Evaluation of surface sampling techniques for collection of Bacillus spores on common drinking water pipe materials†‡
Benjamin H.
Packard
*a and
Margaret J.
Kupferle
b
aNational Homeland Security Research Center, Office of Research and Development, U.S. Environmental Protection Agency, Cincinnati, OH 45268, USA. E-mail: packard.benjamin@epa.gov
bDepartment of Civil and Environmental Engineering, University of Cincinnati, Cincinnati, OH 45221-0071, USA
First published on 10th December 2009
Abstract
Drinking water utilities may face biological contamination of the distribution system from a natural incident or deliberate contamination. Determining the extent of contamination or the efficacy of decontamination is a challenge, because it may require sampling of the wetted surfaces of distribution infrastructure. This study evaluated two sampling techniques that utilities might use to sample exhumed pipe sections. Polyvinyl chloride (PVC), cement-lined ductile iron, and ductile iron pipe coupons (3 cm × 14 cm) cut from new water main piping were conditioned for three months in dechlorinated Cincinnati, Ohio tap water. Coupons were spiked with Bacillus atrophaeus subsp. globigii, a surrogate for Bacillus anthracis. Brushing and scraping were used to recover the inoculated spores from the coupons. Mean recoveries for all materials ranged from 37 ± 30% to 43 ± 20% for brushing vs. 24 ± 10% to 51 ± 29% for scraping. On cement-lined pipe, brushing yielded a significantly different recovery than scraping. No differences were seen between brushing and scraping the PVC and iron pipe coupons. Mean brushing and scraping recoveries from PVC coupons were more variable than mean recoveries from cement-lined and iron coupons. Spore retention differed between pipe materials and the presence of established biofilms also had an impact. Conditioned PVC coupons (with established biofilms) had significantly lower spore retention (31 ± 11%) than conditioned cement-lined coupons (61 ± 14%) and conditioned iron coupons (71 ± 8%).
Environmental impactTreated water available to communities through a water supply network is an important resource that not only impacts the human health in an area, but also the natural environment. Ultimately, generated wastewater must be collected, treated, and discharged into the watershed. Thus, if the system is intentionally or unintentionally contaminated with a persistent pathogen, understanding the fate and transport of the pathogen within the system, as well as outside the system, becomes crucial. It is vital to assess the extent of deposition on wetted internal surfaces like pipes, as well as the efficacy of any attempts to decontaminate those surfaces. In order to take into account pathogen and particle interaction with corrosion and/or biofilm micro-environments, it is essential to evaluate surface sampling methods on various materials, each with unique surface properties. |
Introduction
In the past decade, especially following the terrorist events of September 11, 2001 and subsequent anthrax mailings, the possibility that persistent biological agents might be used to disable critical infrastructures has received increasing attention. This paper examines the biological aspect of this scenario with a specific focus on bacterial endospores. Even in the presence of chlorine, bacterial endospores are persistent on internal pipe surfaces. It has been shown that they are able to persist for long periods even in the presence of chlorine following a contamination event.1,2 It has also been shown that the presence of biofilms and corroding surfaces in drinking water distribution systems may influence adhesion of planktonic bacteria.3There are currently protocols for collecting bulk water samples from a distribution system to detect microbial contamination. However, there are no standardized methods that include sampling of the internal surfaces of drinking water infrastructure for either intentional or unintentional contamination. The purpose of this study was to evaluate the precision and recovery efficiency of two different surface sampling techniques that could potentially be used to sample the internal surfaces of water distribution pipes contaminated with bacterial endospores. Research on drinking water biofilms has generally used standard microbiological surface sampling techniques such as swabbing to recover the biofilm from wetted pipe surfaces.4 However, to evaluate other surface sampling methods, brushing and scraping of three different types of pipe material were compared to determine which was the most effective in removing spores. Spore retention on different pipe materials was also evaluated.
Experimental
Pipe material
PVC, unlined ductile iron, and cement-lined ductile iron were used in this study. These materials were chosen based on the results of a database search of the 2002 American Water Works Association (AWWA) survey of 337 small, medium-sized, and large utilities in the United States and Canada. The search showed that PVC, iron (including cast iron), and cement-lined pipes were found in the United States' and Canada's distribution systems 8%, 15%, and 50% of the time, respectively.5 Each of the pipe materials came from new 20.32 cm water mains obtained from IPEX (Mississauga, ON) (PVC) and American Ductile Iron Pipe Company (Birmingham, AL) (ductile iron and cement-lined ductile iron). Coupons (3 cm × 13 cm) were cut from 20.32 cm pipes using a high-pressure water jet cutter, which kept the coupon surfaces cool as they were cut. Each coupon provided a 39 cm2 area to sample. Prior to use, coupons were washed and disinfected using the National Sanitation Foundation (NSF) International procedure for conditioning drinking water contact materials prior to determining the toxicity of the surface.6 This involved scrubbing the coupons in tap water with a test tube brush to remove any debris, spraying the coupons with 200 mg L−1 sodium hypochlorite solution coating all surfaces of the coupon, and then after 30 min, rinsing the coupons in deionized water. The coupons were then placed into the recirculation tank.Recirculation tank
A 1136-liter recirculation tank, with a residence time of approximately 14 h, was used to expose the coupons to dechlorinated Cincinnati tap water. The average chlorine concentration of the incoming tap water was 0.99 mg L−1 with a standard deviation of 0.11 during the time period. A chemical feed pump delivered approximately five grams of sodium thiosulfate into the recirculation tank every 24 h to achieve 0.01 to 0.05 mg L−1 of detectable free chlorine in the tank. The tank chlorine levels were measured weekly and the sodium thiosulfate feed rate was adjusted to keep the chlorine residual in the tank between 0.01 and 0.05 mg L−1. The tank was gently mixed with a small electric mixer, which exposed the coupons to some shear forces.Stainless steel racks were built to suspend each type of coupon material vertically in the tank. Each rack supported approximately 30 coupons stacked on top of each other and held in place with a stainless steel rod running through the middle of the rack. The curvature of the coupons made it possible for the rod to hold the coupons in place with contact only to the edges of the coupon. The rack and coupons are shown in Fig. 1.
 | ||
| Fig. 1 Racks holding coupons in the recirculation tank. Pipe material (from left to right) is PVC, cement-lined, and iron. | ||
Biofilm enumeration
Three coupons of each type of pipe material were sampled to collect biofilms for enumeration. The biofilm was brushed from the surfaces using adult soft toothbrushes (CVS Pharmacy, Inc., Woonsocket, RI, catalog no. 100982). Each coupon was brushed and subsequently washed a total of four times using a total of 50 mL volume of the phosphate buffer (Standard Methods Section 9216 B).7 Samples were diluted in phosphate buffer and cultured on R2A agar using the spread plate method and incubated at room temperature (21–24) for 7 days (Section 9215 C).7 Mean HPC counts for the biofilm developed on each material were: PVC, 1 × 105 CFU/cm2, cement-lined, 4 × 105 CFU/cm2, and iron, 3 × 108 CFU/cm2. Simoes et al.8 found that biofilms grown in chlorine-free tap water on PVC under laminar conditions generated 104 CFU/cm2. Also, according to a literature review conducted by Ollos et al.,9 a bacterial cell count of 104 CFU/cm2 or higher represents a viable drinking water biofilm.Bacillus spore selection
A literature search was conducted to find a spore-forming Bacillus species that could be used as a surrogate for B. anthracis. One study showed that Bacillus atrophaeus subsp. globigii (BG) spores have a mean CT (C is the concentration of chlorine in mg/liter, and T is the exposure time in minutes) value higher than the mean CT values for B. anthracis Sterne, B. cereus, and B. thuringiensis along with the virulent Ames strain of B. anthracis.10 Because of its increased resistance to chlorine, BG was selected since it represents a conservative surrogate for future drinking water pipe disinfection research.Spore preparation, growth, and enumeration
BG spores were obtained from the U.S. Army's Dugway Proving Ground (Dugway, Utah) and were grown according to the methods described in Coroller et al.11 and Nicholson and Setlow.12 Generic spore media was inoculated with vegetative BG cells and incubated for five days at 35 °C with gentle shaking in a rotary shaker. Purified BG endospores were produced using gradient separation as described by Nicholson and Setlow12 and the presence of spores was confirmed using phase contrast microscopy (<0.1% vegetative cells). Spores were stored in 40% ethanol at 4 °C until use. Spores used in this study came from the same batch and container.Spores were enumerated using serial dilution and the spread plate method (Section 9215 C)7 with 0.1 mL of the sample on tryptic soy agar (TSA). The diluent used was phosphate buffer (Standard Methods Section 9216 B)7 with 0.01% Tween® 80 (Fisher Scientific, Pittsburgh, PA, catalog no. AC278630000). Tween® 80 is a surfactant that has been shown to improve spore recovery.13 All samples, including quality control (QC) samples, were spread plated in triplicate with the exception of the tap water spore suspension, which was spread plated using 5 replicate plates to generate a more precise value. Spore samples from coupons were heat shocked at 80 °C for 10 min to inactivate vegetative organisms. Samples were cooled and vortexed prior to plating. Plates were incubated for 24 ± 2 h at 35 °C. Because it forms orange colonies, BG was easily distinguished from other biofilm organisms that survived the heat shock.
Tap water spore suspensions
Fresh spore suspensions were made on the day of the experiment. Suspensions were made by adding 40 μL of the concentrated spore stock suspension, described above, to 50 mL of dechlorinated Cincinnati tap water resulting in a 6 × 105 CFU/mL suspension. Dechlorinated water from the tank was used as the diluent so that the recirculation tank water quality was represented.Water parameter monitoring
Conductivity, temperature, and pH of the recirculation tank water were measured at each coupon sampling event. Average values of 340 μS cm−1 (SD = 190) for conductivity and 13.7 °C (SD = 0.015) for temperature were recorded using an Extech conductivity meter (Extech instruments, Waltham, MA, catalog no. 407303). pH ranged from 8.22–9.00 with a median value of 8.27 and was measured using an Orion 555A pH/ORP/Conductivity Meter (Thermo Fisher Scientific Inc., Waltham, MA). Total chlorine was measured twice a week using the Hach total chlorine method 8167(4500-Cl Chlorine DPD Colorimetric Method)7 using Hach DPD free chlorine reagent AccuVac® ampules (Hach, Loveland, CO, Catalog no. 25020-25), with a Nalco 2800 DR spectrophotometer (Nalco Company, Naperville, IL). Chlorine was found to be less than 0.05 mg L−1 in the tank due to the introduction of sodium thiosulfate. On four occasions, additional recirculation tank water samples were collected, heat shocked, and spread plated on TSA to determine whether spores were present in the bulk water in the tank.Coupon collection and spore loading
Individual coupons were removed from the coupon rack and placed into a sterile 1000 mL beaker while still submerged in the tank. Each coupon was then placed in a sterile 14 cm diameter Petri dish and was leveled using a sterile level. A two step procedure was used to load the coupons with spores: the suspension on the coupons was spiked; then, the coupons were rinsed to remove spores that had not been retained on the surface.Coupon sampling
Sampling techniques were compared on the three different pipe surfaces to determine differences in recovery and precision. These methods were chosen due to their low cost and availability, as well as their potential to remove biofilm and loose corrosion from porous and non-porous surfaces.Each coupon was brushed or scraped, then washed, four times. A combined 50 mL of the phosphate buffer and 0.01% Tween® 80 was used in the process, resulting in a 50 mL sample that was collected in the Petri dish. This suspension was transferred to a 100 mL sample container. The quantity of spores found in this sample was compared to the number of spores that were calculated to be on the surface prior to sampling, resulting in a recovery efficiency value.
Other sampling and quality control checks
Additional spore sampling was conducted to determine where losses may have occurred throughout the sampling and analysis process. Quality control checks were also routinely done to check for potential contamination issues.Mass balance calculation
A mass balance approach was used to calculate the recovery efficiency of the sampling methods. The specific components (Spike Co, rinse Cr, brush or scrape Cs, and sides and back Cb) are summarized in Table 1.| Samples | Description | Reason for sample | |
|---|---|---|---|
| a All samples are spread plated in triplicate. | |||
| Coupon inoculation spore suspension (Co) | Sample | Tap water spore suspensions used to inoculate coupons were quantified to determine the initial number of spores inoculated onto coupon | To quantify the total number of spores that were spiked onto the coupon |
| Rinse (Cr) | Sample | Following inoculation, coupons were rinsed with 100 mL of dechlorinated tap water to remove spores that have not been retained on the surface | To quantify the number of spores that were rinsed off of the coupon with dechlorinated tap water |
| Petri dish check | Determination of the number of spores that stay attached to the Petri dish | To quantify spores retained to Petri dish from rinse sample | |
| Brush or scrape (Cs) | Sample | Following rinsing, the side of the coupon corresponding to the internal pipe surface was brushed or scraped | To quantify spores that were brushed or scraped from the coupon |
| Toothbrush or scraper check | Determination of the number of spores that stay attached to the toothbrushes and scrapers | To quantify spores on toothbrush or scraper | |
| Petri dish check | Determination of the number of spores that stay attached to the Petri dish | To quantify spores retained to Petri dish from toothbrush or scraper sample | |
| Sides and back (Cb) | Sample | Following brushing or scraping, the sides and back of each coupon were brushed | To quantify the number of spores found on the sides and back of each coupon |
| Toothbrush check | Determination of the number of spores that stay attached to the toothbrushes and scrapers | To quantify spores on toothbrush | |
| Petri dish check | Determination of the number of spores that stay attached to the Petri dish | To quantify spores retained on Petri dishes from sides and back sample |
Spore recovery efficiency (RE) was computed as shown in eqn (1) using the terms defined in Table 1. Percent retention (PR) of spores was computed as shown in eqn (2).
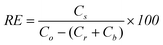 | (1) |
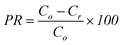 | (2) |
Experimental design
Seventy two coupons including quality control coupons were used in the experiments. Twelve of the 72 coupons were conditioning control coupons described above. An additional twelve of the recirculation tank conditioned coupons were sacrificed in order to enumerate biofilm and conduct quality control checks. The remaining 48 coupons were used for the sampling experiments as shown in Table 2.| Conditioning control coupons (no biofilm) | Recirculation tank conditioned coupons (established biofilm) | TOTAL | |||
|---|---|---|---|---|---|
| Biofilm and procedural blank samples | Brushing | Scraping | |||
| a Numbers in parentheses are actual numbers of coupons used in recovery efficiency and percent retention experiments. | |||||
| PVC | 4 | 4 | 8 (7) | 8 (7) | 24 (18) |
| Cement - lined | 4 | 4 | 8 (8) | 8 (7) | 24 (19) |
| Iron | 4 | 4 | 8 (8) | 8 (7) | 24 (19) |
| 72 (56) | |||||
Statistical analysis and calculations
The experiment was designed for analysis using a 2-way analysis of variance (ANOVA). However, spore recovery efficiency (RE) data were not equally variable among pipe materials (see data in Fig. 2), violating one of the underlying assumptions for an ANOVA analysis. Therefore, recovery efficiency data were analyzed with t-tests using SigmaPlot® software (SYSTAT Software, Inc., San Jose, CA) with a 0.05 significance level. The experimental design enabled testing of the null hypothesis that assumed there were no differences in recovery efficiencies between the sampling techniques for each pipe material. Percent retention (eqn (2)) was tested using a one-way analysis of variance. The null hypothesis for this design was the assumption that there would be no significant differences in retention among pipe materials. | ||
| Fig. 2 Recovery of B. globigii spores from PVC (P), cement-lined (C), and iron (I) pipe coupons using brushing (Br) and scraping (S). The line in the middle of the box indicates the median and the ends of the boxes define the 25th and 75th percentiles. Whisker bars are not shown due to sample size ≤ n = 8. | ||
Results
Recovery efficiency
Recovery efficiency values were calculated using eqn (1) and are shown in Table 3 and Fig. 2. Data were then arcsin squareroot transformed prior to conducting statistical tests. Mean recovery efficiency values for brushing and scraping for combined pipe materials were 0.41 (sd = 0.20) for brushing, and 0.40 (sd = 0.21) for scraping. Combined recovery efficiency for brushing and scraping on all pipe materials was 0.40 (sd = 0.20). t-test results showed significant differences between cement-lined brushing and scraping (P = 0.003). There were no significant differences between brushing and scraping for PVC (P = 0.092) and iron (P = 0.691).| PVC | Cement | Iron | ||||
|---|---|---|---|---|---|---|
| Brush | Scrape | Brush | Scrape | Brush | Scrape | |
| Sample size | 7 | 7 | 8 | 7 | 8 | 6 |
| Mean | 0.37 | 0.51 | 0.42 | 0.24 | 0.43 | 0.46 |
| Median | 0.29 | 0.47 | 0.42 | 0.21 | 0.44 | 0.46 |
| SD | 0.30 | 0.29 | 0.09 | 0.10 | 0.20 | 0.04 |
Percent retention
The percent retention of B. globigii was calculated using eqn (2). Fig. 3 illustrates the differences in retention between the various pipe materials. | ||
| Fig. 3 Comparison of B. globigii retention on PVC (P), cement-lined (C), and iron (I) conditioned (Con) and unconditioned pipe coupons. The line in the middle of the box indicates the median and the ends of the boxes define the 25th and 75th percentiles. Whisker bars define the 10th and 90th percentiles. | ||
There were significant differences between PVC and cement-lined and PVC and iron coupons that had been conditioned. In contrast, no significant differences between conditioned cement-lined and iron pipe coupons were found. Additionally, significant differences were seen between conditioned and unconditioned PVC, and conditioned and unconditioned iron pipe material.
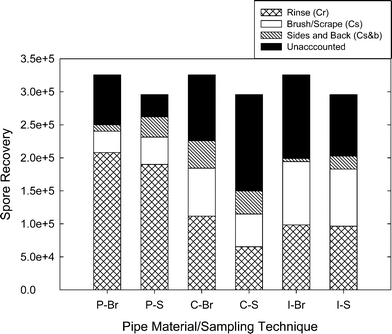 | ||
| Fig. 4 Mean spore recovery for all techniques. Each bar represents the mean number of spores spiked on the coupon (8 brushed (Br) and 7 scraped (S) coupons). Relative numbers of spores recovered as mean values for Cr, Cs, and Cb are shown in relation to the number of spores spiked on PVC (P), cement-lined (C), and iron (I) coupons. | ||
Spore losses to petri dishes, toothbrushes, and scrapers
Spore losses to retention on Petri dishes, toothbrushes, and scrapers, are shown in Table 4. Percentages indicate the number of spores recovered with respect to the total number spiked on the coupons.| Rinse Petri dishes (no Tween®) | Scrape Petri dishes | Sides and back Petri dishes | Toothbrushes | Scrapers | |
|---|---|---|---|---|---|
| Avg. No. spores recovered (SD) | 13557 (10172) | 4078 (4851) | 3423 (5651) | 515 (482) | 568 (912) |
| Percentage of spores recovered | 4.30% | 1.30% | 1.10% | 0.20% | 0.20% |
Impact of Tween® 80 and corrosion on spore recovery
In preliminary studies, it was determined that there were significant losses due to spores clumping and adhesion to sample containers. Thus, 0.01% Tween® 80, was included in all phosphate buffer used to collect and enumerate spores. The purpose of the spore spiking and coupon rinsing steps was to simulate contaminated tap water and subsequent water movement in a pipe. Therefore, out of necessity, the spore spiking and rinsing steps did not include Tween® 80. To confirm the effect that adding a surfactant has on BG spore recovery, a phosphate buffer spore suspension was split into two identical samples and Tween® 80 was added to one of the samples. The suspensions were then spread plated on TSA. Approximately two times the number of spores were recovered in the presence of Tween® 80 (3153 per mL with vs. 1603 spores per mL without).In contrast to the effects of Tween® 80 on spore recovery, iron corrosion was shown to have an adverse effect. Corrosion and/or biofilm was collected from uncontaminated but conditioned pipe coupons using the same procedure used for sampling coupons via brushing without Tween® 80. Then an aliquot from the same spore stock suspension was spiked into each sample to determine spore recovery in the presence of the corrosion and/or biofilm. Fig. 5 shows that there is a considerable difference between spore recovery in the presence of iron corrosion and recovery in the presence of biofilm alone when brushed from the PVC and cement-lined pipe coupons. Furthermore, if samples containing iron corrosion were diluted by half phosphate buffer solution (and, later Tween® 80), spores were able to grow more readily on TSA. The dilution and plating protocol for the iron coupons was adjusted accordingly for the reported experiments.
 | ||
| Fig. 5 Spore enumeration in the presence of biofilm and corrosion (3 plates per sample). Error bars represent standard deviations. | ||
Discussion
This study examined two ways to sample attached bacterial spores from wetted porous and non-porous surfaces. When the mean recovery for all the brushing data is compared with the mean recovery for all the scraping data (for all three pipe materials), it is clear that one sampling technique did not stand out as being more effective than the other. Brushing vs. scraping recovery efficiency data for all three pipe materials combined showed that one sampling technique was not more effective than the other. However, brushing cement-lined pipe coupons was shown to be more effective than scraping cement-lined pipe coupons. Results for recovery efficiency also differed in variability among the three pipe materials, 0.29 for PVC vs. 0.10 and 0.12 for cement-lined and iron, respectively. This suggests that pipe material influences the variability of the recovery efficiency. Recovery efficiency standard deviations for iron coupons varied for brushing and scraping (0.22 vs. 0.04) while PVC (0.30 vs. 0.29) and cement-lined (0.09 vs. 0.10) coupons had approximately the same standard deviation for brushing and scraping, respectively. This difference between brushing and scraping observed for the iron coupons could be attributed to the layer of rust that absorbed and stabilized the spore suspension. It was noted that scraping removed the majority of the biofilm and rust layer with one stroke. In contrast, brushing required 3–4 strokes to remove the same amount of corrosion. Although there were no statistically significant differences between the mean recovery efficiencies for brushing and scraping on iron, variability in the data indicate that scraping may be the more desirable technique due to the higher level of precision.One of the limitations in this study is that the corroded iron coupons may not be representative of tuberculated iron pipes found in real world distribution systems. Based on research involving characterization of iron pipe surface deposits found in pilot scale and real distribution systems,14 the layer of tuberculation would likely be harder to remove than the iron corrosion formed on the iron coupons used in this study. Thus, for exhumed tuberculated iron pipe, a better surface sampling strategy might be to brush the outer layer of pipe scale to collect the soft corrosion and biofilm present on the surface. If spores are able to penetrate past the outer layer of corrosion, removing deeper layers of corrosion using a heavier chisel, scalpel, or spatula may be required to remove the entire deposit.14 Due to the large amounts of iron corrosion that would be present in these samples spore growth would most likely be inhibited. Therefore, understanding the impact of corrosion on spore recovery from exhumed pipe should be verified through the use of positive controls, matrix spikes, and standard additions.
Recovery results for the cement-lined pipe coupons showed similar brushing and scraping variability. The significant differences between mean recovery efficiency of brushing and scraping were expected (42% vs. 24%, respectively) due to the porous cement-lined surface. The toothbrush was likely more effective because it removed the spores that may have been stuck in the interstitial spaces and could not be accessed by the scraper. Therefore, for hard porous surfaces, brushing appears to be a more effective sampling technique that will produce the highest recoveries.
Recovery efficiency variability was highest for PVC pipe coupons for both brushing and scraping. Additionally, conditioned PVC had a lower level of spore retention than cement-lined or iron. This was expected for two reasons. First, PVC had lower HPC (less biofilm) than the cement-lined coupons. Second, the PVC surface was notably smoother, providing less opportunity for spore retention. Iron pipe coupons had a higher HPC (3 × 108 CFU/cm2). The large amount of corrosion and biofilm on the iron coupons may have provided additional surface area for immobilizing the spores within the matrix.
The 24 h conditioned iron coupons did not have the opportunity to develop the same amount of corrosion and surface roughness as did the coupons conditioned for three months, and, not surprisingly, they did not retain spores as well as the iron coupons with more rust. PVC conditioning had the opposite effect. Spores were retained more readily on coupons with minimal conditioning than they were on the conditioned coupons with 3 months of biofilm growth (1 × 105 CFU/cm2 for PVC). This may have been due to the physiochemical properties of the material such as charge or hydrophobicity of the PVC. Spores are generally hydrophobic and negatively charged which may account for a stronger attraction to the unconditioned PVC.15,16 Also, while not directly observed, biofilm sloughing may have occurred more readily from the smooth, hydrophobic surface of the PVC than from the cement-lined or iron coupons, taking the spores with it during the rinse.
The unclosed mass balances (Fig. 4) could be attributed to a number of different causes in addition to sampling inefficiency. First, spores collected during coupon rinsing (Cr) were exposed to tap water without Tween® 80. As seen by increased Petri dish swab counts for the rinse samples, a proportion of the spores that were not recovered probably had been retained on the sample containers and Petri dishes. This illustrates the importance of using a surfactant such as Tween® 80 in every step to increase recovery. The second loss may be attributed to incomplete recovery of spores from the sides and backs of the coupons. Recovery efficiency of spores from the sides and backs was not quantifiable. Thus, the proportion of the spores that were actually removed and enumerated may not have been representative of the true number of spores present on the sides and backs.
The reported data illustrate the potential for spore retention on different wetted pipe materials and associated sampling recovery from those materials. Since spore losses can be encountered throughout the sampling and analysis process, it is important to consider the recovery efficiency of the method used. Furthermore, in real-world situations, very low concentrations of surface contamination may be present making high recovery efficiency and precision of recovery all the more important. In selecting sampling techniques, this study shows that the pipe material characteristics and the presence of a biofilm will influence recovery efficiency and recovery precision. Accordingly, the sampling technique should be selected based on its ability to remove spores from the particular surface with an acceptable level of precision. Brushing vs. scraping cement-lined coupons illustrates the differences in recovery efficiency that may be obtained for the same surface. Bacterial spore retention and sample collection from wetted surfaces is complex. Future work in this area should evaluate additional surface sampling methods for wetted pipe surfaces and methods for quantifying pathogens in the presence of pipe corrosion.
Disclaimer
The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development funded and conducted the research described. It has been reviewed by the Agency but does not necessarily reflect the Agency's views. No official endorsement should be inferred. EPA does not endorse the purchase or sale of any commercial products or services.Acknowledgements
The authors would like to thank the following people and organizations for their support, without which this work would not have been possible: Dr. Hiba Ernst, U.S. EPA/NHSRC, Dr. Alan Lindquist, U.S. EPA/NHSRC, Dr. Nick Ashbolt, U.S.EPA/NERL, Dr. Jeff Szabo, U.S.EPA/NHSRC, John Hall, U.S.EPA/NHSRC, Noreen Adcock, U.S.EPA/NRMRLReferences
- J. G. Szabo, E. W. Rice and P. L. Bishop, Appl. Environ. Microbiol., 2007, 73, 2451–2457 CrossRef CAS.
- J. B. Morrow, J. L. Almeida, L. A. Fitzgerald and K. D. Cole, Water Res., 2008, 42, 5011–5021 CrossRef CAS.
- L. C. Simões, M. Simões, R. Oliveira and M. J. Vieira, J. Basic Microbiol., 2007, 47, 174–183 CrossRef CAS.
- N. B. Hallam, J. R. West, C. F. Forster and J. Simms, Water Res., 2001, 35, 4063–4071 CrossRef CAS.
- American Water Works Association, Water:\Stats, 2003 edition on CD-ROM, ISBN 1-58321-306-6-Catalog No. 53007 Search PubMed.
- NSF/American National Standards Institute (ANSI), 61-2000a Drinking Water System Components - Health Effects, NSF International, Ann Arbor, Michigan Search PubMed.
- Standard Methods for the Examination of Water and Wastewater, ed. A. D. Eaton, L. S. Clesceri, E. W. Rice and A. E. Greenberg, American Public Health Association, Water Environment Federation, and American Water Works Association, 21st edition, 2005 Search PubMed.
- L. C. Simões, N. Azevedo, A. Pacheco, C. W. Keevil and M. J. Vieira, Biofouling, 2006, 22, 91–99 CrossRef.
- P. J. Ollos, P. M. Huck and R. M. Slawson, J. Water Works Assoc., 2003, 95, 87–97 Search PubMed.
- M. Sivaganesan, N. J. Adcock and E. W. Rice, J. Water Supply: Res. Technol.—AQUA, 2006, 55, 33–43 Search PubMed.
- L. Coroller, I. Leguerinel and P. Mafart, Appl. Environ. Microbiol., 2001, 67, 317–322 CrossRef CAS.
- W. L. Nicholson and P. Setlow, in Molecular Biological Methods for Bacillus, ed. C. Harwood and S. Cutting, John Wiley & Sons Ltd., Chichester, England, 1990, 391–450 Search PubMed.
- G. S. Brown, R. G. Betty, J. E. Brockmann, D. A. Lucero, C. A. Souza, K. S. Walsh, R. M. Boucher, M. Tezak, M. C. Wilson and T. Rudolph, Appl. Environ. Microbiol., 2007, 73, 706–710 CrossRef CAS.
- S. E. Smith, J. S. Colbourne, D. Holt, B. J. Lloyd, A. Bisset, Water Quality Technology Conference proceedings AWWA 1996 Search PubMed.
- D. A. Lytle, C. H. Johnson and E. W. Rice, Colloids Surf., B, 2002, 24, 91–101 CrossRef CAS.
- G. Tauveron, C. Slomianny, C. Henry and C. Faille, Int. J. Food Microbiol., 2006, 110, 254–262 CrossRef.
Footnotes |
| † Part of a themed issue dealing with water and water related issues. |
| ‡ Electronic supplementary information (ESI) available: Quality control details. See DOI: 10.1039/b917570a |
| This journal is © The Royal Society of Chemistry 2010 |
