 nm), possessing better accessibility and lower mass-transfer resistance for the target molecules. The fluorescence properties of ZnPP around the imprinted sites will vary upon rebinding of atrazine to these imprinted sites, realizing the conversion of rebinding events into detectable signals by monitoring fluorescence spectra. This MIP probe showed a limit of detection (LOD) of about 1.8 μM for atrazine detection. The core-shell nanostructured MIP method not only improves the sensitivity, but also shows high selectivity for atrazine detection when compared with the non-molecular imprinted counterparts.
1. Introduction
Template polymerization techniques such as molecular imprinting have attracted considerable attention as a method for preparing molecular recognition systems and mimicking the specific binding events of biological receptors.1–10 Molecular imprinting is most commonly carried out by the copolymerization of functional monomers and cross-linking agents in the presence of template molecules. Subsequent removal of templates from the polymeric network generates recognition cavities that are complementary to the size, shape and functionality of template molecules. Therefore, the template molecules can selectively rebind into the molecularly imprinted polymers through the specific interaction with these imprinted sites. As artificial molecular recognition materials, MIPs offer a greater stability, lower cost and better engineering possibility than biological receptors such as enzyme and antibody,11 which gives them potential for applications in chemosensors, separation, catalysis and drug delivery.1–5
Although the conventional imprinting protocol is simple and effective, there are two critical factors to limit the applications of molecularly imprinted materials. The first concern is that traditional imprinted materials are usually bulk polymers without regular shape and size, and therefore the extraction of original templates located at interior area is quite difficult due to the highly cross-linking nature.9,10 The second one is that the rigid polymeric matrix greatly restrains target species into those deep imprinted cavities and thus reduces the conformational freedom of molecular recognition. The main approaches to address these concerns have recently been explored by surface imprinting,4,5,8 monomolecular dendritic imprinting,3 and nanostructured imprinting such as nanospheres,12 nanowires,13 nanotube,14 nanofilms15 and core-shell imprinted nanoparticles.9,16 Of these molecularly imprinted materials, core-shell nanoparticles are particularly interesting because they exhibit high surface-to-volume ratio, good dispersion and rapid binding kinetics9 and the templates within the thin shells can be completely removed to form effective recognition sites.
Furthermore, the most challenging way to make molecular imprinting-based chemosensor is probably the conversion of binding or recognition events into optical signals, such as fluorescence or absorbance, which can be easily monitored and quantified.17 Fluorescence techniques allow us to realize high sensitivity for measurement. Herein, we have designed and synthesized a core-shell molecular imprinting-based fluorescent chemosensor via direct imprinting polymerization at the surface of vinyl functionalized silica nanoparticles. Fluorescent ZnPP was selected as the binding event reporter because its fluorescence properties are highly sensitive to the surrounding changes. In most cases, the fluorescence spectra of metalloporphyrins can be changed upon the coordination of axial ligands.18 In this research, we have demonstrated the incorporation of ZnPP into the pre-polymerization system resulting in the MIPs that can produce a quenching response to the rebinding of target analyte. Moreover, such MIP chemosensor shows highly sensitive and selective responses toward atrazine, a herbicide which has become a major concern due to its contamination of drinking water and agricultural products over the past years.19,20
2. Experimental section
2.1 Materials
Zinc(II) protoporphyrin, tetraethylorthosilicate (TEOS), 3-methacryloxypropyl trimethoxysilane (MPS), ethylene glycol dimethacrylate (EGDMA), atrazine, simazine, triazine were used as received from Sigma-Aldrich. Methacrylic acid (MAA), ammonium hydroxide (25%), dimethyl sulfoxide (DMSO), ethanol, anhydrous toluene, acetic acid and methanol were purchased from Shanghai Chemicals Ltd. Deionized (DI) water with a resistivity of 10 MΩ·cm was used.
2.2 Synthesis of silica particles and surface functionalization with MPS
Uniform silica nanoparticles with an average diameter of 130 nm were synthesized using the Stöber method through hydrolysis of TEOS with aqueous ammonia.21 Subsequently, the monodispersive silica particles were chemically modified with MPS using the following procedure. Briefly, 0.1 g of silica nanoparticles and 265 μL of MPS were added into anhydrous toluene to make a 50 mL mixture solution. After the mixture was refluxed for 12 h under dry nitrogen, the MPS-silica nanoparticles were obtained. The resulting MPS-silica nanoparticles were separated and purified by centrifugation (6000 rpm for 8 min) followed by washing three times with DMSO and ethanol, respectively.
2.3 Molecular imprinting at the surface of MPS-silica nanoparticles
Typically, MPS-silica nanoparticles (100 mg) were dispersed in 40 mL of DMSO by ultrasonication. Atrazine (5.176 mg, 0.024 mmol), MAA (8 μL, 0.09 mmol), ZnPP (3.75 mg, 0.006 mmol), EGDMA (90 μL, 0.48 mmol) and AIBN (10 mg) were then added into the above solution followed by purging with nitrogen for 15 min. A two-step temperature polymerization reaction was carried out in an incubating shaker with a rate of 300 rpm.22 The polymerization was first done at 50 °C for 6 h, and then maintained at 60 °C for 24 h. The products were further aged at 80 °C for 6 h to obtain high cross-linking density. The resultant nanoparticles were separated from the mixed solution by centrifugation followed by washing in sequence with DMSO and methanol. The templates in the imprinted polymers were subsequently removed by washing six times with a mixture of methanol/acetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to obtain the chemosensor (SiO2@atrazine-MIP). The non-imprinted counterparts were also prepared for comparison using the same procedures but without atrazine templates (SiO2@atrazine-NIP). Both the SiO2@atrazine-MIP and SiO2@atrazine-NIP nanoparticles were dried in vacuum for future use.
1, v/v) to obtain the chemosensor (SiO2@atrazine-MIP). The non-imprinted counterparts were also prepared for comparison using the same procedures but without atrazine templates (SiO2@atrazine-NIP). Both the SiO2@atrazine-MIP and SiO2@atrazine-NIP nanoparticles were dried in vacuum for future use.
2.4 Instrumentation and characterizations
The morphology of the nanoparticles was examined by FEI Sirion-200 field-emission scanning electron microscope (SEM) and JEOL 2010 transmission electron microscope (TEM). Fluorescence emission and excitation spectra were recorded using Perkin-Elmer LS-45 Luminescence Spectrometer. The infrared spectra were recorded with Nicolet Nexus-670 FT-IR spectrometer using KBr method. UV-visible absorbance spectra were measured with a UNIC UV-4802 spectrometer.
The working solutions were prepared by adding 10 μL of 5 mg mL−1 imprinted particle suspension in 5 mL of DMSO. The initial fluorescence spectra and intensities of the working solutions were recorded. Atrazine at different concentrations was then added to the working solutions on a rocking table with shaking rate at 300 rpm. After the mixtures were incubated at room temperature for one hour the fluorescence spectra and intensities were recorded. The identical analysis procedure was also performed for the two structure-like analogues, triazine and simazine. All measurements were carried out 3 times and the average maximum intensity at 573 nm was recorded.
3. Results and discussion
3.1 Preparation and surface functionalization of silica nanoparticles with vinyl groups
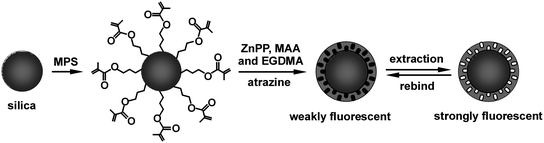
Core-shell imprinted nanoparticles are most commonly prepared via the chemical immobilization of azo-initiators/chain-transfer agents or vinyl functional monomers at the surface of inorganic/organic template colloidal particles,23 followed by initiating a polymerization reaction of the monomers. Recently, we developed a successful surface functional monomer-directing strategy for the formation of uniform core-shell imprinted particles and clearly showed the advantages of core-shell nanostructures over conventional MIP structures, the preparation procedure is complicated and tedious.9 Herein we report a simple and effective method to prepare core-shell MIPs by direct imprinting polymerization at the surface of vinyl functionalized silica particles. As illustrated in Fig. 1, the surface of silica nanoparticles was chemically functionalized with vinyl groups through the reaction of silanol groups with MPS, this lead to the formation of MPS monolayers, which can be further copolymerized with functional monomers in the presence of a template to form MIP shells. The surface-functionalized silica are still monodispersive, as shown in the SEM image of Fig. 2A. As can be seen in the inset TEM image, the surface of the silica particles is smooth and uniform, which ensures the formation of uniform core-shell structures in the imprinting polymerization. Fig. 2B represents the infrared spectra of unmodified silica and MPS-silica nanoparticles. Clearly, two new bands at 2986 cm−1 and 1698 cm−1 appear in the MPS-silica sample and can be attributed to asymmetry stretching vibration of C–H bonds of methylene and the stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the carbonyl group, respectively. The appearances of these bands indicate the covalent grafting of MPS to silica nanoparticle surface.
O bond of the carbonyl group, respectively. The appearances of these bands indicate the covalent grafting of MPS to silica nanoparticle surface.
3.2 Molecular imprinting through copolymerization of two functional monomers at the surface of MPS-silica
The target herbicide, atrazine, was used as the template compound for the synthesis of MIP chemosensor. Fluorescent ZnPP was employed as the reporter of the atrazine binding event because its fluorescence properties could be altered upon the coordination of its axial binding site Zn with atrazine (Fig. 3). In addition, ZnPP also contains two vinyl groups necessary for the copolymerization to synthesize the MIP-based optical sensors. MAA was used as the cofunctional monomer to prepare the copolymer framework for its hydrogen bonding interactions with atrazine, which could further enhance the molecular imprinting effect.24 The combination utilization of the two functional monomers not only strongly enhances the molecular imprinting effect but also realizes the conversion of binding events into detectable signals. Fig. 3 illustrates the monomer-template complex (1), molecular imprinting polymerization (2), and the extraction and rebinding of template molecules of the MIP chemosensor (3). The zinc center of ZnPP is a relatively strong Lewis acid which is capable of binding with the nitrogen of atrazine to form a complex through the acid–base pairing interactions. Moreover, the dual hydrogen bonding interactions between template molecules and MAA moieties further increase the molecular imprinting capacity.
 |
| | Fig. 3 Schematic representation of molecular imprinting of atrazine using both ZnPP and MAA as functional monomers. | |
The molecular imprinting system was accomplished by direct copolymerization and cross-linking reactions in the presence of functional monomers (MAA and ZnPP), cross-linking agents (EGDMA), template molecules (atrazine), and initiator (AIBN). The final atrazine-imprinted core-shell nanostructured fluorescent chemosensor (SiO2@atrazine-MIP nanoparticles) was obtained after the templates were removed from the polymer shells by solvent extraction in the mixture of methanol and acetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The copolymerization of MPS monolayers with functional monomers resulted in selective polymerization at the surface of silica particles, and hence the formation of uniform shells. During the imprinting polymerization and cross-linking process, atrazine in the form of complex was embedded into the cross-linked polymer matrix, as shown in Fig. 3 (2). The template molecules can be extracted to generate atrazine-imprinted sites in the polymer network matrix when they are protonated in acidified solvent. Thus the resultant imprinted sites are complementary to the shape, size, and chemical functionality of the atrazine target analyte. Under appropriate conditions, atrazine can rebind to these cavities through hydrogen bonding with the carboxyl groups of MAA and coordination with the zinc metal of ZnPP, altering the fluorescence properties of ZnPP. In this way, the rebind and coordination of atrazine can be easily monitored and quantified by recording the variation of its fluorescence spectra and intensity.
1, v/v). The copolymerization of MPS monolayers with functional monomers resulted in selective polymerization at the surface of silica particles, and hence the formation of uniform shells. During the imprinting polymerization and cross-linking process, atrazine in the form of complex was embedded into the cross-linked polymer matrix, as shown in Fig. 3 (2). The template molecules can be extracted to generate atrazine-imprinted sites in the polymer network matrix when they are protonated in acidified solvent. Thus the resultant imprinted sites are complementary to the shape, size, and chemical functionality of the atrazine target analyte. Under appropriate conditions, atrazine can rebind to these cavities through hydrogen bonding with the carboxyl groups of MAA and coordination with the zinc metal of ZnPP, altering the fluorescence properties of ZnPP. In this way, the rebind and coordination of atrazine can be easily monitored and quantified by recording the variation of its fluorescence spectra and intensity.
3.3 Morphology and spectral properties of SiO2@atrazine-MIP nanoparticles
Fig. 4A shows the TEM images of the SiO2@atrazine-MIP nanoparticles and it can be seen that most of the particles remain spherical and monodispersive. The thickness of the uniform shell is estimated to be ∼8 nm. The solution of SiO2@atrazine-MIP particles possesses the similar maxima of absorption bands to ZnPP at 414, 543, and 578 nm, respectively (Fig. 4B). These bands are assigned as the Soret (or B) band and the Q bands, traditionally identified as the S2 ← S0 and S1 ← S0 transitions, respectively. The curve 3 in Fig. 4B represents the emission spectra of SiO2@atrazine-MIP particles in solution when excited at 407 nm. It exhibits a typical mirror image of the Q band of the corresponding absorption spectra. Accordingly, the fluorescence maxima are assigned as Q (0, 0) at 573 nm and Q (0, 1) at 623 nm. Due to the strong fluorescence intensity of ZnPP, the red fluorescence can be observed when the solution of SiO2@atrazine-MIP particles is under a UV lamp (inset image of Fig. 4B). The fluorescence stability of SiO2@atrazine-MIP particles in DMSO was also examined, as presented in Fig. 5. Clearly, the fluorescence is very stable and the intensity is virtually unchanged within one hour, which ensures the measurement reliability and improves the LOD of the chemosensor.
 |
| | Fig. 4 (A) TEM image of SiO2@atrazine-MIP nanoparticles. (B) UV absorption (curve 1), excitation (curve 2) and emission (curve 3) spectra of SiO2@atrazine-MIP nanoparticles in DMSO solution. The inset represents the photography of SiO2@atrazine-MIP solution under irradiation of 365 nm UV lamp. | |
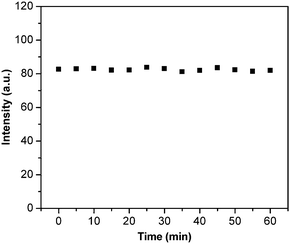 |
| | Fig. 5 Fluorescence stability of SiO2@atrazine-MIP nanoparticles against time in DMSO solutions. | |
The fluorescence quenching behaviour in these systems follow the Stern–Volmer equation at low concentration ranges: (I0/I) = 1 + KSV[A], where I0 and I are the fluorescence intensities in the absence and presence of analyte, respectively. [A] represents the concentration of the analyte, and KSV is the quenching constant of the analyte. As shown in Fig. 6B, the Stern–Volmer plots of SiO2@atrazine-MIP, SiO2@atrazine-NIP particles, and pure ZnPP dye all exhibit linear relationship when the concentration of atrazine is below 10−4 M. The quenching constants were calculated to be about 3499 M−1, 1079 M−1, and 204 M−1 for SiO2@atrazine-MIP, SiO2@atrazine-NIP particles, and pure ZnPP dye, respectively. The quenching efficiency for SiO2@atrazine-MIP particles is about 3-fold that for SiO2@atrazine-NIP particles, which could be attributed to the specific binding affinity of atrazine due to an efficient imprinting effect. These above results indicate that atrazine could be quantitatively detected using SiO2@atrazine-MIP particles by the measurement of fluorescence quenching of the embedded ZnPP. In the case of core-shell structure, the imprinted sites are situated in the shell of the materials, hence provide excellent site accessibility and low mass-transfer resistance for the target atrazine to rebind. The rebinding events of the target species into the imprinted sites are driven by the formation of hydrogen bonding and the coordination with the metal centers of ZnPP. Therefore, the core-shell imprinted particles exhibit very high sensitivity to target species and the LOD for atrazine is calculated as low as 1.8 μM.
3.5 Molecular selectivity of the atrazine-imprinted nanoparticles
The fluorescence of SiO2@atrazine-MIP chemosensor shows a selective quenching effect by atrazine over other structural analogues including simazine and triazine. The fluorescence of the probe was much less quenched by both simazine and triazine when compared with atrazine. The difference could be due to the size variation of the two analogues from the molecular imprinted cavities. It can be seen from Fig. 7, atrazine and simazine have nearly identical chemical and spectral properties, but different only in size and shape. Simazine has slightly bigger ethyl groups than atrazine and it is hard to diffuse into the imprinting cavities, resulting in much low quenching effect (Fig. 8A). On the other hand, the compound triazine is much smaller than atrazine and almost sterically unencumbered to enter into the imprinting site, leading to the fluorescence quenched slightly more than simazine. In contrast, the SiO2@atrazine-NIP probe has no selectivity among the three analogues (Fig. 8B). The three compounds all can coordinate randomly with the ZnPP situated in the proximity of the shell surface of the probe, resulting in fluorescence quenching at the similar level.
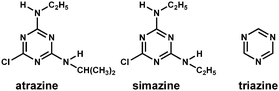 |
| | Fig. 7 Chemical structures of the herbicides used. | |
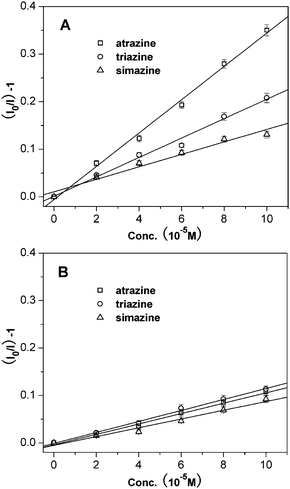 |
| | Fig. 8 Stern–Volmer plots from (A) SiO2@atrazine-MIP particles, and (B) SiO2@atrazine-NIP particles with atrazine (□), triazine (○) and simazine (△). | |
The Stern–Volmer quenching constants of the probes by herbicides are summarized in Fig. 9. In the case of non-templated core-shell structure of SiO2@atrazine-NIP nanoparticles, the fluorescence quenching constants for atrazine, triazine, and simazine are very low and nearly identical. This further suggests that the probe has no selectivity among the three compounds. However, the big difference in the quenching constants are observed in the case of atrazine templated core-shell structure of SiO2@atrazine-MIP nanoparticles. The results indicate an efficient imprinting effect responsible for the highly selective fluorescence quenching toward atrazine.
3.6 Recovery test of atrazine in real lake water
The recovery test of atrazine was conducted in DI water and real lake water, respectively, to examine if there is any positive or negative interference in real drinking water samples. We first examined the effect of water on the fluorescence stability and found no such effect. The DI water was used directly for the recovery test. The real lake water collected from a local lake was filtered first through 0.45 μm Supor filters to remove any particulate suspension. The recovery study was carried out on the mixture of water and DMSO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) spiked with 0, 10, 20 and 50 μM atrazine. Each concentration was done in triplicate and the average was presented with standard deviation. The analysis results for the two types of sample with and without spiked atrazine are given in Table 1.
1, v/v) spiked with 0, 10, 20 and 50 μM atrazine. Each concentration was done in triplicate and the average was presented with standard deviation. The analysis results for the two types of sample with and without spiked atrazine are given in Table 1.
| Spiked concentration/μM |
DI water/DMSO |
Lake water/DMSO |
| Found by MIP probe/μM |
Recovery (%) |
Found by MIP probe/μM |
Recovery (%) |
| 0 |
0.6 |
— |
0.8 |
— |
| 10 |
9.1 |
91.0 ± 1.8 |
11.8 |
118.0 ± 2.7 |
| 20 |
21.3 |
106.5 ± 2.0 |
18.4 |
92.0 ± 1.6 |
| 50 |
48.8 |
97.6 ± 1.5 |
45.3 |
90.6 ± 1.1 |
The contents of atrazine in both DI water and lake water without spiked atrazine are below the LOD of the method, so the values are not considered in the calculation of recoveries. It can be seen that the recoveries of atrazine for lake water samples are statistically close to those values for DI water samples, suggesting no serious positive or negative interferences due to humic materials present in real lake water.
4. Conclusions
A core-shell nanostructured molecularly imprinted polymer based chemosensor was designed, synthesized, and investigated for sensitive and selective detection of atrazine. The chemosensor was constructed by imprinting template molecules and fluorescent rebinding event reporters in the polymer shells on the silica cores, and it has been demonstrated that the utilization of thin polymer shells in the chemosensor improves template extraction efficiency and imprinted site accessibility. The MIP-based chemosensor successfully realized the conversion of detectable fluorescence signals from rebinding and recognition events by embedding fluorescent zinc(II) protoporphyrin in the shell structures. The analytical performance of the core-shell MIP chemosensor for atrazine detection was also assessed and it was found that the limit of detection was 1.8 μM. The core-shell nanostructured chemosensor also showed stereoselective and size-selective detection for atrazine among other analogues such as triazine and simazine due to the imprinted cavities in the thin polymer shells.
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 20925518, 20875090, 20807042, 30901008), China-Singapore Joint Project (2009DFA51810), 863 High Technology Project of China (2007AA10Z434), Innovation Project of Chinese Academy of Science (KSCX2-YW-G-058) and the Natural Science Foundation of Anhui Province (090411022).
References
- G. Wulff, Chem. Rev., 2002, 102, 1–27 CrossRef CAS; K. Haupt and K. Mosbach, Chem. Rev., 2000, 100, 2495–2504 CrossRef CAS.
- A. Katz and M. E. Davis, Nature, 2000, 403, 286–289 CrossRef CAS; J. D. Bass and A. Katz, Chem. Mater., 2003, 15, 2757–2763 CrossRef CAS.
- S. C. Zimmerman, M. S. Wendland, N. A. Rakow, I. Zharov and K. S. Suslick, Nature, 2002, 418, 399–403 CrossRef CAS.
- E. Yilmaz, K. Haupt and K. Mosbach, Angew. Chem., Int. Ed., 2000, 39, 2115–2118 CrossRef CAS.
- M. Tatemichi, M. A. Sakamoto, M. Mizuhata, S. Deki and T. Takeuchi, J. Am. Chem. Soc., 2007, 129, 10906–10910 CrossRef CAS.
- G. Wulff, Angew. Chem., Int. Ed. Engl., 1995, 34, 1812–1832 CrossRef CAS.
- K. Mosbach and O. Ramström, Bio/Technology, 1996, 14, 163–170 CrossRef CAS.
- H. Q. Shi, W. B. Tsai, M. D. Garrison, S. Ferrari and B. D. Ratner, Nature, 1999, 398, 593–597 CrossRef CAS.
- D. M. Gao, Z. P. Zhang, M. H. Wu, C. G. Xie, G. J. Guan and D. P. Wang, J. Am. Chem. Soc., 2007, 129, 7859–7866 CrossRef CAS.
- C. G. Xie, B. H. Liu, Z. Y. Wang, D. M. Gao, G. J. Guan and Z. P. Zhang, Anal. Chem., 2008, 80, 437–443 CrossRef CAS; G. J. Guan, Z. P. Zhang, Z. Y. Wang, B. H. Liu, D. M. Gao and C. G. Xie, Adv. Mater., 2007, 19, 2370–2374 CrossRef CAS.
- N. A. O'Connor, D. A. Paisner, D. Huryn and K. J. Shea, J. Am. Chem. Soc., 2007, 129, 1680–1689 CrossRef CAS; C. Ayela, F. Vandevelde, D. Lagrange, K. Haupt and L. Nicu, Angew. Chem., Int. Ed., 2007, 46, 9271–9274 CrossRef CAS; O. Hayden, K. J. Mann, S. Krassnig and F. L. Dickert, Angew. Chem., Int. Ed., 2006, 45, 2626–2629 CrossRef CAS.
- Z. Li, J. F. Ding, M. Day and Y. Tao, Macromolecules, 2006, 39, 2629–2636 CrossRef CAS; H. Kempe and M. Kempe, Anal. Chem., 2006, 78, 3659–3666 CrossRef CAS; G. Ciardelli, C. Borrelli, D. Silvestri, C. Cristallini, N. Barbani and P. Giusti, Biosens. Bioelectron., 2006, 21, 2329–2338 CrossRef CAS.
- H. H. Yang, S. Q. Zhang, F. Tan, Z. X. Zhuang and X. R. Wang, J. Am. Chem. Soc., 2005, 127, 1378–1379 CrossRef CAS; C. G. Xie, Z. P. Zhang, D. P. Wang, G. J. Guan, D. M. Gao and J. H. Liu, Anal. Chem., 2006, 78, 8339–8346 CrossRef CAS.
- H. J. Wang, W. H. Zhou, X. F. Yin, Z. X. Zhuang, H. H. Yang and X. R. Wang, J. Am. Chem. Soc., 2006, 128, 15954–15955 CrossRef CAS.
- F. Shi, Z. Liu, G. L. Wu, M. Zhang, H. Chen, Z. Q. Wang, X. Zhang and I. Willner, Adv. Funct. Mater., 2007, 17, 1821–1827 CrossRef CAS; T. Piacham, A. Josell, H. Arwin, V. Prachayasittikul and L. Ye, Anal. Chim. Acta, 2005, 536, 191–196 CrossRef CAS; K. Das, J. Penelle and V. M. Rotello, Langmuir, 2003, 19, 3921–3925 CrossRef CAS; T. Kunitake and S. W. Lee, Anal. Chim. Acta, 2004, 504, 1–6 CrossRef CAS.
- C. J. Tan, H. G. Chua, K. H. Ker and Y. W. Tong, Anal. Chem., 2008, 80, 683–692 CrossRef CAS; C. J. Tan and Y. W. Tong, Anal. Chem., 2007, 79, 299–306 CrossRef CAS.
- W. Wang, S. H. Gao and B. H. Wang, Org. Lett., 1999, 1, 1209–1212 CrossRef CAS; C. A. Carlson, J. A. Lloyd, S. L. Dean, N. R. Walker and P. L. Edmiston, Anal. Chem., 2006, 78, 3537–3542 CrossRef CAS; D. L. Rathbone and A. Bains, Biosens. Bioelectron., 2005, 20, 1438–1442 CrossRef CAS; H. Kubo, N. Yoshioka and T. Takeuchi, Org. Lett., 2005, 7, 359–362 CrossRef CAS; M. K. P. Leung, C. F. Chow and M. H. W. Lam, J. Mater. Chem., 2001, 11, 2985–2991 RSC.
- Y. Aoyama, M. Asakawa, A. Yamagishi, H. Toi and H. Ogoshi, J. Am. Chem. Soc., 1990, 112, 3145–3151 CrossRef CAS; T. Mizutani, T. Kurahashi, T. Murakami, N. Matsumi and H. Ogoshi, J. Am. Chem. Soc., 1997, 119, 8991–9001 CrossRef.
- T. A. Sergeyeva, S. A. Piletsky, A. A. Brovko, E. A. Slinchenko, L. M. Sergeeva and A. V. El'skaya, Anal. Chim. Acta, 1999, 392, 105–111 CrossRef CAS; P. Kueseng, M. L. Noir, B. Mattiasson, P. Thavarungkul and P. Kanatharana, J. Environ. Sci. Health, Part B, 2009, 44, 772–780 CrossRef CAS; D. Djozan and B. Ebrahimi, Anal. Chim. Acta, 2008, 616, 152–159 CrossRef CAS; R. G. C. Silva, C. R. M. Vigna, C. B. G. Bottoli, C. H. Collins and F. Augusto, J. Sep. Sci., 2010, 33, 1319–1324; J. Matsui, K. Fujiwara and T. Takeuchi, Anal. Chem., 2000, 72, 1810–1813 CrossRef CAS.
- W. B. Shim, Z. Y. Yang, J. Y. Kim, J. G. Choi, J. H. Je, S. J. Kang, A. Y. Kolosova, S. A. Eremin and D. H. Chung, J. Agric. Food Chem., 2006, 54, 9728–9734 CrossRef CAS; W. Fan, T. Yanase, H. Morinaga, S. Gondo, T. Okabe, M. Nomura, T. Hayes, R. Takayanagi and H. Nawata, Biochem. Biophys. Res. Commun., 2007, 355, 1012–1018 CrossRef CAS.
- W. Stöber, A. Finker and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- Q. Lu, X. M. Chen, L. Nie, J. Luo, H. J. Jiang, L. N. Chen, Q. Hu, S. H. Du and Z. P. Zhang, Talanta, 2010, 81, 959–966 CrossRef CAS.
- C. Sulitzky, B. Rückert, A. J. Hall, F. Lanza, K. Unger and B. Sellergren, Macromolecules, 2002, 35, 79–91 CrossRef CAS; C. H. Lu, W. H. Zhou, B. Han, H. H. Yang, X. Chen and X. R. Wang, Anal. Chem., 2007, 79, 5457–5461 CrossRef CAS.
- J. Matsui, M. Higashi and T. Takeuchi, J. Am. Chem. Soc., 2000, 122, 5218–5219 CrossRef CAS; T. Takeuchi, T. Mukawa, J. Matsui, M. Higashi and K. D. Shimizu, Anal. Chem., 2001, 73, 3869–3874 CrossRef CAS; A. J. Tong, H. Dong and L. D. Li, Anal. Chim. Acta, 2002, 466, 31–37 CrossRef CAS; S. K. Chou and M. J. Syu, Biomaterials, 2009, 30, 1255–1262 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to obtain the chemosensor (SiO2@atrazine-MIP). The non-imprinted counterparts were also prepared for comparison using the same procedures but without atrazine templates (SiO2@atrazine-NIP). Both the SiO2@atrazine-MIP and SiO2@atrazine-NIP nanoparticles were dried in vacuum for future use.
1, v/v) to obtain the chemosensor (SiO2@atrazine-MIP). The non-imprinted counterparts were also prepared for comparison using the same procedures but without atrazine templates (SiO2@atrazine-NIP). Both the SiO2@atrazine-MIP and SiO2@atrazine-NIP nanoparticles were dried in vacuum for future use.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the carbonyl group, respectively. The appearances of these bands indicate the covalent grafting of MPS to silica nanoparticle surface.
O bond of the carbonyl group, respectively. The appearances of these bands indicate the covalent grafting of MPS to silica nanoparticle surface.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The copolymerization of MPS monolayers with functional monomers resulted in selective polymerization at the surface of silica particles, and hence the formation of uniform shells. During the imprinting polymerization and cross-linking process, atrazine in the form of complex was embedded into the cross-linked polymer matrix, as shown in Fig. 3 (2). The template molecules can be extracted to generate atrazine-imprinted sites in the polymer network matrix when they are protonated in acidified solvent. Thus the resultant imprinted sites are complementary to the shape, size, and chemical functionality of the atrazine target analyte. Under appropriate conditions, atrazine can rebind to these cavities through hydrogen bonding with the carboxyl groups of MAA and coordination with the zinc metal of ZnPP, altering the fluorescence properties of ZnPP. In this way, the rebind and coordination of atrazine can be easily monitored and quantified by recording the variation of its fluorescence spectra and intensity.
1, v/v). The copolymerization of MPS monolayers with functional monomers resulted in selective polymerization at the surface of silica particles, and hence the formation of uniform shells. During the imprinting polymerization and cross-linking process, atrazine in the form of complex was embedded into the cross-linked polymer matrix, as shown in Fig. 3 (2). The template molecules can be extracted to generate atrazine-imprinted sites in the polymer network matrix when they are protonated in acidified solvent. Thus the resultant imprinted sites are complementary to the shape, size, and chemical functionality of the atrazine target analyte. Under appropriate conditions, atrazine can rebind to these cavities through hydrogen bonding with the carboxyl groups of MAA and coordination with the zinc metal of ZnPP, altering the fluorescence properties of ZnPP. In this way, the rebind and coordination of atrazine can be easily monitored and quantified by recording the variation of its fluorescence spectra and intensity.





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) spiked with 0, 10, 20 and 50 μM atrazine. Each concentration was done in triplicate and the average was presented with standard deviation. The analysis results for the two types of sample with and without spiked atrazine are given in Table 1.
1, v/v) spiked with 0, 10, 20 and 50 μM atrazine. Each concentration was done in triplicate and the average was presented with standard deviation. The analysis results for the two types of sample with and without spiked atrazine are given in Table 1.
