Disaggregation of single-walled carbon nanotubes (SWNTs) promoted by the ionic liquid-based surfactant 1-hexadecyl-3-vinyl-imidazolium bromide in aqueous solution†
Antonello
Di Crescenzo
a,
Davide
Demurtas
b,
Andrea
Renzetti
a,
Gabriella
Siani
a,
Paolo
De Maria
a,
Moreno
Meneghetti
c,
Maurizio
Prato
d and
Antonella
Fontana
*a
aDipartimento di Scienze del Farmaco, Università “G. d'Annunzio”, Via dei Vestini, I-66013, Chieti, Italy. E-mail: fontana@unich.it; Fax: +39 0871 3554461; Tel: +39 0871 4554790
bCentre Intégratif de Génomique (CIG), University of Lausanne, CH-1015, Lausanne, Switzerland
cDipartimento di Scienze Chimiche, Università degli Studi di Padova, Via Marzolo 1, I-33151, Padova, Italy
dDipartimento di Scienze Farmaceutiche, Università degli Studi di Trieste, Piazzale Europa 1, I-34127, Trieste, Italy
First published on 24th September 2008
Abstract
Stable homogeneous aqueous dispersions of pristine single-walled carbon nanotubes (SWNTs) are obtained by using a water-soluble long-chain imidazolium ionic liquid (hvimBr) above its critical micelle concentration. The amount of hivmBr and the sonication time are two essential factors to obtain a good dispersion. The effective concentration of exfoliated SWNTs in aqueous solution is determined by simple, convenient and rapid UV-visible spectrophotometric measurements.
In the last decade, carbon nanotubes1 have received much attention due to their unique physical, electronic2 and optical3 properties, particularly for applications in materials science and sensor technology.4 In the biomedical field carbon nanotubes have also been extensively studied as cellular carriers of biomolecules in diagnostics and therapeutics,5,6 as immobilizers of protein and biosensors7 and as transporters of genetic material.8
However, the major limitation related to their use is their low solubility in most organic and aqueous solvents, and their tendency to aggregate into packed ropes or entangled networks. Thus, the dispersion and dissolution of SWNTs is recognized to be an important step for their application.
To favor the disaggregation of bundles and the dispersion of individual nanotubes in solution different approaches have been followed. Covalent modifications have allowed the introduction of various organic groups onto the π-conjugated backbone of the SWNTs.9 When the group introduced on the SWNTs surface is hydrophilic, dispersion in water is favored. The disadvantage of this approach is that covalent functionalization may alter the valuable electronic properties of SWNTs. An alternative strategy to achieve better processability of SWNTs is non-covalent modification. The non-covalent approach is based on the adsorption of suitable molecules on the nanotube surface, and results in either a proper functionalization of the nanotubes or in an efficient intercalation among bundled SWNTs, and consequently in an effective dispersion of individual SWNTs. The advantage of this strategy is the preservation of the extended π-electron networks of SWNTs. Up to now, dispersing agents used for the preparation of unbundled SWNT solution have been ionic (sodium dodecylsulfate, SDS;3,10sodium dodecyl benzenesulfonate, SDBS;3cetyl trimethylammonium bromide, CTAB)3 and non-ionic surfactants (Triton X-100),3polysaccharides such as gum arabic,11water-soluble polymers,12 polycyclic aromatics,13DNA14 and proteins.15 The hydrophobic portions of these molecules interact with the nanotube surface through van der Waals, π–π, CH–π, cation–π and other interactions, while the hydrophilic portions orient themselves toward the aqueous phase.
Room-temperature ionic liquids (RTILs) are promising non-volatile, non-flammable, environmentally safe alternatives to conventional organic solvents.16 Nowadays, ionic liquids have been extensively used as “green” solvents in organic synthesis.17 Fukushima et al. have demonstrated18 that RTILs based on the imidazolium cation, upon grinding with SWNTs, form physical gels called “bucky gels”. The heavily entangled nanotube bundles were found to segregate within the gel to form much finer bundles. However, these black gels are hydrophobic and highly viscous, and therefore unsuitable for use in the biological field. We thought it interesting to use a water-soluble long-chain ionic liquid (IL) with surfactant properties,19–221-hexadecyl-3-vinylimidazolium bromide (hvimBr), in order to exploit both its features of being a surfactant and an IL, and hopefully obtain a very effective dispersing agent for SWNTs. Kocharova et al. have recently produced23water-soluble, positively charged nanotubes with pendent surface-active thiol groups, by using an imidazolium IL derivative. Our aim is i) to evaluate the capacity of hvimBr to solubilize and unbundle SWNTs with respect to other dispersing agents; ii) to measure the stability of the obtained dispersions; and iii) to study the effect that a physical dispersion process such as sonication has on SWNT dispersions.
The IL 1-hexadecyl-3-vinylimidazolium bromide was synthesized according to literature procedures (See NMR spectra in ESI†).24 Aqueous solutions of hvimBr were prepared by adding water to the proper amount of IL and heating the solution at 39 °C for 5 minutes in order to favor the salt dissolution. These aqueous solutions are stable for hours at room temperature. The critical micelle concentration (CMC) of hvimBr was measured by both fluorimetric and conductimetric measurements (see ESI† for experimental details) and an average value of 0.13(±0.02) mg mL−1 [= 3.3(±0.6) × 10−4 M] was obtained in good agreement with the published value of 3.2 × 10−4 M24 (see Fig. S3 and S4 of ESI†I).
Aqueous dispersions of SWNTs25 were prepared by adding 5 mL of hvimBr aqueous solutions of different concentrations to 1 mg of pristine SWNTs placed into a glass centrifuge tube. The sample was then sonicated with a ultrasonic bath sonicator (Transsonic 310 Elma, 35 KHz) for several hours. The obtained suspension was centrifuged for 10 min at 4000 RPM by using a Universal 32 (Hettich Zentrifugen) centrifuge in order to accelerate the separation of the supernatant aqueous solution from the precipitate. The former consists of mostly exfoliated or fine bundled SWNTs, while the latter contains essentially non-dispersed SWNTs, graphite, amorphous carbon and metal catalysts.26
The absorption spectra of the suspended individual SWNTs were obtained by using a Cary 100 (Varian) UV-visible spectrophotometer with 1 mm quartz cuvettes. In recent years, UV-vis spectroscopy has been extensively used to analyze SWNT dispersions.27–29 In the UV-vis-NIR region, SWNTs exhibit characteristic electronic adsorption spectra that show more pronounced structures with increasing percentage of exfoliated SWNTs in the suspension.3,30 As a matter of fact, the broadening of the peaks referring to bundled SWNTs has been attributed to side-by-side van der Waals contacts,31 while the series of sharp van Hove maxima at energies dependent mainly on tube diameter have been attributed to the electronic density of states of the quasi one-dimensionality of SWNTs.3
We used UV-vis spectroscopy in order to quantify the amount of exfoliated SWNTs dispersed in the IL aqueous solution. We measured the extinction coefficient of SWNTs dispersed in SDBS at 377 nm by taking advantage of the calibration curve already proposed by Attal et al.26 for pristine HiPCO SWNTs at 273 nm. Indeed, the absorbance spectrum of hvimBr is characterized by one absorption peak at 220 nm (ε = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 mol−1 L cm−1) but the absorbance of hvimBr at 273 nm at the investigated concentrations is not zero (at λ = 273 nm, ε = 1460 mol−1 L cm−1). Therefore we preferred to move our measurements at 377 nm, a wavelength where hvimBr does not absorb. The alternative was to employ the reference substraction method,26 which allows us to eliminate the contribution of IL absorbance by using the aqueous IL solution as the reference. Our choice was dictated by the fact that, during the separation of the supernatant from the precipitate, a small amount of the surfactant might be trapped26 in the solid residue, thereby causing a mismatch between the concentration of IL in the reference and in the supernatant aqueous solution. By plotting the absorbance of exfoliated SWNT–SDBS aqueous solutions against the true concentrations of SWNTs (calculated from the absorbance of the supernatant at λ = 273 nm and the extinction coefficient for the same system at λ = 273 nm derivable from ref. 26) an average extinction coefficient of 106.0 ± 1.3 mL mg−1 cm−1 has been obtained at 377 nm (see as an example Fig. 1).
100 mol−1 L cm−1) but the absorbance of hvimBr at 273 nm at the investigated concentrations is not zero (at λ = 273 nm, ε = 1460 mol−1 L cm−1). Therefore we preferred to move our measurements at 377 nm, a wavelength where hvimBr does not absorb. The alternative was to employ the reference substraction method,26 which allows us to eliminate the contribution of IL absorbance by using the aqueous IL solution as the reference. Our choice was dictated by the fact that, during the separation of the supernatant from the precipitate, a small amount of the surfactant might be trapped26 in the solid residue, thereby causing a mismatch between the concentration of IL in the reference and in the supernatant aqueous solution. By plotting the absorbance of exfoliated SWNT–SDBS aqueous solutions against the true concentrations of SWNTs (calculated from the absorbance of the supernatant at λ = 273 nm and the extinction coefficient for the same system at λ = 273 nm derivable from ref. 26) an average extinction coefficient of 106.0 ± 1.3 mL mg−1 cm−1 has been obtained at 377 nm (see as an example Fig. 1).
 | ||
| Fig. 1 Calibration curve of HiPCO–SDBS at λ = 377 nm. The true concentration of SWNTs (lower than the concentration used in the preparation of the dispersion due to the formation of a precipitate) has been obtained form the absorbance of HiPCO–SDBS supernatant at λ = 273 nm by using the calibration curve reported by Attal et al.26 for the same system (see main text). | ||
Fig. 2 reports the UV-vis spectra of SWNTs in the presence of increasing concentration of hvimBr (traces a, b, c and e) and of SDBS (trace d). Each spectrum was recorded on the supernatant of the sample once it had undergone the proper sonication and centrifugation protocols. The absorption peaks in these five spectra match exactly in wavelength but differ in intensity. As the exfoliation and dispersing activities of SDBS towards SWNTs are well known,3,26 this indicates that, at comparable concentration of dispersing agent (compare curve e and d for hvimBr and SDBS, respectively), the solubilizing power of hvimBr is 20% higher than that of SDBS. Moreover, the good exfoliation activity of hvimBr is confirmed by the well resolved and structured spectra obtained on increasing the concentration of hvimBr as compared to the broad absorption generally observed for tube bundles.3,32–34
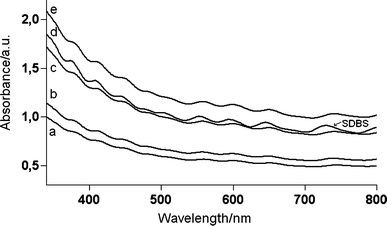 | ||
| Fig. 2 UV-vis spectra of the supernatant obtained from 1 mg of SWNTs dispersed with increasing initial hvimBr concentration (a = 0.272 mg mL−1; b = 0.816 mg mL−1; c = 1.77 mg mL−1; e = 2.58 mg mL−1) after 5 h of sonication and with an initial SDBS concentration of 2.60 mg mL−1 (curve d) after 8 h of sonication. | ||
The first step toward solubilization was to find suitable concentration ratios of nanotube to surfactant. To this end, we mixed 1 mg of carbon nanotube with various concentrations of aqueous solutions of hvimBr and sonicated the suspension for about 5 h (see Fig. S5, ESI†). The ratio of nanotube to surfactant varied from about 1 : 0.1 to 1 : 5.5 by weight. A great improvement of SWNT dispersion was obtained for surfactant concentration exceeding the CMC. An almost complete dispersion of nanotubes can be obtained when the concentration of hvimBr is approximately 20 times its CMC (Table 1). We could not, however, observe evidence of the presence of surfactant micelles or other phases. Presumably, as previously reported,10,35,36 the hydrophobic part of hvimBr is adsorbed on the graphite by van der Waals interactions, likely following the carbon network, and the hydrophilic part of the surfactant is oriented toward the aqueous phase, forming half-cylinders on the surface of the graphite plane.37
| hvimBr conc./mg mL−1 | hvimBr conc. (× CMC) | Conc. of dispersed SWNTs/mg mL−1 | Sonication time/h | % of dispersed SWNTs |
|---|---|---|---|---|
| 0.0272 | 0.2 | 0.0115 | 7 | 5.32 |
| 0.136 | 1.0 | 0.0232 | 5 | 11.2 |
| 0.272 | 2.1 | 0.0799 | 5 | 39.9 |
| 0.816 | 6.2 | 0.0974 | 6 | 50.2 |
| 1.77 | 13 | 0.160 | 7.5 | 79.7 |
| 2.58 | 20 | 0.197 | 17 | 98.7 |
In order to confirm the exfoliation activity of hvimBr we performed some cryo-TEM38 (transmission electron microscopy) measurements on the hvimBr–SWNT suspensions. This technique, making use of vitrified specimens, prevents component segregation and rearrangement, preserves the original fluid microstructure of the sample, and has proven to be well suited to structural investigations of lipid and surfactant systems.39 The cryo-TEM micrograph reported, as an example, in Fig. 3 clearly points to a fairly good unbundle activity of hvimBr on aggregated SWNTs.
 | ||
| Fig. 3 Cryo-TEM micrograph of the supernatant obtained from 1 mg of SWNTs dispersed in a 0.816 mg mL−1 hvimBr solution after 11 h of sonication. Black dots are due to catalyst particles from the HiPCO SWNTs. | ||
The NIR spectrum confirmed the solubilization of both semiconducting and metallic SWNTs and the good exfoliation activity of IL (see sharp van Hove singularity bands in Fig. S6, ESI†).
Raman spectroscopy gives relevant information on the type of nanotubes present in a sample. The comparison between the Raman spectra of the original SWNTs, as a solid sample, and that of the nanotubes dispersed in hvimBr does not evidence any change of the main features like the G band at 1590 cm−1, the D band at 1315 cm−1, its overtone, G', the RBM (radial breathing modes) spectral region below 400 cm−1 (See Fig. S7, ESI†). A luminescence is observed as a background in the spectrum of the wrapped nanotubes, which disappears when the nanotubes are thorougly washed with acetone. This causes the precipitation of the nanotubes from the solution and removes the wrapping IL. Fig. 4 shows the RBM spectral region in more detail. The wrapped nanotubes show a band at 220 cm−1, corresponding to that at 217 cm−1 in the original SWNT sample, with a strong intensity decrease. Using the experimental Kataura plots,40,41 which allow us to assign42 the individual RBM bands to specific nanotubes, the band at 217 cm−1 can be assigned to (12,3) and (13,1) metallic tubes. Excitation with the 633 nm laser line reveals large diameter metallic nanotubes below 230 cm−1 and semiconducting nanotubes with smaller diameters above this frequency. The intensity decrease is, therefore, found only for some type of metallic nanotubes. The intensity variation of an RBM band can derive both from the variation of the concentration of the corresponding nanotubes and/or from the variation of the resonance condition of the registered spectrum. In the second case the variation is a consequence of the variation of the electronic structure of the nanotubes interacting with the environment, i.e. with the ionic liquid molecules in the present case. To understand the reason of the decreasing intensity of the 217 cm−1 band, we report the comparison of the spectrum of the wrapped nanotubes (after substraction of the luminescence background) in Fig. 4 and the spectrum of the nanotubes thorougly washed with acetone (all bands normalized to the G band). Although we see some minor variations of the intensity of some bands, in particular those assigned to the metallic nanotubes (13,4) and (12,6), the intensity of the band at 217 cm−1 continues to remain low with respect to that of the original SWNTs. Therefore, we can draw the conclusion that wrapping with hvimBr leads to some selection of nanotubes perhaps due to their specific interaction with the ionic liquid, as it was noted for example with some polymers which have been reported43 to be able to wrap around nanotube with definite chiralities. However, further investigations are needed to understand which are the parameters (diameters, helicities, metallic or semiconducting properties) that govern the preferential interactions of the ionic liquid with nanotubes.
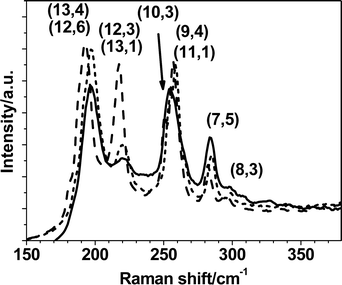 | ||
| Fig. 4 RBM bands (normalized on the G band) in the Raman spectra for solid SWNTs (dotted curve), for SWNTs dispersed in 2.58 mg mL−1 hvimBr and sonicated for 17 h (solid curve) and for SWNTs washed from the ionic liquid (dashed, luminescence background substracted). Assignment are based on experimental Kataura plots (see main text). | ||
Last, we investigated the relative efficacy of different sonication times on the dispersion of nanotubes. It has been reported3,44,45 that the mechanical energy supplied by ultrasonication helps the particles to overcome the attractive van der Waals force at contact. The standard approach was to disperse 1 mg of SWNTs with the proper amount of hvimBr using the same bath sonicator (35 kHz) for different times (from 1 to about 20 h). As can be concluded from the example of Fig. 5, the percentage of dispersed SWNTs increases markedly upon increasing the sonication time (up to 5–7 h) and then remains essentially unchanged (up to 18 h). A sonication time of 5–8 h can be considered an optimum compromise at all the investigated concentrations of hvimBr. It must be pointed out that the sonication process was associated with a moderate increase of the temperature, which might further favor the dispersion of SWNTs and the solubilization of hvimBr.
 | ||
| Fig. 5 Effect of the sonication time on the dispersion of SWNTs in aqueous solution at constant 2.58 mg mL−1 hvimBr. | ||
The dissolution activity of hvimBr has been compared to that of SDS, SBDS and CTAB in Table 2. It turns out that our ionic liquid, even at very low concentrations, is a very effective dispersing agent for SWNTs. At comparable surfactant : SWNT ratios the dissolution activity is twice as much as that of SDS (compare entries 7 and 1), 20% higher than that of CTAB (compare entries 6 and 5) and similar to that of SDBS (compare entries 6 and 2). However it turns out that the suspendability depends also on the absolute concentration of surfactant: at approximately 20 times their CMC the dissolution activity towards 0.02 mg% SWNTs is 20% higher for hvimBr with respect to SDBS (compare entries 8 and 3).
| Entry | Surfactant | Surfactant conc./mg% | Surfactant conc. (× CMC) | Initial SWNT conc./mg% | Surfactant : SWNT ratio | % of dispersed SWNTs |
|---|---|---|---|---|---|---|
| a Ref. 26. | ||||||
| 1 | SDSa | 1 | 4 | 0.1 | 10 : 1 | 45% |
| 2 | SDBS | 0.08 | 1.4 | 0.02 | 4 : 1 | 60% |
| 3 | SDBS | 0.26 | 4.7 | 0.02 | 13 : 1 | 79% |
| 4 | SDBSa | 1 | 18 | 0.1 | 10 : 1 | 65% |
| 5 | CTAB a | 0.5 | 15 | 0.1 | 5 : 1 | 40% |
| 6 | hvimBr | 0.08 | 6.2 | 0.02 | 4 : 1 | 50% |
| 7 | hvimBr | 0.18 | 13 | 0.02 | 9 : 1 | 80% |
| 8 | hvimBr | 0.26 | 20 | 0.02 | 13 : 1 | 99% |
To assess the stability at room temperature of the obtained SWNT dispersions, the UV-vis spectrum of each dispersion was monitored over time. The spectrum was recorded after centrifuging each sample for 10 min at 4000 rpm. Over a period of 2–3 months the SWNT–hvimBr dispersions are fairly stable as the SWNT concentration (measured from the absorbance at λ = 377 nm) remained constant in the presence of hvimBr 2.58, 1.77 and 0.816 mg mL−1 over 2 months (see Fig. S8, ESI†). A precipitation of more than 33% of the initially dispersed SWNTs was detected only for the sample obtained in the presence of a concentration (0.0272 mg mL−1) of hvimBr well below its CMC.
The ζ-potential is often used as an index of the magnitude of electrostatic interactions between colloidal particles and it is thus considered a further measure of the colloidal stability of a suspension. Particles with ζ-potential less than −15 mV or more than 15 mV are expected to be stabilized by electrostatic repulsion interactions.46 The ζ-potentials of hvimBr–SWNT, and, for sake of comparison, of SDBS–SWNT dispersions, were measured by using a Zeta Plus apparatus (Zeta Potential Analyzer, Brookhaven Instruments Corporation). For the SDBS–SWNT suspension (with a surfactant concentration of 0.25 mg mL−1) a ζ-potential of −51.7 ± 1.8 mV was obtained; this value is consistent with that reported in the literature for such an anionic surfactant.30 For the hvimBr–SWNT dispersion, at an IL concentration above the CMC (0.272 mg mL−1), the recorded ζ-potential was equal to 28.4 ± 1.8 mV. These highly negative and highly positive values further confirm the stability observed over time for SWNT dispersions of SDBS and hvimBr, respectively.
In conclusion, we have demonstrated a simple scheme to obtain exfoliated and stable suspensions of SWNTs in water. The concentration of the surfactant hvimBr is critical in order to solubilize high weight fraction single-wall carbon nanotubes. Suspension concentrations were improved by a factor of at least 20% with respect to commonly used surfactants and sonication for about 6 h increases further the dispersing power of hvimBr. Single SWNTs prepared at high concentration by these means can now be used for different applications, from the creation of novel composite materials, to their use as sensors or as drug carriers in water.
Acknowledgements
This work has been supported by MIUR (PRIN 2006, project 2006034372).References
- S. Iijima, Nature, 1991, 354, 56–58A CrossRef CAS.
- R. Saito, M. Fujita, G. Dresselhaus and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 1804–1811 CrossRef CAS.
- M. J. O'Connell, S. M. Bachilo, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. P. Ma, R. H. Hauge, R. B. Weissman and R. E. Smalley, Science, 2002, 297, 593–596 CrossRef CAS.
- J. Kong, N. R. Franklin, C. W. Zhou, M. G. Chapline, S. Peng, K. J. Cho and H. Dai, Science, 2000, 287, 622–625 CrossRef CAS.
- M. Prato, K. Kostarelos and A. Bianco, Acc. Chem. Res., 2008, 41, 60–68 CrossRef CAS.
- D. Pantarotto, J.-P. Briand, M. Prato and A. Bianco, Chem. Commun., 2004, 16–17 RSC.
- R. J. Chen, Y. Zhang, D. Wang and H. Dai, J. Am. Chem. Soc., 2001, 123, 3838–3839 CrossRef CAS.
- D. Pantarotto, R. Singh, D. McCarthy, M. Erhardt, J.-P. Briand, M. Prato, K. Kostarelos and A. Bianco, Angew. Chem., Int. Ed., 2004, 43, 5242–5246 CrossRef CAS.
- J. Chen, M. A. Hamon, H. Hu, Y. Chen, A. M. Rao, P. C. Eklund and R. C. Haddon, Science, 1998, 282, 95–98 CrossRef CAS; V. Georgakilas, K. Kordatos, M. Prato, D. M. Guldi, M. Holzinger and A. Hirsch, J. Am. Chem. Soc., 2002, 124, 760–761 CrossRef CAS; D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS.
- M. F. Islam, E. Rojas, D. M. Bergey, A. T. Johnson and A. G. Yodh, Nano Lett., 2003, 3, 269–273 CrossRef CAS.
- R. Bandyopadhyaya, E. Nativ-Roth, O. Regev and R. Yerushalmi-Rozen, Nano Lett., 2002, 2, 25–28 CrossRef CAS.
- V. A. Sinani, M. K. Gheith, A. A. Yaroslavov, A. Rakhnyanskaya, K. Sum, A. Mamedov, J. P. Wicksted and N. A. Kotov, J. Am. Chem. Soc., 2005, 127, 3463–3472 CrossRef CAS.
- A. Mateo-Alonso, C. Ehli, K. H. Chen, D. M. Guldi and M. Prato, J. Phys. Chem. A, 2007, 111, 12669–12673 CrossRef CAS.
- J. N. Barisci, M. Tabhan, G. G. Wallace, S. Badaire, T. Vaugien, M. Maugey and P. Poulin, Adv. Mater., 2004, 14, 130–133.
- S. S. Karajanagi, H. Yang, P. Asuri, E. Sellitto, J. S. Dordick and R. S. Kane, Langmuir, 2006, 22, 1392–1395 CrossRef CAS.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773–3789 CrossRef.
- C. Chiappe, D. Pieraccini and P. Saullo, J. Org. Chem., 2003, 68, 6710–6715 CrossRef CAS.
- T. Fukushima, A. Kosaka, Y. Ishimura, T. Yamamoto, T. Takigawa, N. Ishii and T. Aida, Science, 2003, 300, 2072–2074 CrossRef CAS.
- J. Bowers, C. P. Butts, P. J. Martin and M. C. Vergara-Gutierrez, Langmuir, 2004, 20, 2191–2198 CrossRef CAS.
- Z. Miskolczy, K. Sebők-Nagy, L. Biczók and S. Göktürk, Chem. Phys. Lett., 2004, 400, 296–300 CrossRef CAS.
- J. Sieriex-Plénet, L. Gaillon and P. Letellier, Talanta, 2004, 63, 979–986 CAS.
- R. Vanyúr, L. Biczók and Z. Miskolczy, Colloids Surf., A, 2007, 299, 256–261 CrossRef CAS.
- N. Kocharova, T. Ääritalo, J. Leiro, J. Kankare and J. Lukkari, Langmuir, 2007, 23, 3363–3371 CrossRef CAS.
- P. Mukherjee, J. A. Crank, M. Halder, D. W. Armstrong and J. W. Petrich, J. Phys. Chem. A, 2006, 110, 10725–10730 CrossRef CAS.
- Pristine HiPCO SWNTs were provided by Carbon Nanotechnologies, Inc. (CNI Lot #: P0257) Houston, USA.
- S. Attal, R. Thiruvengadathan and O. Regev, Anal. Chem., 2006, 78, 8098–8104 CrossRef CAS.
- L. Jiang, L. Gao and J. Sun, J. Colloid Interface Sci., 2003, 260, 89–94 CrossRef CAS.
- B. J. Landi, H. J. Ruf, C. M. Evans, C. D. Cress and R. P. Raffaelle, J. Phys. Chem. B, 2005, 109, 9952–9965 CrossRef CAS.
- V. C. Moore, M. S. Strano, E. H. Haroz, R. H. Hauge and R. E. Smalley, Nano Lett., 2003, 3, 1379–1382 CrossRef CAS.
- B. White, S. Banerjee, S. O'Brien, N. J. Turro and I. P. Herman, J. Phys. Chem. C, 2007, 111, 13684–13690 CrossRef CAS.
- S. Reich, C. Thomsen and P. Ordejon, Phys. Rev. B, 2002, 65, 155411/1–155411/11 CAS.
- X. Zhang, T. Liu, T. V. Sreekumar, S. Kumar, V. C. Moore, R. H. Hauge and R. E. Smalley, Nano Lett., 2003, 3, 1285–1288 CrossRef CAS.
- A. M. Rao, E. Richter, S. Bandow, B. Chase, P. C. Eklund, K. A. Williams, S. Fang, K. R. Subbaswamy, M. Menon, A. Thess, R. E. Smalley, G. Dresselhaus and M. S. Dresselhaus, Science, 1997, 275, 187–191 CrossRef CAS.
- A. G. Ryabenko, T. V. Dorofeeva and G. I. Zvereva, Carbon, 2004, 42, 1523–1535 CrossRef CAS.
- S. Manne, J. P. Cleveland, H. E. Gaub, G. D. Stucky and P. K. Hansma, Langmuir, 1994, 10, 4409–4413 CrossRef CAS.
- E. J. Wanless and W. A. Ducker, J. Phys. Chem., 1996, 100, 3207–3214 CrossRef CAS.
- C. Richard, F. Balavoine, P. Schultz, T. W. Ebbesen and C. Mioskowski, Science, 2003, 300, 775–778 CrossRef CAS.
- The specimens were prepared by applying a drop of the aqueous dispersion on a microcopper grid coated with a porous carbon film. Excess dispersion was blotted away, resulting in a 30–100 nm thick film that spans the holes of the porous carbon support. The sample was then rapidly vitrified by immersion in liquid ethane and eventually transferred into the cryo-electron microscope (Philips CM100, holder-specimen Gatan 626) operating at 100 kV in transmission mode at a temperature never exceeding −160 °C. Images were recorded on a CCD camera 2024 × 2024.
- J. Dubochet, M. Adrian, J. Chang, J. Homo, J. Lepault, A. W. McDowall and P. Schultz, Q. Rev. Biophys., 1988, 21, 129–228 CrossRef CAS.
- C. Fantini, A. Jorio, M. Souza, M. S. Strano, M. S. Dresselhaus and M. Pimenta, Phys. Rev. Lett., 2004, 93, 147406/1–147406/4 CAS.
- J. Maultzsch, H. Telg, S. Reich and C. Thomsen, Phys. Rev. B, 2005, 72, 205438/1–205438/16 CAS.
- S. Campidelli, M. Meneghetti and M. Prato, Small, 2007, 3, 1672–1676 CrossRef CAS.
- A. Nish, J.-Y. Hwang, J. Doig and R. J. Nicholas, Nat. Nanotechnol., 2007, 2, 640–646 Search PubMed.
- M. Sano, A. Kamino, J. Okamura and S. Shinkai, Science, 2001, 293, 1299–1301 CrossRef CAS.
- J.-M. Bonard, T. Stora, J.-P. Salvetat, F. Maier, T. Stöckli, C. Duschl, L. Forró, W. A. Heer and A. Châtelain, Adv. Mater., 1997, 9, 827–831 CrossRef CAS.
- P. C. Hiemez and R. Rajagopalan, Principles of Colloid and Surface Chemistry, Marcel Dekker, New York, 3rd edn, 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra and m.p. of the synthesized hvimBr. Fluorimetric (Fig. S3) and conductimetric (Fig. S4) determinations of the CMC of hvimBr. Effect of the concentration of hvimBr on the dispersion of SWNTs (Fig. S5). NIR spectra (Fig. S6). Raman spectra (Fig. S7). Stability of SWNT dispersions over time (Fig. S8). See DOI: 10.1039/b812022f |
| This journal is © The Royal Society of Chemistry 2009 |
