Tuning the morphologies of amphiphilic metallo-supramolecular triblock terpolymers: from spherical micelles to switchable vesicles
Christina
Ott
a,
Richard
Hoogenboom
a,
Stephanie
Hoeppener
a,
Daan
Wouters
a,
Jean-François
Gohy
b and
Ulrich S.
Schubert
*ac
aLaboratory of Macromolecular Chemistry and Nanoscience, Eindhoven University of Technology, PO Box 513, 5600 MB Eindhoven, Netherlands. Tel: +(31)-402474186E-mail: u.s.schubert@tue.nl
bUnité de Chimie des Matériaux Inorganiques et Organiques (CMAT), Université catholique de Louvain (UCL), Place Pasteur 1, 1348 Louvain-la-Neuve, Belgium
cLaboratory of Organic and Macromolecular Chemistry, Friedrich-Schiller-University Jena, Humboldtstr. 10, 07743 Jena, Germany
First published on 7th November 2008
Abstract
A well-defined poly(styrene)-block-poly(para-trifluoromethylstyrene)-[Ru]-poly(ethylene glycol) (PS–b–PTFMS–[Ru]–PEG) amphiphilic metallo-supramolecular triblock terpolymer containing a bis(2,2′:6′,2-terpyridine)ruthenium(II) complex (–[Ru]–) as a connection between the PS–b–PTFMS and the PEG block was successfully synthesized using the concepts of controlled radical polymerization and supramolecular chemistry. Spherical micelles, wormlike micelles, vesicles and hollow tubes were all accessed from this copolymer by varying the polarity of the solvent which provokes a change in the solvophilic to solvophilic ratio within the same macromolecule by dissolution or precipitation of the PTFMS-block. The self-assembled structures with both PS and PTFMS as insoluble blocks are presumed to be multicompartmentalized based on the incompatibility of these blocks. Furthermore, the thermoresponsive behavior of PTFMS allows reversible control over the size and morphology of the self-assembled structures.
Introduction
The self-assembly process of amphiphilic block copolymers has been investigated intensively in the past years by many research groups. This sustained interest mainly arises from the numerous potential applications of the resulting micelles in various fields ranging from drug delivery,1,2 separation techniques3 to pharmaceutical technologies3 and nanolithography.4Self-assembly of amphiphilic block copolymers in aqueous or organic media led to the formation of micellar aggregates with a large variety of different morphologies.5–9 Control over the micellar morphology can be achieved by adjusting several parameters including the copolymer composition and architecture, the solvent nature, the presence of additives (such as salts) and the use of mixed solvents.10–12 Zhang and Eisenberg have pioneered the field of micellar morphology control with the so-called “crew-cut” micelles. These authors observed transitions from spheres-to-rods-to-vesicles by varying the block length ratio of polystyrene-block-poly(acrylic acid) (PS-PAA) copolymers, evidencing the role of block copolymer composition on the micellar morphology.13 Accordingly, the morphology of a micelle basically depends on three factors, i.e. (i) the stretching of the core-forming chains, (ii) the core-corona interfacial energy and (iii) the repulsion among coronal chains.14 A change in one of these three parameters directly affects the free energy of the micelles which leads to the transformation of their morphology in order to reach another stable state.Advances in the field of controlled radical polymerization (CRP) techniques simplify nowadays the design of macromolecules with well-defined molecular characteristic features and chain architectures.15–19 These techniques have thus emerged as “golden” tools to engineer amphiphilic copolymers since important macromolecular features such as molecular weight, hydrophobic-to-hydrophilic block length, and chain architecture play an important role with respect to the size and the morphology of the micelles.20–24
In recent years, research focused on more and more complex structures, such as e.g. amphiphilic ABC triblock terpolymers.25 If two of the three polymer blocks are soluble in the solvent, the formation of micelles is observed featuring a mixed-arm soluble corona.26–28 On the other hand, if only one of the three segments is soluble in the solvent, phase separation can occur in the micellar core between the two non-soluble blocks leading to the so-called multicompartment micelles.22–34 Recently, Hillmyer and coworkers showed that micelles consisting of a phase-separated core are able to selectively store and release different hydrophobes.35 The hydrophobic microdomains of multicompartment micelles must therefore substantially differ from each other which can be achieved when hydrocarbon and fluorocarbon blocks are combined within one copolymer.36,37 Due to the structural complexity of multicompartment micelles, they may find wide applications in the fields of drug delivery, nanotechnology and catalysis.2,38
Stimuli-responsive micelles are also of major interest for the development of smart materials. The vast majority of thermoresponsive micelles are based on lower critical solution temperature (LCST) transitions, while only a very limited number of upper critical solution temperature (UCST)-based thermoresponsive micelles were reported.39–42
In this contribution, we report on the synthesis and characterization of a new metallo-supramolecular triblock terpolymer consisting of a hydrophilic, a fluorophilic and a hydrophobic block by combining nitroxide mediated polymerization (NMP) and supramolecular chemistry. In addition, micelles were obtained from this copolymer using various alcohols as solvents; direct visualization was enabled by transmission electron microscopy (TEM). We demonstrate that different micellar morphologies are observed upon varying the solvent; these morphologies originate from the unique solution behavior of the triblock terpolymer. The fluorinated polymer block exhibits an UCST behavior which results in the formation of thermoreversible micelles upon changing the temperature.
Results and discussion
Nitroxide mediated polymerization (NMP) has been proven to be a valuable technique for the preparation of well-defined (block co)polymers using simple experimental protocols and a wide range of monomers.17,43 In this study, a unimolecular terpyridine-functionalized alkoxyamine initiator44–49 has been applied for the polymerization of para-trifluoromethylstyrene (Scheme 1). The polymerization has been performed up to 51% conversion as determined by 1H NMR spectroscopy. The obtained polymer exhibits a narrow molecular weight distribution indicated by a polydispersity index below 1.20 (Fig. 1). This terpyridine-functionalized polymer was further utilized as a macroinitiator to polymerize styrene (Scheme 1). An important aspect for a successful block copolymerization is an efficient cross-over reaction from the macroinitiator to the second monomer. The unimodal gel permeation chromatography (GPC)-trace of the obtained block copolymer demonstrates the efficient reinitiation of the macroinitiator resulting in a successful chain extension with good control over the polymerization judging from the polydispersity index of 1.18 for the accordingly obtained ]–PTFMS–b–PS diblock copolymer (Fig. 1). The respective block ratio and the molecular weight of the block copolymer were calculated from the 1H NMR spectrum, revealing an average degree of polymerization of 76 for the styrene block and of 42 for the trifluoromethylstyrene block, respectively. | ||
| Scheme 1 Schematic representation of the synthetic approach for the polymerization of para-trifluoromethylstyrene and the corresponding block copolymer with styrene. | ||
![GPC-traces of the ]–PTFMS homopolymer and the corresponding ]–PTFMS–b–PS block copolymer. Eluent: DMA with LiCl (2.1 g/L).](/image/article/2009/SM/b813161a/b813161a-f1.gif) | ||
| Fig. 1 GPC-traces of the ]–PTFMS homopolymer and the corresponding ]–PTFMS–b–PS block copolymer. Eluent: DMA with LiCl (2.1 g/L). | ||
The bulk morphology of the ]–PTFMS42–b–PS76diblock copolymer was investigated by atomic force microscopy (AFM). The diblock copolymer was spin-coated from chloroform (2 mg/mL) onto a silicon wafer and annealed at 120 °C. Based on the volume fractions (48% PTFMS, 52% PS) a lamellar phase separation is expected. Although the AFM images revealed phase separation, the structural morphology is difficult to assign (Fig. 2). Nonetheless, the clear phase separation demonstrates the demixing of the PS and PTFMS phases.
![Height image of a ]–PTFMS–b–PS film after annealing at 120 °C.](/image/article/2009/SM/b813161a/b813161a-f2.gif) | ||
| Fig. 2 Height image of a ]–PTFMS–b–PS film after annealing at 120 °C. | ||
The obtained block copolymer offers two useful functionalities: the nitroxide moiety for further controlled radical polymerizations at one chain end and the terpyridine ligand for supramolecular self-assembly processes at the other end. This latter approach has been used in this study for the construction of a PEG–[Ru]–PTFMS–b–PS metallo-supramolecular triblock terpolymer, where the metal-complex is located at the junction between the PTFMS and poly(ethylene glycol) (PEG) blocks. In order to prepare heteroleptic bis-terpyridine ruthenium complexes, a two-step synthesis is required. The directed strategy includes the formation of a polymeric ruthenium(III) mono-complex which is subsequently reduced in situ to the corresponding ruthenium(II) complex.50,51 For this purpose, the terpyridine-functionalized diblock copolymer (]–PTFMS42–b–PS76) and the poly(ethylene glycol) ruthenium mono-complex (PEG70–[RuCl3) were solubilized in a solvent mixture of methanol and tetrahydrofuran (1:2) containing a catalytic amount of N-ethylmorpholine (Scheme 2). The reaction mixture was stirred at 85 °C in a closed reaction vessel overnight. The GPC-traces recorded after purification by preparative size exclusion chromatography (BioBeads SX1) and column chromatography (silica) revealed a successful chain extension (Fig. 3). Furthermore, the combination of GPC with a photo-diode array detector (PDA) gives evidence of the formation of the bis-terpyridine ruthenium complex by the characteristic metal-to-ligand charge transfer band at 490 nm (Fig. 3). The formed metal-complex can be regarded as a dormant switch due to its high complex stability. Nonetheless, the non-covalent metal–ligand interaction can be cleaved under harsh conditions, i.e. either by the addition of large amounts of a competitive ligand such as N-hydroxyethylethylene-diamine triacetic acid sodium salt (HEEDTA)51,52 or by the addition of an aqueous Ce(SO4)2 solution which can oxidize Ru(II) to Ru(III) ions.53
 | ||
| Scheme 2 Schematic representation of the synthesis of the triblock copolymer. | ||
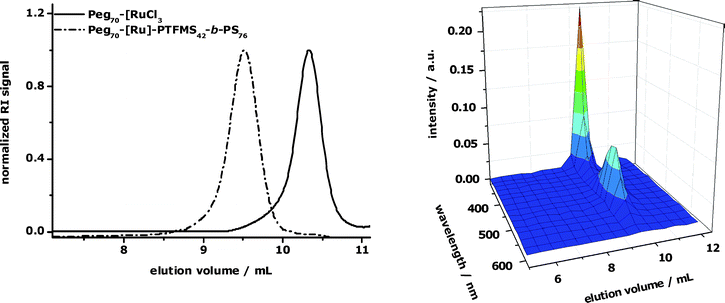 | ||
| Fig. 3 Left: GPC-traces of the PEG mono-complex and the resulting triblock copolymer using the RI detector. Right: GPC (PDA) chromatogram of the desired metallo-supramolecular block copolymer indicating the effective complexation reaction. Eluent: DMF with NH4PF6 (0.8 g/L). | ||
The key to self-assembling materials into a variety of morphologies, i.e. tuning the micellar shape, is based on the targeted design and synthesis of amphiphilic macromolecules. In this work, the influence of the solvent on micelle formation was investigated by applying a single solvent procedure7 onto the above described metallo-supramolecular triblock copolymer which consists of a hydrophilic, a fluorophilic and a hydrophobic polymer block. Various alcohols including methanol, ethanol, 1-propanol, 2-propanol and 1-butanol were selected as potential solvents for micellization. Since the investigated triblock terpolymer was poorly soluble in those alcohols at room temperature, the solutions (1 mg/mL) were heated in capped microwave vials to 140 °C for 5 minutes and cooled slowly back to room temperature. The resulting self-assembly into discrete nanostructures upon cooling was then visualized by transmission electron microscopy (TEM, Fig. 4). From the results shown in Fig. 4, it is clear that the micellar morphology is strongly dependent on the alcohol used. In methanol, a vast majority of small spherical micelles was formed whereas in ethanol a coexistence of spherical micelles and wormlike micelles was observed. However, much larger aggregates including vesicles, hollow tubes and large compound micelles (LCMs) were detected in the solutions of 1-propanol, 2-propanol and 1-butanol, respectively. The micellar solutions in those latter solvents were macroscopically investigated by a cloudy appearance while the methanol and ethanol-based solutions seemed to be optically transparent. The reason for the different morphologies has to be found in the solubility of the constituent polymer blocks of the triblock terpolymer in the respective solvents. The poly(ethylene glycol) block is soluble in all utilized solvents already at room temperature. As expected, the polystyrene block is neither soluble at room temperature nor at higher temperature in all the solvents used (up to 90 °C). On the other hand, the polytrifluoromethylstyrene (PTFMS) block is characterized by an upper critical solution temperature (UCST) in the less polar alcohols, i.e.1-propanol, 2-propanol and 1-butanol. In order to shed light on this temperature-dependent behavior, solubility measurements were performed by measuring the turbidity of a synthesized model polymer ]-PTFMS81 in the above mentioned alcohols as a function of temperature over a wide temperature range (Fig. 5). The resulting turbidity curves, together with a visual inspection of the solutions, revealed complete solubility of PTFMS in methanol and ethanol from −20 °C to 90 °C. Basically, transparent solutions, i.e. solutions in which the polymer is fully dissolved, reveal a transmittance above 75% which is the case for both solvents. Furthermore, it can be assumed that methanol is a slightly better solvent for the polymer since the polarity is higher. In this case, the major part of the PS76–b–PTFMS42–[Ru]–PEG70 triblock terpolymer is solubilized in the solvent. Therefore it is not surprising to observe a spherical morphology in methanol for the micelles consisting of a PS76 core surrounded by PTFMS42–[Ru]–PEG70 coronal chains. Since the solubility of the PTFMS is slightly reduced in ethanol compared to methanol, the coexistence of spherical micelles and wormlike micelles in ethanol can be explained by the decreased hydrodynamic volume of the PTFMS-block. With a decreasing polarity of the solvents, PTFMS became less soluble; the polymer only dissolved in 2-propanol, 1-propanol and 1-butanol at higher temperatures, indicating that PTFMS exhibits an UCST in these solvents (48.6 °C in 2-propanol, 72.5 °C in 1-propanol and 82.6 °C in 1-butanol, respectively; determined at 50% transmittance during cooling), whereby the UCST increases with decreasing solvent polarity. In other words, the investigated metallo-supramolecular triblock terpolymer (PS76–b–PTFMS42–[Ru]–PEG70) is able to change the solvophilic to solvophobic block ratio depending on the solvent system utilized; here a transition from spherical micelles to wormlike micelles to vesicles and hollow tubes can be observed. Using methanol and ethanol as solvents for the triblock terpolymer, two polymer blocks are solvophilic (PEG and PTFMS), whereas only the PEG block is solvophilic at room temperature in the less polar solvents, i.e.1-propanol, 2-propanol and 1-butanol (Fig. 6). On closer inspection, the turbidity curves of PTFMS in 1-propanol and 1-butanol in Fig. 5 reveal strong hysteresis, which is absent for the UCST transition observed in 2-propanol. This difference might be due to the hydrogen bond acidity of the different solvents, which affect the formation of weak hydrogen bonds between the hydroxyl-group of the solvent and the fluorine atoms of the PTFMS. It might be speculated that the higher hydrogen-bond acidity of 1-propanol and 1-butanol results in better solvation of the precipitated polymer upon heating compared to the 2-propanol that has a lower hydrogen bond acidity.54 In contrast, the precipitation of the polymer upon cooling seems to be related to the solvent polarity. The exact nature of these observed differences will be investigated in detail in future work. These future studies should also focus on the multiple transitions that are observed at around 62 °C in the cooling run for 1-propanol and 1-butanol that might be related to the formation of large highly solvated aggregates followed by the formation of denser aggregates upon further cooling and finally macroscopic precipitation resulting in 0% transmittance. Nonetheless, the existence of an upper critical solution temperature in 1-propanol, 2-propanol and 1-butanol is the main important observation for the current study since it explains the observed morphological transformations for the triblock terpolymer.
 | ||
| Fig. 4 Unstained TEM images of the triblock copolymer in different solvents. | ||
 | ||
| Fig. 5 Transmission measurements of polytrifluoromethylstyrene in methanol, ethanol, 1-propanol, 2-propanol and 1-butanol in the range from −20 °C to 100 °C. | ||
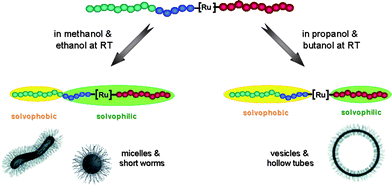 | ||
| Fig. 6 Schematic representation of the soluble blocks of the triblock copolymer in different solvents. | ||
In general, transitions from spherical micelles to wormlike micelles to vesicles go along with a decrease in the core-corona interfacial curvature. A decrease in interfacial curvature could directly result from an increase in hydrophobic block length.10,35 This is actually the situation happening in this study: the solvophobic block length in the investigated triblock terpolymer increases when altering the solvent from methanol or ethanol to propanol or butanol (Fig. 6). The decrease in the solvent compatible block leads to reduced corona crowding and, therefore, allows a larger aggregation number. However, an unlimited expansion of the micelle is prevented due to a large entropy penalty caused by the stretching of the solvophobic blocks in the core domain. In order to satisfy the interfacial curvature requirement, the micelle undergoes a transition towards wormlike micelles and vesicles rather then forming larger spherical micelles (Fig. 4). Since those morphologies are formed during the cooling of the solution, which is realized at a given speed, we cannot exclude the kinetic trapping of non-equilibrium morphologies during this step. This could explain why different morphologies are coexisting in each TEM picture of Fig. 4.
The nanostructures formed at room temperature in 1-propanol, 2-propanol and 1-butanol possess the PS-block and the PTFMS-block in the core domain. Since those blocks phase-separate into coexisting hydrocarbon and fluorocarbon microdomains (as was confirmed by the bulk phase separation, see Fig. 2), it might be speculated that multicompartment micelles and vesicles are formed in these solvents. Unfortunately, it was not possible to visualize these multicompartment structures by staining in TEM due to the presence of ruthenium in the polymer, but future investigations will be directed to prove the formation of multicompartment structures by e.g.dye-encapsulation studies.35
Due to the fact that PTFMS exhibits an UCST in 1-propanol, 2-propanol and 1-butanol, thermoreversible switching of the morphologies was investigated in these solvents. Therefore, the micellar solution (in 2-propanol) was not cooled to room temperature after heating to 140 °C but only to 75 °C. The solution was left at 75 °C for 12 hours in order to allow the system to reach equilibrium. Afterwards, the solution was drop-cast onto a heated TEM-grid. After evaporation of the solvent in the oven, the morphologies are kinetically frozen and the corresponding TEM image clearly reveals the formation of spherical micelles instead of vesicles that were present at room temperature (Fig. 7).
 | ||
| Fig. 7 TEM images in 2-propanol at room temperature (left) and at 75 °C (right) representing the thermoreversible character of the formed aggregates. | ||
Above the UCST temperature in less polar solvents, the triblock terpolymer behaves as it does in methanol and ethanol at room temperature, i.e. the PEG-block and the PTFMS-block are solubilized and accommodated in the corona of the micelle. Thus, the interfacial curvature increases and causes a transition from vesicles to spherical micelles. The larger aggregates in the image are believed to have been formed upon cooling of the micelle solution during the blotting onto the TEM grid since the clear solution turned slightly cloudy indicating changes in the particle size.
Experimental section
para-Trifluoromethylstyrene was obtained from Apollo Scientific Ltd and styrene from Sigma-Aldrich. Both monomers were purified by column chromatography (basic AlOx) before usage. All other chemicals were of reagent grade and used as received unless otherwise specified. The nitroxide initiator was synthesized as described previously.45GPC measurements were either performed on a Shimadzu system with a SCL-10A system controller, a LC-10AD pump, a RID-6A refractive index detector and a Polymer Laboratories PLgel 5 µm Mixed-D column using N,N-dimethylacetamide (DMA) as eluent at a flow rate of 1 mL/min or on a Waters system with a 1515 pump, a 2414 refractive index detector, a 2996 photo-diode array (PDA) detector and a Waters Styragel HT4 column utilizing a N,N-dimethylformamide (DMF)/5 mM NH4PF6 mixture as eluent at a flow rate of 0.5 mL/min at 50 °C. Molecular weights were calculated against polystyrene standards.
1H-NMR spectra were recorded on a Varian Gemini 400 spectrometer using deuterated methylene chloride (Cambridge Isotopes Laboratories) at room temperature.
The solubility measurements were performed by heating the polymer (2.0 ± 0.1 mg) in the corresponding solvent (1.0 mL) from −20 °C to 90 °C (methanol and ethanol) or from 20 to 100 °C (1-propanol, 2-propanol, 1-butanol) with a heating rate of 1.0 °C per minute followed by cooling at a cooling rate of 1.0 °C per minute after keeping the temperature for 10 minutes at the maximum/minimum temperature. During these controlled heating cycles the transmission through the solutions was monitored in a Crystal16 from Avantium Technologies.55 All vials were visually inspected after the heating program to facilitate interpretation of the observed transmission profiles. The upper critical solution temperature (UCST) was determined at 50% transmittance in the second cooling cycle.
Transmission microscopy measurements were performed on a FEI Tecnai 20, type Sphera TEM operating at 200 kV (LaB6 filament). Images were recorded with a bottom mounted 1 k × 1 k Gatan CCD camera. 200 mesh carbon coated copper grids for TEM were purchased from SPI. Prior to blotting, the grids were made hydrophilic by surface plasma treatment using a Cressington 208 carbon coater operating at 5 mA for 40 s. For sample preparation a droplet of the micelle solution was blotted onto the grid and subsequently excess liquid was manually removed with filter paper. The samples for TEM measurements were not stained.
AFM investigations were conducted on a NanoScope IIIa Multimode system (DI, Santa Barbara, US) in tapping mode. Commercially available AFM tips, purchased from µ-masch (Estonia), were used for imaging.
Terpyridine-functionalized poly(trifluoromethylstyrene) (]–PTFMS42)
The unimolecular nitroxide initiator (100 mg, 1.7 × 10−4 mol) was dissolved in p-trifluoromethylstyrene (3.0 g, 0.017 mol, M/I = 100). Three freeze-pump-thaw cycles were applied for removal of oxygen before the closed reaction vessel was immersed in an oilbath at 120 °C for 1.6 hours. The polymer was precipitated twice from CH2Cl2 into cold methanol.1H NMR (400 MHz, CD2Cl2, 25 °C, TMS): δH = 8.67 (m, 2H; H6:6″), 8.64 (m, 2H; H3:3″), 8.14 (m, 2H; H3′:5′), 7.88 (m, 2H; H4:4″), 7.60–6.30 (m, 179H; HPTFMS backbone, Haromatic, H5,5″), 5.30–5.20 (m, 2H; tpyOCH2), 4.78 (m, 1H; HC–ON, both diastereomers), 3.54–3.20 (m, 1H; ON–CH, major & minor), 2.30–0.00 (m, 145H; HPTFMS backbone, C(CH3)3; CH3CHCH3 major and minor, CH3CHCH3; CH3CH–ON).
GPC (eluent DMA with LiCl 2.1 g/L): Mn = 7,100 g/mol, PDI = 1.16.
Terpyridine-functionalized poly(trifluoromethylstyrene-b-styrene) (]–PTFMS42–b–PS76)
The poly(trifluoromethylstyrene) macroinitiator (250 mg, 3.2 × 10−5 mol, Mn = 7,900 g/mol) was dissolved in purified styrene (0.9 g, 8.6 × 10−3 mol, M/I = 260) and 0.5 mL dry toluene. Three freeze-pump-thaw cycles were applied, and the reaction mixture was heated for 2 hours in a closed reaction vessel at 120 °C and then stopped. The block copolymer was precipitated twice from dichloromethane into methanol. The precipitate was collected and dried under vacuum to yield the desired block copolymer.1H NMR (400 MHz, CD2Cl2, 25 °C, TMS): δH = 8.68 (m, 2H; H6:6″), 8.65 (m, 2H; H3:3″), 8.14 (m, 2H; H3′:5′), 7.88 (m, 2H; H4:4″), 7.58–6.21 (m, 559H; HPS backbone, HPTFMS backbone, Haromatic, H5,5″), 5.30–5.20 (m, 2H; tpyOCH2), 4.78 (m, 1H; HC–ON, both diastereomers), 3.54–3.20 (m, 1H; ON–CH, major & minor), 2.37–0.10 (m, 373H; HPTFMS & PS aliphatic backbone, CH3CHCH3 major, C(CH3)3; CH3CHCH3 minor, CH3CHCH3; CH3CH–ON).
GPC (eluent DMA with LiCl 2.1 g/L): Mn = 13,100 g/mol, PDI = 1.18.
Metallo-supramolecular triblock terpolymer (PS76–b–PTFMS42–[Ru]–PEG70)
PEG70–[RuCl3 (22.7 mg, 6.5 × 10−6 mol)56 and terpyridine-functionalized PS76–b–PTFMS42 (95 mg, Mn = 15,800 g/mol, 6.0 × 10−6 mol) were dissolved in a mixture of THF:MeOH (2:1, 1.5 mL). The reaction mixture was heated to 85 °C for 30 minutes. A few drops of N-ethylmorpholine were added to the solution. Stirring under reflux was continued overnight, after which an excess of NH4PF6 was added. The solvent was removed in vacuo and the reaction mixture was partitioned between 25 mL water and 25 mL methylene chloride (CH2Cl2). The organic layer was washed with water (3 × 25 mL), dried over Na2SO4 and finally removed in vacuo. The triblock terpolymer was further purified by preparative size exclusion chromatography (BioBeads SX-1) and column chromatography (AlOx). Yield: 48%.1H NMR (400 MHz, CD2Cl2, 25 °C, TMS): δH = 8.60–8.37 (m, 8H; H3′:5′, H3:3″), 7.86 (m, 4H; H4:4″), 7.58–6.21 (m, 565H; HPS & PTFMS aromatic backbone, Haromatic, H6,6″, H5,5″), 5.30–5.20 (m, 2H; tpyOCH2), 4.78 (m, 1H; HC–ON, both diastereomers), 3.90–3.15 (m, 281H; ON–CH, major and minor, OCH2 PEG backbone), 2.37–0.10 (m, 373H; HPTFMS & PS aliphatic backbone, CH3CHCH3 major, C(CH3)3; CH3CHCH3 minor, CH3CHCH3; CH3CH–ON); GPC (eluent DMF with NH4PF6 0.8 g/L): Mn = 11,100 g/mol, PDI = 1.10; UV-vis (CHCl3): λ/nm (ε/L mol−1 cm −1): 489 (12800), 446 (7700), 343 (6100), 305 (46600), 270 (56000).
Conclusions
In summary, we have demonstrated that a well-defined metallo-supramolecular triblock terpolymer can be successfully synthesized using a combination of synthetic concepts from polymer and supramolecular chemistry. The amphiphilic character of this copolymer has been used to prepare micelles in various alcohols as solvents. The solubility of the fluorinated middle block could be tuned depending on the polarity of the alcohol. This finally resulted in micelles with various morphologies. All basic micellar morphologies, i.e. spherical micelles, wormlike micelles and vesicles, were easily obtained from the same copolymer only by changing the solvent used. Moreover, the UCST behavior of the fluorinated middle block allowed the formation of discrete nano objects whose morphology can be reversibly tuned as a function of temperature. This process is controlled by the fascinating solution characteristics of the PTFMS-block. The obtained block copolymer micelles could be potentially applied for the incorporation and transport of hydrophobic guest molecules as well as for catalytic purposes. Future work will include opening up the heteroleptic ruthenium complex by adding a large excess of a strong competing ligand. In a subsequent reaction, the vacant terpyridine ligand can be easily combined with a different macromolecular building block allowing the construction of block copolymer libraries.Acknowledgements
The authors thank the Dutch Counsel for Scientific Research (NWO, VICI award) and the Fonds der Chemischen Industrie for funding. This research has been carried out with the support of the Soft Matter cryo-TEM Research Unit, Department of Chemical Engineering and Chemistry, Eindhoven University of Technology. JFG is grateful to the STIPOMAT ESF Programme and to the “Politique Scientifique Fédérale” for financial support in the frame of the “Interuniversity Attraction Poles Programme (PAI VI/27): Functional Supramolecular Systems”.References
- R. Savic, L. B. Luo, A. Eisenberg and D. Maysinger, Science, 2003, 300, 615 CrossRef CAS.
- C. Allen, D. Maysinger and A. Eisenberg, Colloids Surf. B, 1999, 16, 3 CrossRef CAS.
- P. Alexandridis and B. Lindman, Amphiphilic Block Copolymers: Self-Assembly and Applications, Elsevier, Amsterdam, 2000 Search PubMed.
- M. Park, C. Harrison, P. M. Chaikin, R. A. Register and D. H. Adamson, Science, 1997, 276, 1401 CrossRef CAS.
- I. W. Hamley, The Physics of Block Copolymers, Oxford University Press,Oxford, 1998 Search PubMed.
- J.-F. Gohy, Adv. Polym. Sci., 2005, 190, 65 CAS.
- L. Desbaumes and A. Eisenberg, Langmuir, 1999, 15, 36 CrossRef CAS.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967 CrossRef CAS.
- T. P. Lodge, J. A. Bang, Z. B. Li, M. A. Hillmyer and Y. Talmon, Faraday Discuss., 2005, 128, 1 RSC.
- L. Zhang and A. Eisenberg, Polym. Adv. Technol., 1998, 9, 677 CrossRef CAS.
- R. Hoogenboom, H. M. L. Thijs, D. Wouters, S. Hoeppener and U. S. Schubert, Macromolecules, 2008, 41, 1581 CrossRef CAS.
- X. Wang, G. Guerin, H. Wang, Y. Wang, I. Manners and M. A. Winnik, Science, 2007, 317, 604 CrossRef.
- L. Zhang and A. Eisenberg, Science, 1995, 268, 1728 CrossRef CAS.
- L. Zhang and A. Eisenberg, J. Am. Chem. Soc., 1996, 118, 3168 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS.
- M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689 CrossRef CAS.
- C. J. Hawker, A. W. Bosman and E. Harth, Chem. Rev., 2001, 101, 3661 CrossRef CAS.
- H. Fischer, Chem. Rev., 2001, 101, 3581 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Polymer, 2008, 49, 1079 CrossRef CAS.
- C. J. Hawker and K. L. Wooley, Science, 2005, 309, 1200 CrossRef CAS.
- Z. B. Li, E. Kesselman, Y. Talmon, M. A. Hillmyer and T. P. Lodge, Science, 2004, 306, 98 CrossRef CAS.
- J.-F. Gohy, N. Willet, S. Varshney, J. X. Zhang and R. Jérôme, Angew. Chem. Int. Ed., 2001, 40, 3214 CrossRef CAS.
- A. Kotzev, A. Laschewsky, P. Adriaensens and J. Gelan, Macromolecules, 2002, 35, 1091 CrossRef CAS.
- Y. He, Z. Li, P. Simone and T. P. Lodge, J. Am. Chem. Soc., 2006, 128, 2745 CrossRef CAS.
- C.-A. Fustin, V. Abetz and J.-F. Gohy, Eur. Phys. J. E, 2005, 16, 291 CrossRef CAS.
- C. S. Patrickios, A. B. Lowe, S. P. Armes and N. C. Billingham, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 617 CrossRef CAS.
- Y. Cai and S. P. Armes, Macromolecules, 2004, 37, 7116 CrossRef CAS.
- V. Sfika, C. Tsitsilianis, A. Kiriy, G. Gorodyska and M. Stamm, Macromolecules, 2004, 37, 9551 CrossRef CAS.
- W. Y. Chen, P. Alexandridis, C. K. Su, C. S. Patrickios, W. R. Hertler and T. A. Hatton, Macromolecules, 1995, 28, 8604 CrossRef CAS.
- J. Kriz, B. Masar, J. Plestil, Z. Tuzar, H. Pospisil and D. Doskocilova, Macromolecules, 1998, 31, 41 CrossRef CAS.
- G.-E. Yu and A. Eisenberg, Macromolecules, 1998, 31, 5546 CrossRef CAS.
- Q. Ma and K. L. Wooley, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4805 CrossRef CAS.
- J.-F. Gohy, B. G. G. Lohmeijer, S. K. Varshney, B. Decamps, E. Leroy, S. Boileau and U. S. Schubert, Macromolecules, 2002, 35, 9748 CrossRef CAS.
- A. K. Brannan and F. S. Bates, Macromolecules, 2004, 37, 8816 CrossRef CAS.
- T. P. Lodge, A. Rasdal, Z. Li and M. A. Hillmyer, J. Am. Chem. Soc., 2005, 127, 17608 CrossRef CAS.
- A. Laschewsky, Curr. Opin. Coll. Interf. Sci., 2003, 8, 274 CrossRef CAS.
- J.-F. Lutz and A. Laschewsky, Macromol. Chem. Phys., 2005, 206, 813 CrossRef CAS.
- G. Riess, Prog. Polym. Sci., 2003, 28, 1107 CrossRef CAS.
- I. Dimitrov, B. Trzebicka, A. H. E. Müller, A. Dworak and C. B Tsvetanov, Prog. Polym. Sci., 2007, 32, 1275 CrossRef CAS.
- M. Arotçaréna, B. Heise, S. Ishaya and A. Laschewsky, J. Am. Chem. Soc., 2002, 124, 3787 CrossRef CAS.
- J. V. M. Weaver, S. P. Armes and V. Bütün, Chem. Commun., 2002, 2122 RSC.
- Y. Maeda, H. Mochiduki and I. Kieda, Macromol. Rapid Commun., 2004, 25, 1330 CrossRef CAS.
- D. Benoit, V. Chaplinski, R. Braslau and C. J. Hawker, J. Am. Chem. Soc., 1999, 121, 3904 CrossRef CAS.
- B. G. G. Lohmeijer and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 1413 CrossRef CAS.
- B. G. G. Lohmeijer and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 4016 CrossRef CAS.
- B. G. G. Lohmeijer and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 6331 CrossRef CAS.
- C. Ott, B. G. G. Lohmeijer and U. S. Schubert, Macromol. Chem. Phys., 2006, 207, 1439 CrossRef CAS.
- C. Ott, R. Hoogenboom and U. S. Schubert, Chem. Commun., 2008, 3516 RSC.
- A. D. Ievins, A. O. Moughton and R. K. O'Reilly, Macromolecules, 2008, 41, 3571 CrossRef CAS.
- B. G. G. Lohmeijer and U. S. Schubert, Angew. Chem. Int. Ed., 2002, 41, 3825 CrossRef CAS.
- J.-F. Gohy, B. G. G. Lohmeijer and U. S. Schubert, Chem. Eur. J., 2003, 9, 3479.
- A. D. Ievins, A. O. Moughton and R. K. O'Reilly, Macromolecules, 2008, 41, 3571 CrossRef CAS.
- C.-A. Fustin, B. G. G. Lohmeijer, A. S. Duwez, A. M. Jonas, U. S. Schubert and J.-F. Gohy, Adv. Mater., 2005, 17, 1162 CrossRef CAS.
- M. H. Abraham, Chem. Soc. Rev., 1993, 73 RSC.
- R. Hoogenboom, H. M. L. Thijs, D. Wouters, S. Hoeppener and U. S. Schubert, SoftMatter, 2008, 4, 103 RSC.
- C. Ott, D. Wouters, H. M. L. Thijs, U. S. Schubert and J. Inorg, Organometal. Polym. Mater., 2007, 17, 241 Search PubMed.
| This journal is © The Royal Society of Chemistry 2009 |
