Colorimetric detection of melamine in complex matrices based on cysteamine-modified gold nanoparticles†
Xiaosheng
Liang
ab,
Hongping
Wei
a,
Zongqiang
Cui
a,
Jiaoyu
Deng
a,
Zhiping
Zhang
a,
Xiangyu
You
ab and
Xian-En
Zhang
*a
aState Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan, 430071, China. E-mail: x.zhang@wh.iov.cn; Fax: +86 27 8719 9492; Tel: +86 27 8719 9115
bGraduate School, Chinese Academy of Sciences, Beijing, 100039, China
First published on 29th September 2010
Abstract
A sensitive assay for melamine in complex matrices is built using cysteamine-modified gold nanoparticles (AuNPs) and an effective sample pretreatment protocol. Citrate-stabilized AuNPs were modified by cysteamine in order to weaken the electrostatic repulsion force between the gold nanoparticles. Detection sensitivity gained through this modification increased about 100 fold compared with the result using the unmodified AuNPs. Direct colorimetric visualizations of melamine in milk products, eggs and feeds was successfully demonstrated within the linear ranges of 1–200 mg L−1 and detection limits below 1 mg L−1. The proposed scheme could be an alternative means for onsite detection of melamine without costly instruments.
Introduction
Melamine is an organic base and a trimer of cyanamide, with a 1,3,5-triazine skeleton. It is used extensively in resin manufacture for its fire retardant properties, and has several other industrial uses.1 Because melamine contains 66% nitrogen by mass, there are frequent reports that melamine has been illegally added into milk products and feeds in order to increase apparent protein content measured by the most common chemical assays used to test for food protein, such as the Kjeldahl method.2 Since ingestion of melamine at levels above the safety limits (2.5 ppm in USA and EU; 1 ppm for infant formula in China) may cause kidney malfunction,3melamine adulteration is considered as a serious food safety issue. Therefore, it is very useful to develop simple, rapid and sensitive methods for the detection of melamine in real samples, such as milk products, eggs and feeds.Currently there are a number of analytical techniques for detecting melamine in milk products. Among them, high performance liquid chromatography methods (HPLC)4 and gas chromatography coupled with mass spectrometry (GC-MS)5 are the most commonly used techniques. But these methods are expensive and in need of dedicated instruments.
Recently, gold nanoparticles (AuNPs) have received great attention in the development of visual sensing schemes. The use of AuNPs as a colorimetric reporter relies on their unique surface plasmon resonance (SPR) with colors of red or blue corresponding to their dispersion or aggregation state, respectively. Based on this principle, several colorimetric assays have been developed for the detection of DNA, proteins, ions, amino acids,6–9TNT, viruses, cancerous cells and β-lactamase.10–13 Several assays based on AuNPs have also been developed for melamine detection. In one of the assays, triple hydrogen-bonding recognition between melamine and a cyanuric acid derivative grafted on the surface of AuNPs has been used for reliable detection of melamine.14 In other reports, electrostatic interactions between melamine and citrate-capped AuNPs have been exploited for the detection.15,16,17 Because citrate-capped AuNPs are easily synthesized, the assays based on electrostatic interactions between melamine and citrate-capped AuNPs can be easily performed and appear to be a promising method for real applications.
Herein, we demonstrate a more sensitive assay for melamine based on electrostatic interactions between melamine and citrate-capped AuNPs. In this study, citrate-stabilized AuNPs were further modified by cysteamine in order to weaken the electrostatic repulsion force between AuNPs. Through controlling the concentration of cysteamine used for the modification, the AuNPs could be modified to a critical state, at which a tiny amount of melamine existing in the sample could induce the modified AuNPs to aggregate. In previous similar research,15 AuNP modification was anticipated to be more sensitive. In this work, compared with unmodified AuNPs, the sensitivity could be improved about 100 times for the detection of melamine using the cysteamine-modified AuNPs. Meanwhile, a pretreatment procedure for universal samples was proposed and excellently performed in formula products, eggs and feeds.
Experimental section
General information
Tetrachloroauric acid, sodium citrate, urea and hydrogen chloride were purchased from Sinopharm Chemical Reagent Co, Ltd. (Shanghai, China). Melamine, poly-L-lysine and cysteamine were purchased from Sigma-Aldrich (Milwaukee, WI, USA). Glycine and lysine were obtained from Merck Co. Ltd. (USA). dNTP (deoxyadenosine ribonucleoside triphosphate, deoxycytidine ribonucleoside triphosphate, deoxyguanosine ribonucleoside triphosphate and deoxythymidine ribonucleoside triphosphate) were obtained from Takara (Japan). Milk was purchased from Mengniu Dairy Group Co. Ltd. (Huhhot, China). Milk powder was purchased from Guangming Dairy Group Co. Ltd. (Shanghai, China). Pedigree adult dog food was obtained from Mars Foods Inc (McLean, VA, USA). Eggs were purchased from a local market. Other reagents and chemicals were at least of analytical grade. Serological plates were obtained from JET Biochemicals Int'l, Inc. (Canada). Spectrometric measurements were performed on a Synergy HT microplate reader (Bio-Tek Instrument, USA).Preparation of 13 nm citrate-stabilized AuNPs
AuNPs with a diameter of 13.0 nm were prepared by a trisodium citrate reduction method as reported before.6,18 Briefly, trisodium citrate (5 mL, 38.8 mM) was rapidly added to a boiling solution of HAuCl4 (50 mL, 1 mM) and the solution was continually boiled for another 30 min to give a wine-red solution. After filtering the solution through a 0.22-μm Millipore syringe filter to remove the precipitate, the filtrate was stored in a refrigerator at 4 °C. The concentration of the prepared AuNPs was 10 nM as determined by UV-Vis spectrometry.AuNP modification
The 13 nm citrate-stabilized AuNPs were modified with cysteamine by mixing 50 μM cysteamine solution with the AuNP solution at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The modification was finished after the mixed solution was wrapped in foil and shaken gently for 2 h.
100. The modification was finished after the mixed solution was wrapped in foil and shaken gently for 2 h.
Milk sample pretreatment
The pretreatment procedures were similar to that reported for chromatography.19 Briefly, proteins in the samples were removed first by adding 1% (v/v) trichloroacetic acid into the milk samples with a 20 min sonication at a frequency of 20 KHz and a power of 277 W (Sonics VC 750, Vibra-Cell, France). After centrifuging the sample at 8000 g for 10 min, the supernatant was filtered through a 0.22 μm membrane to remove lipids. Then, 1% (wt/vol) granular active charcoal was added into the filtrate and the samples were centrifuged at 8000 g for 10 min again. The pH of the supernatant was adjusted to 7 by adding 1 M HCl. Finally, the supernatant was filtered through a 0.22 μm membrane after vortexing.Colorimetric assay
To measure melamine in the samples, 80 μL of the cysteamine-modified AuNPs and 10 μL of glycine-Cl−buffer (0.2 M, pH 3.2) were pipetted into wells of a serological plate and then mixed together. Then, 10 μL of melamine solution or the pretreated samples spiked with melamine were added into the wells, respectively. After a few minutes of development, the color changes were observed either by the naked eye or from the absorbance values of the solutions measured using the microplate reader (synergy HT Biotek).Results and discussion
Modification of AuNPs
As reported previously, melamine has a strong electrostatic interaction with citrate-capped AuNPs, which decreases the stability of the AuNPs, thus causing visible color changes indicating the existence of melamine. We speculated that the sensitivity of the colorimetric signal could be improved by adjusting the surface charges of the citrate-capped AuNPs (Fig. 1). To demonstrate this, cysteamine-modified AuNPs were prepared. The mercapto group of cysteamine could be easily attached to the surface of the AuNPs by the formation of Au–S bonds between the –NH2groups exposed on the outer surface of the citrate-capped AuNPs. Therefore, the surface charges of the AuNPs could be adjusted by controlling the modification process. Ideally, the cysteamine-modified AuNPs should be sensitive enough to fit the detection needs and be stable enough for storage. Fig. 2 shows the color changes and UV-spectra of the AuNP solution modified by ligand exchange with different concentrations of cysteamine. It could be found that the UV-Vis spectrum of the AuNPs modified with 0.5 μM cysteamine (AuNP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cysteamine = 50) showed very little difference to that of the unmodified AuNPs. However, modification with higher concentrations of cysteamine decreased the specific surface plasmon resonance of the AuNPs at 520 nm, which indicated a decreased stability of the AuNPs. Based on these results, AuNPs modified at a concentration ratio of 50 (AuNP
cysteamine = 50) showed very little difference to that of the unmodified AuNPs. However, modification with higher concentrations of cysteamine decreased the specific surface plasmon resonance of the AuNPs at 520 nm, which indicated a decreased stability of the AuNPs. Based on these results, AuNPs modified at a concentration ratio of 50 (AuNP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cysteamine) were used throughout the following experiments, unless otherwise indicated.
cysteamine) were used throughout the following experiments, unless otherwise indicated.
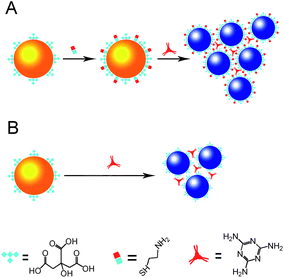 | ||
| Fig. 1 Colorimetric detection of melamine using cysteamine-modified (A) and unmodified (B) gold nanoparticles. | ||
 | ||
| Fig. 2 (A) Visual color change of AuNPs modified with cysteamine at different concentrations (from left to right: 0, 0.5 μM, 0.6 μM, 0.7 μM, 0.8 μM, 0.9 μM, 1 μM, and 10 μM.). The concentration of AuNPs in the solutions was 10 nM; (B) UV-Vis spectra of the corresponding AuNP solutions modified with cysteamine at different concentrations. | ||
pH dependence and specificity
Different melamine solutions were tested using the cysteamine-modified AuNPs. Similar to the detection based on unmodified AuNPs,15 the interaction between melamine and the cysteamine-modified AuNPs showed pH dependence. As shown in Fig. 3A, the optimum pH was between pH 3.0 and pH 4.0 for melamine detection with a detection limit of about 0.04 mg L−1 (signal/noise = 3) and a linear range up to 2.5 mg L−1. When the pH was below 3, the AuNPs became less sensitive to melamine. When the pH was above 4, the linear range of the detection became narrow. Therefore, pH 3.3 was chosen as the optimum pH for melamine detection. It is worthy of note that pH 3.3 is within the optimum pH range (pH 3–pH 4) for melamine detection using citrate-capped AuNPs, as reported previously. Fig. 3B shows that a dramatic redshift of the modified AuNPs could be observed for melamine, but not for urea, dNTP or lysine. But poly-L-lysine could induce a strong color change. These results are similar to that of the unmodified AuNPs and means that other molecules with similar exocyclic amines could interfere with the melamine detection.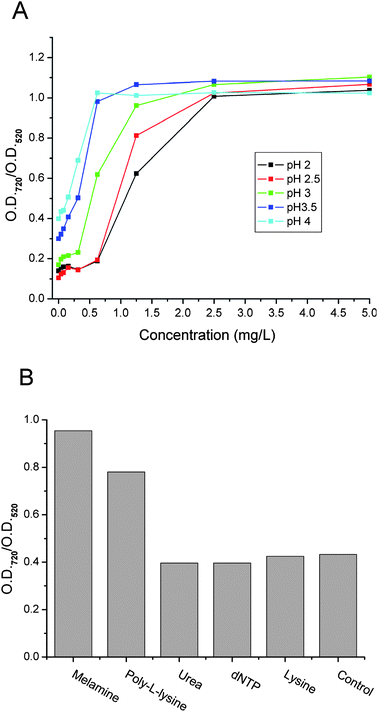 | ||
| Fig. 3 (A) Colorimetric concentration profiles (ratio of optical density readings of cysteamine modified AuNPs at 720 nm to that at 520 nm vs.melamine concentration) obtained under different pH conditions. (B) The ratio of absorbance readings at 720 nm to those at 520 nm in the presence of a dNTP mixture, urea, L-lysine, poly-L-lysine and melamine (from left to right). Concentration: 10 μM each. | ||
Detection sensitivity
Parallel assays were performed to compare the sensitivity of the cysteamine-modified AuNPs with the unmodified AuNPs for melamine detection. From Fig. 4, it can be seen that the sensitivity of the modified AuNPs was about 100 times greater than that of the unmodified AuNPs (based on the distinct color change region). Besides the improvement of sensitivity, the response range of the modified AuNPs to melamine also seems wider than that of the unmodified AuNPs, providing an additional advantage for quantitative measurement. | ||
| Fig. 4 The colorimetric concentration profiles (ratio of optical density readings at 720 nm to those at 520 nm) at pH 3.3 for cysteamine-modified AuNPs and unmodified AuNPs. The inset figure shows the visual color changes of cysteamine-modified AuNPs (upper row) and unmodified AuNPs (lower row) in the presence of melamine at different concentrations. | ||
Detection of melamine spiked into milk products and feeds
Milk products and feeds are complicated matrices of various compounds that could interfere with the colorimetric detection of melamine by the modified AuNPs. Therefore, it is very important to have a good method to extract melamine and remove interferents from the milk products and feeds. Here, we developed a sample pretreatment that could be used for various milk products and feeds. The detailed procedures were described in the experimental section. In the pretreatment process, proteins were precipitated by trichloroacetic acid, and lipid micelles were removed by repeated filtrations. Lipophilic vitamins were removed together with lipids. Pigments that exist in other kinds of samples such as feeds were removed by granular active charcoal absorption. After such a pretreatment, melamine was extracted and added into the cysteamine-modified AuNP solutions for melamine detection. Although the pretreatment may look a little complicated, it works well for different kinds of milk products and feeds, as demonstrated in the following experiments.Milk samples were spiked with melamine and analyzed first by the cysteamine-modified AuNPs. As shown in Fig. 5, we can see that increased redshifts could be observed for the milk samples spiked with increasing concentrations of melamine. To quantitate the melamine concentration in the samples, the ratio of absorption readings at 720 nm to those at 520 nm, or at 620 nm to those at 520 nm, have been used widely.20 The AuNPs status in the absence or presence of melamine was characterized by TEM (Fig. S1, ESI†). As shown in Fig. 5B, the colorimetric concentration plot based on the ratio of 720 nm to 520 nm gave a wider linear range, but was less sensitive than the plot based on the ratio of 620 nm to 520 nm. Based on the ratio of absorption readings at 720 nm to that at 520 nm, melamine in the milk samples could be quantified within the range of 1 mg L−1 to 200 mg L−1 with a detection limit of 0.6 mg L−1 (this concentration is the actual concentration of melamine in the spiked milk samples) and a correlation coefficient of 0.95737. Meanwhile, based on the ratio of absorption readings at 620 nm to those at 520 nm, melamine in the milk samples could be quantified within the range of 1 mg L−1 to 20 mg L−1 with a detection limit of 0.4 mg L−1 and a correlation coefficient of 0.98867. Similar experiments were carried out in melamine spiked milk powders, eggs and pet foods. The melamine concentrations in all of the spiked samples could be well quantified within the linear range of 1–200 mg L−1 with good correlation coefficients after the sample pretreatment (Fig. S2, ESI†). As we know the calibrated ranges for melamine detection by using extinction measurement ratios of both 720 nm to 520 nm and 620 nm to 520 nm are larger than the previous AuNP based melamine detections.
 | ||
| Fig. 5 Colorimetric assay of melamine in milk samples. A) UV-Vis spectra of cysteamine-modified AuNPs in the presence of a series of concentrations of melamine spiked into milk samples. B) The calibration curves of melamine in spiked milk samples. C) Visual color changes of cysteamine-modified AuNPs in the presence of a series of concentrations of melamine spiked into milk samples. | ||
Conclusions
In summary, with the cysteamine-modified AuNPs and effective sample pretreatment, we presented a new scheme for sensitive, low cost and onsite detection of melamine in real samples.Acknowledgements
This work was funded by the National Natural Science Foundation of China (NSFC, 30700745 and 90606028) and the Ministry of Health China (Infectious Disease Control, Project No. 2008ZX10004-004). Zhiping Zhang was supported by the National Basic Research Program of China (2006CB933100) and the Nanoscience Project of China Academy of Science (No. kjcx2-sw-hl2).Notes and references
- M. Birnbaum, J. Schulman and L. Seren, Rev. Sci. Instrum., 1955, 26, 457 CrossRef CAS.
- J. Junsomboon and J. Jakmunee, Anal. Chim. Acta, 2008, 627, 232 CrossRef CAS.
- L. Zhu, G. Gamez, H. W. Chen, K. Chingin and R. Zenobi, Chem. Commun., 2009, 559 RSC.
- J. H. Zhou, J. Zhao, X. F. Xue, F. Chen, J. Z. Zhang, Y. Li, L. M. Wu and L. Z. Chen, J. Sep. Sci., 2010, 33, 167 CrossRef CAS.
- W. Goodman and J. Neal-Kababick, LC GC Europe, 2008, 17.
- H. D. Hill and C. A. Mirkin, Nat. Protoc., 2006, 1, 324 CrossRef CAS.
- W. L. Daniel, M. S. Han, J. S. Lee and C. A. Mirkin, J. Am. Chem. Soc., 2009, 131, 6362 CrossRef CAS.
- P. Kaur, S. Kaur and K. Singh, Inorg. Chem. Commun., 2009, 12, 978 CrossRef CAS.
- J. S. Lee, P. A. Ulmann, M. S. Han and C. A. Mirkin, Nano Lett., 2008, 8, 529 CrossRef CAS.
- Y. Jiang, H. Zhao, N. N. Zhu, Y. Q. Lin, P. Yu and L. Q. Mao, Angew. Chem., Int. Ed., 2008, 47, 8601 CrossRef CAS.
- C. D. Medley, J. E. Smith, Z. Tang, Y. Wu, S. Bamrungsap and W. H. Tan, Anal. Chem., 2008, 80, 1067 CrossRef CAS.
- R. R. Liu, R. S. Liew, H. Zhou and B. G. Xing, Angew. Chem., Int. Ed., 2007, 46, 8799 CrossRef CAS.
- K. Niikura, K. Nagakawa, N. Ohtake, T. Suzuki, Y. Matsuo, H. Sawa and K. Ijiro, Bioconjugate Chem., 2009, 20, 1848 CrossRef CAS.
- K. L. Ai, Y. L. Liu and L. H. Lu, J. Am. Chem. Soc., 2009, 131, 9496 CrossRef CAS.
- F. Wei, R. Lam, S. Cheng, S. Lu, D. A. Ho and N. Li, Appl. Phys. Lett., 2010, 96, 3702.
- B. L. Li, D. Cheng and L. Mao, Food Chem., 2010, 122, 895 CrossRef.
- H. Chi, B. Liu, G. Guan, Z. Zhang and M.-Y. Han, Analyst, 2010, 135, 1070 RSC.
- K. C. Grabar, R. G. Freeman, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735 CrossRef CAS.
- X. M. Xu, Y. P. Ren, Y. Zhu, Z. X. Cai, J. L. Han, B. F. Huang and Y. Zhu, Anal. Chim. Acta, 2009, 650, 39 CrossRef CAS.
- Y. Y. Qi, L. Li and B. X. Li, Spectrochim. Acta, Part A, 2009, 74, 127 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images of modified nanoparticles and calibration curves of melamine. See DOI: 10.1039/c0an00432d |
| This journal is © The Royal Society of Chemistry 2011 |
