Environmental effects of ozone depletion and its interactions with climate change: Progress report, 2008†
United Nations Environment Programme, Environmental Effects Assessment Panel‡
First published on 13th December 2008
Abstract
After the enthusiastic celebration of the 20th Anniversary of the Montreal Protocol on Substances that Deplete the Ozone Layer in 2007, the work for the protection of the ozone layer continues. The Environmental Effects Assessment Panel is one of the three expert panels within the Montreal Protocol. This “EEAP” deals with the increase of the UV irradiance on the Earth's surface and its effects on human health, animals, plants, biogeochemistry, air quality and materials. For the past few years, interactions of ozone depletion with climate change have also been considered. It has become clear that the environmental problems will be long-lasting. In spite of the fact that the worldwide production of ozone depleting chemicals has already been reduced by 95%, the environmental disturbances are expected to persist for about the next half a century, even if the protective work is actively continued, and completed. The latest full report was published in Photochem. Photobiol. Sci., 2007, 6, 201–332, and the last progress report in Photochem. Photobiol. Sci., 2008, 7, 15–27. The next full report on environmental effects is scheduled for the year 2010. The present progress report 2008 is one of the short interim reports, appearing annually.
Ozone and changes in biologically active UV radiation reaching the Earth's surface
• The Montreal Protocol continues to be effective in protecting the stratospheric ozone layer. However, the timing of the return to pre-1980 ozone and UV values cannot yet be predicted precisely and continuation of monitoring and improvements of models are necessary
Evidence for the success of the Montreal Protocol continues to mount. The concentrations of ozone depleting substances are decreasing, and the concentrations of replacement chemicals are increasing.1,2 Stratospheric ozone also continues to recover. A European study showed that, at mid-latitudes in the northern hemisphere and in the Arctic, there was an almost monotonic negative trend from the late 1970s to the mid 1990s followed by an increase, as expected from the changes in ozone depleting substances, which peaked in 1997. However, changes in atmospheric circulation are also playing a key role in the observed turnaround.3 Improved models that include better estimates of atmospheric circulation predict a slightly faster ozone layer recovery at mid latitudes, and a slightly slower recovery at high latitudes compared with earlier model results.4 Unfortunately, few high-quality long-term measurements are available to confirm the ongoing success of the Montreal Protocol in terms of UV radiation received at the Earth's surface.• The effectiveness of the Montreal Protocol in countering global warming has been further established
As reported last year, the climate protection already achieved by the Montreal Protocol alone is larger than the reduction target of the first commitment period of the Kyoto Protocol (ending 2012).5 This climate protection is achieved by reductions in the concentrations of ozone-depleting substances that are also greenhouse gases.6 Furthermore, without the Montreal Protocol the more extensive ozone depletion would have led to changes in present-day surface temperatures similar in magnitude to those expected by 2025 due to increasing greenhouse gases. At polar latitudes in both hemispheres, ozone depletion would have led to a warmer surface on average (see Fig. 1). However, there are substantial regional differences: some regions would have warmed, while others would have cooled.7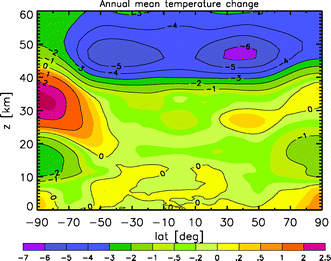 | ||
| Fig. 1 Mean difference in temperature, in K, between the simulation with 9 ppbv chlorine (no Montreal Protocol) and 3.5 ppbv chlorine (present day). From O. Morgenstern, P. Braesicke, M. M. Hurwitz, F. M. O'Connor, A. C. Bushell, C. E. Johnson and J. A. Pyle, The World Avoided by the Montreal Protocol, Geophys. Res. Lett., 35, L16811, DOI:10.1029/2008GL034590 (ref. 7). Reproduced by permission of the American Geophysical Union. | ||
• Changes in ozone and aerosols affect climate. These forcing agents should be included in future climate models
Interactions between ozone depletion and climate change are more complex than previously thought. For accurate prediction of future changes in climate, all forcing agents must be included, rather than the principal greenhouse gases alone. These forcing agents should include changes in ozone with altitude8 and longitude,9 changes in ozone depleting substances,10 and changes in aerosol.11 For example, climate models that include stratospheric chemistry predict that the observed increase in westerly winds at southern high latitudes will not continue as previously thought, but will decrease in the next few decades as ozone recovers12—see Tropospheric air quality, below.• UV radiation at high latitudes will be affected by the reduction in sea ice due to global warming
The extent of sea-ice in the Arctic is decreasing rapidly due to global warming and models suggest that the summertime ice cover will disappear within the next few decades.13,14 Thus organisms originally living below the ice will be exposed to increased UV doses, but organisms above the surface will receive decreased UV doses due to the reduced reflectivity—see Biogeochemical cycles, below.• The atmosphere is a complex system and any deliberate interventions should be treated with great care as they may have unanticipated adverse effects
It has been suggested that climate change can be counteracted by injection of sulfur compounds directly into the stratosphere to produce aerosols that reflect incoming solar radiation. However, this geo-engineering strategy would increase Arctic ozone depletion during the 21st century and delay Antarctic ozone recovery by 30 to 70 years.15Human health: Effects of solar UV radiation and interactions with climate change
• Climate change can amplify several damaging effects of solar UV-B radiation on human health
The impacts of temperature and UV-B dose on the incidence of non-melanoma skin cancer in subjects living in 10 regions of the USA were estimated separately. It was concluded that for the same UV dose, each one degree C increase in temperature would result in estimated increases in the incidences of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) of 3% and 6%, respectively.16 High temperatures and humidity, as experienced in the tropics, may increase the deleterious effects of UV-B radiation on human health, including suppression of immunity to infectious diseases and skin cancers.17• The incidence of melanoma in young Caucasian adults is increasing in some parts of the world but not in others (where sun protection campaigns appear to be working). Population-based studies in various countries continue to indicate that sun exposure is an important factor in the induction of melanoma
The annual age-adjusted incidence of cutaneous melanoma (CM) in Caucasian women, aged 15–39 years, living in nine areas of the USA, has increased over the last 20 years.18 This increased incidence correlates with reported increases in risk-related behaviours associated with increased exposure to solar UV radiation, the primary environmental cause of CM. The prevalence of sunburn in recent years has increased significantly amongst adults in 20 states of the USA.19 The number of days per year spent in outdoor activity by individuals less than 15 or more than 30 years of age is associated with increased CM risk,20 and the majority of the USA population engage in multiple risk-related behaviours.21 In contrast to the results from the USA, the incidence of CM in younger people in Queensland, Australia is stabilising currently—a situation attributed to public health campaigns directed at reducing harmful solar exposure.22 This outcome clearly shows that such campaigns can be effective at reducing the incidence of CM.• Trend analyses conducted in several countries demonstrate an increasing incidence of the non-melanoma skin cancers, squamous cell carcinoma and basal cell carcinoma, in which sun exposure is the major risk factor
For example, in the UK, there has been an annual increase in the incidence of basal cell carcinoma (BCC) over the past ten years, with the largest rise in the 30–39 year age group.23 In Sweden, the incidence of squamous cell carcinoma (SCC) has become four times greater in both men and women over the past 40 years; it was concluded that this rise may reflect behavioural and societal changes, particularly intentional tanning.24 In subtropical Australia, the annual incidence of primary BCCs in Caucasians was 1.5% in 2006.25 This is ten times higher than that observed in equivalent populations living in temperate climates. Overall, these results indicate that changes in human behaviour are still needed with regard to sun exposure in order to reduce the incidences of BCC and SCC.• Evidence continues to accumulate indicating that sun exposure can also have beneficial effects in protecting humans against several internal cancers and autoimmune diseases
Patients with BCC or SCC who had the highest accumulated sun exposure were shown to have a markedly decreased risk of developing colorectal cancer.26 In addition, exposure to sunlight was associated with a reduced risk of advanced breast cancer in women with fair skin,27 and sun exposure in early life was associated with a lower risk of prostate cancer.28 A study of the relationship between the solar UV-B irradiance at latitudes 46–52° N in Canada and prevalence of the autoimmune disease, multiple sclerosis (MS), confirmed earlier results that the incidence of MS increased with increasing latitude.29 Uniquely, this report showed that low exposure to solar UV-B radiation in early life, particularly in the first year, can be an important risk factor for MS in later life. A common suggestion in these reports is that the observed protective effects are due to the action of vitamin D, synthesized in the skin in response to UV-B radiation. Vitamin D has multiple effects on the immune system, cellular differentiation and cell death that help to control some tumours and autoimmune diseases. Public health agencies and individuals need to be aware that exposures to UV-B radiation are associated with beneficial and detrimental effects, and act accordingly.• The need to protect the eye from cataract and pterygium (two adverse consequences of ocular exposure to UV-B radiation) has been strengthened by results from additional populations and environments
Comparison of the numbers of cases of pterygium and cataract in four indigenous populations in the Amazonian rainforest revealed that differences in the amount of sun exposure are largely responsible for the observed differences in the prevalence of these two diseases.30 A study in a Croatian island population found a prevalence of pterygium of 23% amongst farmers and fishermen compared with none in subjects residing in urban areas.31 In a Tibetan population living at high altitude in China, those individuals who seldom used sunglasses had a five-fold increase in the risk of pterygium compared to the rest of the population.32 Recurrence of pterygium following surgery was reduced in those exposed to less sunlight.33 The public health need for eye protection (sunglasses) was emphasised in all of these studies.• A number of natural products, when given orally, can protect humans from some of the adverse consequences of UV-B radiation
Examples of such products include probiotic bacteria (such as those found in yoghurt),34 vitamin B3,35 carotenoids and flavonoids,36,37 and green tea polyphenols.38• Widespread uses of some new pharmaceuticals have the potential to adversely affect human responses to UV-B radiation
An example is infliximab (Remicade®, a monoclonal antibody against tumour necrosis factor-α) that is frequently used to treat a variety of autoimmune diseases (e.g., rheumatoid arthritis and Crohn's disease). Animals treated with infliximab have impaired DNA repair of UV-B-induced precancerous lesions in the skin. In humans, a similar effect could increase the risk of skin carcinogenesis.39Terrestrial ecosystems: Effects of solar UV radiation and interactions with climate change
• New studies confirm that UV-B radiation interacts with other climate factors and affects plant responses to several environmental stressors
Two studies40,41 combined substantial UV-B radiation stress and water deprivation. Overall, the UV-B and water stress reduced growth considerably, but less than would be predicted as the additive effect of both stressors. Experiments that manipulated CO2 concentration and UV-B radiation showed that increased CO2 stimulates growth and may compensate for some negative effects of high UV-B radiation42,43 as shown in earlier research. Elevated UV-B radiation with four weed species reduced the efficacy of a common herbicide.44 Earlier studies also showed that herbicide efficacy can be reduced by UV-B radiation.• Solar UV-B radiation can be a stress for plants in high-latitude ecosystems
Solar UV-B radiation exclusion studies in northern Greenland showed that ambient UV-B radiation decreased photosynthesis in bog blueberry plants despite increases in protective UV-absorbing pigments. Belowground biomass was decreased in response to solar UV-B, and soil microbial communities were altered.45 Thus, present day solar UV-B radiation may have pronounced effects on plants in this environment.• Systemic responses of plants to UV-B radiation continue to be documented
These responses in plant parts not directly exposed to the UV-B radiation can also have further biological consequences. For example, supplemental UV-B radiation applied to cotton plants did not alter plant growth but did increase content of phenolic substances in roots which, in turn, reduced numbers of nematodes (roundworms) and nematode eggs.46 Thus, the UV-B exposure of the shoot would potentially reduce belowground pest damage. Plants in a peat bog exposed to supplemental UV-B radiation had higher amounts of phenolic substances in roots, reduced carbohydrates in leaves, and increased carbohydrates in belowground storage organs. These changes may be responsible for the observed lower bacterial growth rates and changes in bacterial community composition within the peat where no sunlight penetrates.47 Bacterial activity in peat bogs is important for ecosystem function such as carbon sequestration.• Increasing evidence from arid and semiarid ecosystems suggests a direct role of UV-B radiation in photodegradation of dead plant material
Attenuation of solar UV-B radiation modestly reduced decomposition of twigs and senescent leaves of a desert shrub (Larrea tridentata) in the Mojave Desert48 as also shown to a greater degree in earlier studies in a semiarid steppe.49 Total exclusion of UV (UV-B and UV-A) significantly reduced decomposition of grass litter from the Colorado shortgrass steppe under drought conditions,50 and in a California annual grassland.51 Indirect evidence from studies in arid and semiarid ecosystems supports the idea that loss of litter mass is faster in bare soil patches exposed to high UV, than under the shade of shrubs.52,53 Increased aridity in response to climate change in some regions is likely to increase the importance of litter photodegradation as a driver of carbon emissions and nutrient cycling—see Biogeochemical cycles, below.• UV-B radiation and elevated temperature stimulate emission of methane in plants
Recently, methane (CH4) emissions from terrestrial ecosystems were reported to occur in the presence of oxygen rather than only under conditions of low or no oxygen, as had been documented previously. High temperatures stimulate the CH4 emission rates under solar radiation and especially when the UV radiation is enhanced.54 Experiments conducted without elevated temperatures and UV radiation resulted in little or no CH4 emission.55,56 The CH4 emissions are derived from degradation of plant cell wall components.57–59 McLeod and co-workers concluded that UV-B radiation is more effective in CH4 production than longer wavelengths.59 These plant-mediated effects of UV radiation may increase CH4 in the atmosphere—see Biogeochemical cycles, below.• Changes in solar UV-B radiation can have strong effects on insect herbivores and plant pathogens
A recent study60 found that the diamondback moth (Plutella xylostella), which is an important pest of crops, laid their eggs preferentially on plants grown in low levels of UV-B radiation, confirming previous work.61 Foggo and co-workers60 also showed that plant exposure to UV-B radiation resulted in reduced growth of the P. xylostella caterpillars, and an increased likelihood that the caterpillars would be attacked by parasitic wasps. In another study, enhanced UV-B radiation appeared to reduce pathogen infection in rice.62 Studies involving mammalian herbivores and several species of forages, on the other hand, have found little or no effects of UV-B radiation on plant nutritional value.63,64 Overall, it appears that UV-B radiation can affect many biotic interactions, but the consequences of changes in UV-B irradiance for the functioning of terrestrial food webs are difficult to predict. Recent work has provided additional insights into the mechanisms whereby UV-B radiation interacts with molecular components of plant defense system.65–67 This knowledge can be useful for breeding crop varieties resistant to multiple stresses.• Some animals can detect and react specifically to UV-B in their behavior
Although animal responses to UV radiation have historically been considered to be responses to the UV-A component, recent work has provided additional evidence that a specific response to UV-B occurs. For example, females of a jumping spider species choose a mate based on sex-specific UV-B reflectance patterns.68,69 This finding corroborates earlier reports of behavioral response to UV-B radiation for leaf thrips70,71 and frogs.72 Flight activity of hornets correlated better with daily patterns of UV-B radiation than with other weather variables.73 The finding that some animals can react specifically to UV-B under natural conditions71 raises the possibility that changes in UV-B irradiance may directly affect animal behavior. Thus, ozone column changes could potentially affect animal behavior with important consequences for insect reproduction, herbivory, and predation.Aquatic systems: Effects of solar UV radiation and interactions with climate change
• Increases in UV-B radiation due to human-induced ozone depletion combined with global climate change can negatively impact many aquatic species and ecosystems
These effects have implications for marine and freshwater ecosystems, and the economic and social systems that depend on them. Many new studies, especially from East Asia, India and South America, investigated the effects of solar UV radiation, climate change and their possible interactions, on scales ranging from whole communities to individual organisms at the cellular, biochemical and genetic level. Covering new geographical locations and species, these most recent studies largely confirm previous findings.74• Interactions among stressors are important when considering environmental and climate change on single species, as well as the effects on entire communities75
For example, declines in amphibian populations are likely due to many factors, including habitat loss, disease, contaminants, introduced species and UV-B radiation. The effect of UV-B varies widely among species and can vary within populations of the same species or at different life-history stages. This variation often leads to opposing conclusions about how UV-B affects amphibians. Larvae are more susceptible to damage from UV-B radiation compared with embryos. Salamanders are more susceptible compared with frogs and toads. Furthermore, UV-B radiation interacts synergistically with other environmental stressors in amphibia, including those resulting from climate change, and results in greater than additive effects on survival when two stressors are present.75 Another example, from shallow water corals exposed to high UV radiation, shows that photoinduced toxicity of polycyclic aromatic hydrocarbon is a synergistic stress factor.76–78• Freshwater resources for the rapidly growing human population are being affected by ozone depletion and global climate change
For example, a recent Australian study revealed79 increased solar UV radiation, temperature, and evaporation of water plus changes in precipitation and surface runoff, as some of the outcomes of climate change and ozone depletion. Such changes have an impact upon the aquatic ecosystems in lakes and rivers, and alter the character of dissolved organic matter and, consequently, have the potential to affect the quality (e.g., pathogens), quantity and treatability of freshwater resources.• UV radiation may penetrate to greater depths in the clearest waters than previously thought
New estimates for the absorption coefficients of chemically pure water in the UV region have been shown to be roughly half the values previously accepted. These findings are important for estimating solar UV radiation penetrating into the water column in the clearest natural waters with possible consequences for estimating ozone-depletion effects on phytoplankton productivity, aquatic photochemistry, photobiology and biogeochemical cycles.80• Algorithms have been developed for the retrieval of attenuation coefficients for diffuse UV-A/visible radiation from satellite sensors
A new algorithm permits retrieval of the diffuse attenuation coefficient at key wavelengths from multispectral remote-sensing reflectances. This opens the opportunity for global scale observations in the fields of marine photochemistry and photobiology that will complement in situ studies of the effects of enhanced UV radiation on aquatic systems.81• Fish are an important component of the human food supply and are negatively affected by increased UV-B
Estimating the impact of exposure to increased ambient UV-B levels on marine and freshwater fish in nature is complex. Recent studies in large flow-through tanks, supported by earlier laboratory studies, suggest that increased ambient UV-B exposure has negative impacts on growth of marine and freshwater fish and their resistance to disease.82,83 Any shortfall in fish supplies is likely to affect developing nations more than developed nations.84–86Biogeochemical cycles: Effects of solar UV radiation and interactions with climate change
• Scientific information continues to mount that interactions between climate and ozone changes are affecting the biogeochemical cycles that are vital to the Earth system
These cycles include the biological, chemical, and physical processes that control the exchanges of materials between land, freshwaters, oceans and the atmosphere, which in turn influence climate and the health of ecosystems. These biogeochemical cycles include those of carbon, nitrogen, sulfur, halogens, and metals.• Climate change and solar UV-B radiation can act together to amplify the increase in atmospheric CO2
In aquatic systems, increases in atmospheric CO2 caused by human activities result in acidification of the oceans,87 decreased mixing,88,89 and increased inputs of material from land into freshwaters and the ocean.90–92 These physical and chemical changes can have negative effects on carbon capture by many aquatic organisms,93,94 resulting in decreases in oceanic uptake of atmospheric CO2. In addition, these changes may influence UV-induced decomposition of dissolved organic matter (DOM),95 resulting in increased production of CO2. In terrestrial systems, recent evidence confirms that direct photodegradation by sunlight, primarily UV-B,49,89 plays an important role in the decomposition of dead plant material in low rainfall/high sunlight ecosystems50,51—see Terrestrial ecosystems, above. Future increases in temperature and changes in the distribution of rainfall are expected to lead to increases in the area covered by these low rainfall/high sunlight ecosystems. Modeling the balance of CO2 uptake and release from terrestrial ecosystems will need to take account of this increased direct role of UV-B in decomposition.• UV-induced cycling of halogen compounds affects atmospheric ozone concentrations and thus solar UV radiation and climate
Naturally-produced halogen compounds (CHBr3, CH2Br2, CH3I, CH3Cl, and CH3Br) influence atmospheric ozone depletion (also see discussion in sections on changes in ozone and UV radiation and on tropospheric air quality, composition, and processes). Marine ecosystems are important sources of these halogen compounds.96,97 Methyl chloride (CH3Cl) has been increasing over the South Pole in response to climate change98 and possibly to UV-induced photoreactions involving chloride and colored dissolved organic matter.99• Changes in UV-B radiation influence concentrations of many trace gases that have wide-ranging effects on atmospheric chemistry and climate change
The newly discovered process of methane production from some terrestrial plants under non-waterlogged conditions is stimulated by UV-B radiation (also see Terrestrial ecosystems).59,100 However, the contribution of this process to the global production of this highly important greenhouse gas (IPCC 2007) remains unclear and controversial.101–104 Increased UV-B over a sub-arctic ecosystem caused a significant increase in the release of isoprene,105 a volatile organic compound which is important in the chemistry of tropospheric ozone. Atmospheric radiation and temperature are influenced by changes in aerosols derived from naturally-produced dimethylsulfide. New models of the biogeochemical cycling of dimethylsulfide106–108 have taken into account the range of processes that can be influenced by solar UV radiation, including UV-induced stress of phytoplankton.109 These updates on trace gas biogeochemical cycles should be taken into account in global change models.• UV-B radiation may increase the biological availability of trace nutrients such as iron and the toxicity of metals such as mercury and copper with consequences for aquatic ecosystems and human health
Rainwater is an important source of trace metals (e.g. iron) to aquatic systems.110,111 Solar UV radiation modifies the chemical form of iron in rainwater89 and stabilization of this form by DOM increases its biological availability to aquatic organisms.110 UV induces the methylation and demethylation of mercury,112 and methylmercury may adversely affect human health through bioaccumulation in aquatic food webs.Tropospheric air quality, composition, and processes: Effects of solar UV radiation and interactions with climate change
• The impact of ozone depleting substances and climate change on the chemistry of the stratosphere can change weather patterns and, as a result, regional air quality
The upper troposphere/lower stratosphere has been difficult to characterize in models, but understanding of this region is crucial for modeling the movement of air. New models of global circulation that include tropospheric and stratospheric chemistry better account for movement of ozone and other gasses between the troposphere and lower stratosphere.113 Simulations are now showing that changes in stratospheric chemistry can increase the severity of storms (e.g.114).• Long-term records in Antarctica reveal a small upward trend in the concentration of ground-level ozone115
In Antarctica, atmospheric chemistry can be directly affected by changes in solar radiation and alterations in stratospheric/tropospheric ozone exchange due to the ozone hole. Ground-level ozone is produced photochemically by the reaction of UV-radiation and nitrogen oxides (NOx) released (also by UV-radiation) from the snow,116 and not because of the transport of ozone from the stratosphere. The seasonal changes in ozone concentration at ground level result primarily from increases in the summer. The trend (∼0.2% per year) could be driven by increased transport of reactive nitrogen from mid-latitudes (pollution) or increased photolysis of NOx, or both. This observation challenges the concept that Antarctic air is pristine and unaffected by human activity but the small changes make it difficult to identify the causes.• Regional climate and hence tropospheric air quality can be influenced by both changes in stratospheric ozone and the effects of greenhouse gases
Ozone depleting substances and greenhouse gases can contribute to alterations in global circulation12—see section above Ozone and changes in biologically active UV radiation. Changes in these large-scale atmospheric circulation patterns have been associated with changes in regional climate, for example a reduction in rainfall in SW Australia.117 Such changes will also affect air quality through changes in local climate.• Regional air quality could be affected by stratospheric ozone recovery and climate change
Ozone concentrations at ground level are a function of local, regional, and global sources.118 A variety of modeling studies estimating the impacts of future global warming on tropospheric air quality119–121 have highlighted significant increases in concentration of tropospheric ozone at the global scale. Small increases in background ozone concentrations can result in significantly longer periods when air-quality regulatory standards are exceeded. Current air quality models have yet to explicitly include changes in ozone depleting substances.119• The measurement of alternate chemical reaction rates involving nitrogen oxides challenges the importance of UV-B as the driver of ground-level atmospheric quality
UV-B radiation drives the production of a key atmospheric reactive species (hydroxyl radical, ˙OH) through a well-established process involving tropospheric ozone and water vapour. ˙OH is the primary reactant in the destruction of organic pollutants in the atmosphere. An alternate reaction pathway for production of ˙OH involving nitrogen dioxide (NO2) and water suggests that this pathway plays an important role in the atmosphere.122 However, this conflicts with earlier measurements of the rates.123,124 Another reaction involving nitric oxide (NO) is calculated to lead to a 5–12% reduction in ozone in the troposphere,125 but decreases the agreement between the modeled and measured values. Clearly, there are still significant uncertainties in our understanding of the ˙OH production and its importance in the destruction of air pollutants.• Natural sources of reactive halogens from the oceans have been proposed as having a significant role in the destruction of ozone in the lower atmosphere
Additional sources of reactive forms of bromine and iodine from productive (tropical) oceans have been better inferred from measurements in the Atlantic.126An understanding of the contribution made by such open ocean sources is required to adequately model the balance between production and destruction of tropospheric ozone.127 However, limited satellite measurements suggest that reactive iodine chemistry is confined to small regions near the equator and the poles.128 The consequences of these sources of halogens on the predicted future ozone levels in the troposphere have yet to be evaluated.Materials: Effects of solar UV radiation and interactions with climate change
• Composite plastic materials with wood-powder filler, popularly used in building construction, shown to have good UV stability
Both high-density polyethylene (HDPE)129 and polypropylene (PP)130 plastics work particularly well in this application. Solar UV-B induced damage to the materials based on HDPE (58–59% wood) is reported to be markedly lower than those based on PP. The discoloration was lower by ca. 34% while the chemical resistance was lower by several hundred percent, depending on the exposure time.131 These results help in designing wood-plastic composites that are more durable under exposure to solar radiation.• An emerging trend in stabilizing plastics materials against UV-B damage is the use of nanoparticle fillers
Conventional oxide pigments (ultrafine grades) are used as whiteners or fillers in numerous plastic formulations. Nanoscale oxides such as zinc oxide132 and titanium dioxide133 are successfully used as high-grade stabilizers in plastics. For instance, commercial nano-titania appears to be several-fold more efficient compared to the conventional ultrafine grades of the pigment in controlling discoloration in polypropylene. The higher surface area per unit mass of filler in the nanoscale material is responsible for the increased efficiency of stabilization. However, depending on the choice of surface chemical composition, the light-stabilizer effectiveness can vary widely. This was demonstrated for composites of 5 wt% Bohemite mineral nanopowder in PP where the photooxidation rates increased by up to 41%,134 depending on the nature of surface treatment.• Surface modification of even the conventional grades of titania can alter their stabilizer effectiveness against UV-B
Surface modification of titania can not only stabilize but can also degrade the plastic component depending on the surface chemistry used. Studies on the mechanisms of photoreactions of surface-treated titania with the plastic material as well as additives135,136 have been reported. The findings provide insights into the design of new grades of fillers. Surface modification can also improve dispersion of the pigments, thereby enhancing their efficiency in stabilizing the plastic against UV-B damage.136• The presence of colorants in the plastic can affect the stability of the formulations exposed to solar UV-B
Recent reports on polycarbonates (PC) (used in glazing) and polypropylene (PP) plastics (used outdoors in construction) suggest that diazo-type colorants used in the formulation can improve their stability under laboratory exposure to solar UV-B wavelengths.137 This mechanism can contribute to additional stability even in plastics already stabilized with conventional light stabilizers such as the most-used HALS.138• In general, the UV-induced degradation rates of plastics as well as biomaterials increase with increasing ambient temperature
Increase in average temperatures due to climate change can exacerbate the degradation of plastics exposed to solar UV-B, further reducing their service life outdoors.References
- F. Hendrick, P. V. Johnston, M. De Maziére, C. Fayt, C. Hermans, K. Kreher, N. Theys, A. Thomas and M. Van Roozendael, One-decade trend analysis of stratospheric BrO over Harestua (60° N) and Lauder (45° S) reveals a decline, Geophys. Res. Lett., 2008, 35 DOI:10.1029/2008GL034154.
- S. Reimann, M. K. Vollmer, D. Folini, M. Steinbacher, M. Hill, B. Buchmann, R. Zander and E. Mahieu, Observations of long-lived anthropogenic halocarbons at the high-Alpine site of Jungfraujoch (Switzerland) for assessment of trends and European sources, Sci. Total Environ., 2008, 391, 224–231 CrossRef CAS.
- N. R. P. Harris, E. Kyrö, J. Staehelin, D. Brunner, S.-B. Andersen, S. Godin-Beekmann, S. Dhomse, P. Hadjinicolaou, G. Hansen, I. Isaksen, A. Jrrar, A. Karpetchko, R. Kivi, B. Knudsen, P. Krizan, J. Lastovicka, J. Maeder, Y. Orsolini, J. A. Pyle, M. Rex, K. Vanicek, M. Weber, I. Wohltmann, P. Zanis and C. Zerefos, Ozone trends at northern mid- and high latitudes - a European perspective, Ann. Geophys., 2008, 26, 1207–1220 Search PubMed.
- P. A. Newman, J. S. Daniel, D. W. Waugh and E. R. Nash, A new formulation of equivalent effective stratospheric chlorine (EESC), Atmos. Chem. Phys., 2007, 7, 4537–4552 CAS.
- G. J. M. Velders, S. O. Andersen, J. S. Daniel, D. W. Fahey and M. McFarland, The importance of the Montreal Protocol in protecting climate, Proc. Natl. Acad. Sci. USA, 2007, 104, 4814–4819 CrossRef CAS.
- M. Steinbacher, M. K. Vollmer, B. Buchmann and S. Reimann, An evaluation of the current radiative forcing benefit of the Montreal Protocol at the high-Alpine site Jungfraujoch, Sci. Total Environ., 2008, 391, 217–223 CrossRef CAS.
- O. Morgenstern, P. Braesicke, M. M. Hurwitz, F. M. O'Connor, A. C. Bushell, C. E. Johnson and J. A. Pyle, The World Avoided by the Montreal Protocol, Geophys. Res. Lett., 2008, 35, L16811, DOI:10.1029/2008GL034590.
- P. M. Forster, G. E. Bodeker, R. Schofield, S. Solomon and D. W. J. Thompson, Effects of ozone cooling in the tropical lower stratosphere and upper troposphere, Geophys. Res. Lett., 2007, 34 DOI:10.1029/2007GL031994.
- J. A. Crook, N. P. Gillett and S. P. E. Keeley, Sensitivity of Southern Hemisphere climate to zonal asymmetry in ozone, Geophys. Res. Lett., 2008, 35 DOI:10.1029/2007GL032698.
- T. G. Shepherd and A. I. Jonsson, On the attribution of stratospheric ozone and temperature changes to changes in ozone-depleting substances and well-mixed greenhouse gases, Atmos. Chem. Phys., 2008, 8, 1435–1444 CAS.
- D. Shindell, Climate Change: Cool Ozone, Nat. Geosci., 2008, 1, 85–86 Search PubMed.
- S. W. Son, L. M. Polvani, D. W. Waugh, H. Akiyoshi, R. Garcia, D. Kinnison, S. Pawson, E. Rozanov, T. G. Shepherd and K. Shibata, The impact of stratospheric ozone recovery on the southern hemisphere westerly jet, Science, 2008, 320, 1486–1489 CrossRef CAS.
- J. C. Comiso, C. L. Parkinson, R. Gersten and L. Stock, Accelerated decline in the Arctic sea ice cover, Geophys. Res. Lett., 2008, 35 DOI:10.1029/2007GL031972.
- J. Overland, J. Turner, J. Francis, N. Gillett, G. Marshall and M. Tjernstr, The Arctic and Antarctic: Two faces of climate change, Trans. Am. Geophys. Union, 2008, 89, 177–178 Search PubMed.
- S. Tilmes, R. Müller and R. Salawitch, The sensitivity of polar ozone depletion to proposed geoengineering schemes, Science, 2008, 320, 1201–1204 CrossRef CAS.
- J. C. van der Leun, R. D. Piacentini and F. R. de Gruijl, Climate change and human skin cancer, Photochem. Photobiol. Sci., 2008, 7, 730–733 RSC.
- M. Ilyas, Climate augmentation of erythemal UV-B radiation dose damage in the tropics and global change, Curr. Sci., 2007, 93, 1604–1608 CAS.
- M. P. Purdue, L. E. Freeman, W. F. Anderson and M. A. Tucker, Recent trends in incidence of cutaneous melanoma among US caucasian young adults, J. Invest. Dermatol., 2008, 128, 2905–2908 CrossRef CAS.
- Centers for Disease Control, Sunburn prevalence among adults - United States, 1999, 2003, and 2004, Morbid. Mort. Wkly. Rep., 2007, 56, 524–528 Search PubMed.
- C. S. Lea, J. A. Scotto, P. A. Buffler, J. Fine, R. L. Barnhill and M. Berwick, Ambient UVB and melanoma risk in the United States: a case-control analysis, Ann. Epidemiol., 2007, 17, 447–453 CrossRef.
- E. J. Coups, S. L. Manne and C. J. Heckman, Multiple skin cancer risk behaviors in the U.S. population, Am. J. Prev. Med., 2008, 34, 87–93 CrossRef.
- D. C. Whiteman, C. A. Bray, V. Siskind, A. C. Green, D. J. Hole and R. M. Mackie, Changes in the incidence of cutaneous melanoma in the west of Scotland and Queensland, Australia: hope for health promotion?, Eur. J. Cancer Prev., 2008, 17, 243–250 CrossRef.
- F. Bath-Hextall, J. Leonardi-Bee, C. Smith, A. Meal and R. Hubbard, Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study, Int. J. Cancer, 2007, 121, 2105–2108 CrossRef CAS.
- H. Dal, C. Boldemann and B. Lindelof, Trends during a half century in relative squamous cell carcinoma distribution by body site in the Swedish population: support for accumulated sun exposure as the main risk factor, J. Dermatol., 2008, 35, 55–62 Search PubMed.
- N. M. Richmond-Sinclair, N. Pandeya, R. S. Ware, R. E. Neale, G. M. Williams, J. C. van der Pols and A. C. Green, Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population, J. Invest. Dermatol., 2008, in press Search PubMed.
- I. Soerjomataram, W. J. Louwman, V. E. Lemmens, J. W. Coebergh and E. de Vries, Are patients with skin cancer at lower risk of developing colorectal or breast cancer?, Am. J. Epidemiol., 2008, 167, 1421–1429 CrossRef CAS.
- E. M. John, G. G. Schwartz, J. Koo, W. Wang and S. A. Ingles, Sun exposure, vitamin D receptor gene polymorphisms, and breast cancer risk in a multiethnic population, Am. J. Epidemiol., 2007, 166, 1409–1419 CrossRef.
- E. M. John, J. Koo and G. G. Schwartz, Sun exposure and prostate cancer risk: evidence for a protective effect of early-life exposure, Cancer Epidemiol. Biomarkers Prev., 2007, 16, 1283–1286 CrossRef.
- J. S. Sloka, W. E. Pryse-Phillips and M. Stefanelli, The relation of ultraviolet radiation and multiple sclerosis in Newfoundland, Can. J. Neurosci., 2008, 35, 69–74 Search PubMed.
- J. S. Paula, F. Thorn and A. A. Cruz, Prevalence of pterygium and cataract in indigenous populations of the Brazilian Amazon rain forest, Eye, 2006, 20, 533–536 CrossRef CAS.
- B. Vojnikovic, S. Njiric, M. Coklo, I. Toth, J. Spanjol and M. Marinovic, Sunlight and incidence of pterygium on Croatian Island Rab-epidemiological study, Coll. Antropol., 2007, 31(Suppl. 1), 61–62 Search PubMed.
- P. Lu, X. Chen, Y. Kang, L. Ke, X. Wei and W. Zhang, Pterygium in Tibetans: a population-based study in China, Clin. Exp. Ophthalmol., 2007, 35, 828–833.
- S. Sekelj, I. Dekaris, E. Kondza-Krstonijevic, N. Gabric, J. Predovic and S. Mitrovic, Ultraviolet light and pterygium, Coll. Antropol., 2007, 31(Suppl. 1), 45–47 Search PubMed.
- J. Peguet-Navarro, C. Dezutter-Dambuyant, T. Buetler, J. Leclaire, H. Smola, S. Blum, P. Bastien, L. Breton and A. Gueniche, Supplementation with oral probiotic bacteria protects human cutaneous immune homeostasis after UV exposure-double blind, randomized, placebo controlled clinical trial, Eur. J. Dermatol., 2008, 18, 504–511 Search PubMed.
- D. L. Damian, C. R. Patterson, M. Stapelberg, J. Park, R. S. Barnetson and G. M. Halliday, UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide, J. Invest. Dermatol., 2008, 128, 447–454 CAS.
- W. Stahl and H. Sies, Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight, Mol. Biotechnol., 2007, 37, 26–30 Search PubMed.
- W. Kopcke and J. Krutmann, Protection from sunburn with beta-Carotene - a meta-analysis, Photochem. Photobiol., 2008, 84, 284–288 CrossRef.
- A. Schwarz, A. Maeda, D. Gan, T. Mammone, M. S. Matsui and T. Schwarz, Green tea phenol extracts reduce UVB-induced DNA damage in human cells via interleukin-12, Photochem. Photobiol., 2008, 84, 350–355 CrossRef CAS.
- A. Faurschou, R. Gniadecki and H. C. Wulf, Infliximab inhibits DNA repair in ultraviolet B-irradiated premalignant keratinocytes, Exp. Dermatol., 2008, 7, 933–938 CrossRef.
- I. Cechin, N. Corniani, T. D. Fumis and A. C. Cataneo, Ultraviolet-B and water stress effects on growth, gas exchange and oxidative stress in sunflower plants, Rad. Environ. Phys., 2008, 47, 405–413 Search PubMed.
- H. Y. Feng, S. W. Li, L. G. Xue, L. Z. An and X. F. Wang, The interactive effects of enhanced UV-B radiation and soil drought on spring wheat, S. Afr. J. Bot., 2007, 73, 429–434 Search PubMed.
- S. Koti, K. R. Reddy, V. G. Kakani, D. Zhao and W. Gao, Effects of carbon dioxide, temperature and ultraviolet-B radiation and their interactions on soybean (Glycine max L.) growth and development, Environ. Exp. Bot., 2007, 60, 1–10 CrossRef CAS.
- M. M. Qaderi, D. M. Reid and E. C. Yeung, Morphological and physiological responses of canola (Brassica napus) siliquas and seeds to UVB and CO2 under controlled environment conditions, Environ. Exp. Bot., 2007, 60, 428–437 CrossRef CAS.
- S. Wang, L. Duan, J. Li, X. Tian and Z. Li, UV-B radiation increases paraquat tolerance of two broad-leaved and two grass weeds in relation to changes in herbicide absorption and photosynthesis, Weed Res., 2007, 47, 122–128 CrossRef CAS.
- K. R. Albert, R. Rinnan, H. Ro-Poulsen, T. N. Mikkelsen, K. B. Hakansson, M. F. Arndal and A. Michelsen, in Advances in Ecological Research Vol. 40, Elsevier Academic Press Inc, San Diego, 2008, pp. 421–440 Search PubMed.
- S. Koti, K. Raja Reddy, G. W. Lawrence, V. R. Reddy, V. G. Kakani, D. Zhao and W. Gao, Effect of enhanced UV-B radiation on reniform nematode (Rotylenchus reniformis Linford and Oliveira) populations in cotton (Gossypium hirsutum L.), Plant. Path. J., 2007, 6, 51–59 Search PubMed.
- R. Rinnan, A. M. Nerg, P. Ahtoniemi, H. Suokanerva, T. Holopainen, E. Kyro and E. Baath, Plant-mediated effects of elevated ultraviolet-B radiation on peat microbial communities of a subarctic mire, Global Change Biol., 2008, 14, 925–937 CrossRef.
- T. A. Day, E. T. Zhang and C. T. Ruhland, Exposure to solar UV-B radiation accelerates mass and lignin loss of Larrea tridentata litter in the Sonoran Desert, Plant Ecol., 2007, 193, 185–194 Search PubMed.
- A. T. Austin and L. Vivanco, Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation, Nature, 2006, 442, 555–558 CrossRef CAS.
- L. A. Brandt, J. Y. King and D. G. Milchunas, Effects of ultraviolet radiation on litter decomposition depend on precipitation and litter chemistry in a shortgrass steppe ecosystem, Global Change Biol., 2007, 13, 2193–2205 CrossRef.
- H. A. L. Henry, K. Brizgys and C. B. Field, Litter decomposition in a California annual grassland: Interactions between photodegradation and litter layer thickness, Ecosystems, 2008, 11, 545–554 CrossRef CAS.
- H. L. Throop and S. R. Archer, Interrelationships among shrub encroachment, land management, and litter decomposition in a semidesert grassland, Ecol. Appl., 2007, 17, 1809–1823 CrossRef.
- A. Martinez-Yrizar, S. Nunez and A. Burquez, Leaf litter decomposition in a southern Sonoran Desert ecosystem, northwestern Mexico: Effects of habitat and litter quality, Acta Oecol.-Int. J. Ecol., 2007, 32, 291–300 Search PubMed.
- D. F. Ferretti, J. B. Miller, J. W. C. White, K. R. Lassey, D. C. Lowe and D. M. Etheridge, Stable isotopes provide revised global limits of aerobic methane emissions from plants, Atmos. Chem. Phys., 2007, 7, 237–241 CAS.
- T. A. Dueck, R. de Visser, H. Poorter, S. Persijn, A. Gorissen, W. de Visser, A. Schapendonk, J. Verhagen, J. Snel, F. J. M. Harren, A. K. Y. Ngai, F. Verstappen, H. Bouwmeester, L. Voesenek and A. von der Werf, No evidence for substantial aerobic methane emission by terrestrial plants: a C-13-labelling approach, New Phytol., 2007, 175, 29–35 Search PubMed.
- Z. P. Wang, X. G. Han, G. G. Wang, Y. Song and J. Gulledge, Aerobic methane emission from plants in the Inner Mongolia steppe, Environ. Sci. Technol., 2008, 42, 62–68 CrossRef CAS.
- F. Keppler, J. T. G. Hamilton, W. C. McRoberts, I. Vigano, M. Brass and T. Rockmann, Methoxyl groups of plant pectin as a precursor of atmospheric methane: evidence from deuterium labelling studies, New Phytol., 2008, 178, 808–814 Search PubMed.
- I. Vigano, H. van Weelden, R. Holzinger, F. Keppler, A. McLeod and T. Rockmann, Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components, Biogeoscience, 2008, 5, 937–947 Search PubMed.
- A. R. McLeod, S. C. Fry, G. J. Loake, D. J. Messenger, D. S. Reay, K. A. Smith and B.-W. Yun, Ultraviolet radiation drives methane emissions from terrestrial plant pectins, New Phytol., 2008, 180, 124–132 Search PubMed.
- A. Foggo, S. Higgins, J. J. Wargent and R. A. Coleman, Tri-trophic consequences of UV-B exposure: plants, herbivores and parasitoids, Oecologia, 2007, 154, 505–512 CrossRef.
- C. Caputo, M. Rutitzky and C. L. Ballaré, Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): impacts on oviposition and involvement of the jasmonic acid pathway, Oecologia, 2006, 149, 81–90 CrossRef.
- K. Xu and B. S. Qiu, Responses of superhigh-yield hybrid rice Liangyoupeijiu to enhancement of ultraviolet-B radiation, Plant Sci., 2007, 172, 139–149 CrossRef CAS.
- N. J. Thines, L. A. Shipley, J. H. Bassman, J. K. Fellman, D. S. Mattison, J. R. Slusser and W. Gao, Effects of enhanced UV-B radiation on plant chemistry: nutritional consequences for a specialist and generalist lagomorph, J. Chem. Ecol., 2007, 33, 1025–1039 CrossRef CAS.
- N. J. Thines, L. A. Shipley, J. H. Bassman, J. R. Slusser and W. Gao, UV-B effects on the nutritional chemistry of plants and the responses of a mammalian herbivore, Oecologia, 2008, 156, 125–135 CrossRef.
- M. M. Izaguirre, C. A. Mazza, A. Svatos, I. T. Baldwin and C. L. Ballaré, Solar ultraviolet-B radiation and insect herbivory trigger partially overlapping phenolic responses in Nicotiana attenuata and Nicotiana longiflora, Ann. Bot., 2007, 99, 103–109 CrossRef.
- H. H. Wang, J. J. Hao, X. J. Chen, Z. N. Hao, X. Wang, Y. G. Lou, Y. L. Peng and Z. J. Guo, Overexpression of rice WRKY89 enhances ultraviolet B tolerance and disease resistance in rice plants, Plant Mol. Biol., 2007, 65, 799–815 CrossRef CAS.
- S. P. Pandey and I. T. Baldwin, Silencing RNA-directed RNA polymerase 2 increases the susceptibility of Nicotiana attenuata to UV in the field and in the glasshouse, Plant J., 2008, 54, 845–862 CrossRef CAS.
- J. J. Li, Z. T. Zhang, F. X. Liu, Q. Q. Liu, W. J. Gan, J. Chen, M. L. M. Lim and D. Q. Li, UVB-based mate-choice cues used by females of the jumping spider Phintella vittata, Curr. Biol., 2008, 18, 699–703 CrossRef CAS.
- J. J. Li, M. L. M. Lim, Z. T. Zhang, Q. Q. Liu, F. X. Liu, J. Chen and D. Q. Li, Sexual dichromatism and male colour morph in ultraviolet-B reflectance in two populations of the jumping spider Phintella vittata (Araneae:Salticidae) from tropical China, Biol. J. Linn. Soc., 2008, 94, 7–20 Search PubMed.
- C. A. Mazza, J. Zavala, A. L. Scopel and C. L. Ballaré, Perception of solar UVB radiation by phytophagous insects: Behavioral responses and ecosystem implications, Proc. Natl. Acad. Sci. USA, 1999, 96, 980–985 CrossRef CAS.
- C. A. Mazza, M. M. Izaguirre, J. Zavala, A. L. Scopel and C. L. Ballaré, Insect perception of ambient ultraviolet-B radiation, Ecol. Lett., 2002, 5, 722–726 CrossRef.
- B. A. Han, L. B. Kats, R. C. Pommerening, R. P. Ferrer, M. Murry-Ewers and A. R. Blaustein, Behavioral avoidance of ultraviolet-B radiation by two species of neotropical poison-dart frogs, Biotropica, 2007, 39, 433–435 Search PubMed.
- S. Volynchik, M. Plotkin, D. J. Bergman and J. S. Ishay, Hornet flight activity and its correlation with UVB radiation, temperature and relative humidity, Photochem. Photobiol., 2008, 84, 81–85 CAS.
- D.-P. Häder, H. D. Kumar, R. C. Smith and R. C. Worrest, Effects of solar UV radiation on aquatic ecosystems and interactions with climate change, Photochem. Photobiol. Sci., 2007, 6, 267–285 RSC.
- B. A. Bancroft, N. J. Baker and A. R. Blaustein, A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival, Conserv. Biol., 2008, 22, 987–996 CrossRef.
- M. D. C. Guzmán Martínez, P. Ramírez Romero and A. T. Banaszak, Photoinduced toxicity of the polycyclic aromatic hydrocarbon, fluoranthene, on the coral, Porites divaricata, J. Environ. Sci. Health A, 2007, 42, 1495–1502 Search PubMed.
- J. L. Torres, R. A. Armstrong and E. Weil, Enhanced ultraviolet radiation can terminate sexual reproduction in the broadcasting coral species Acropora cervicornis Lamarck, J. Exp. Mar. Biol. Ecol., 2008, 358, 39–45 CrossRef.
- C. Ferrier-Pages, C. Richard, D. Forcioli, D. Allemand, M. Pichon and J. M. Shick, Effects of temperature and UV radiation increases on the photosynthetic efficiency in four Scleractinian coral species, Biol. Bull., 2007, 213, 76–87 Search PubMed.
- Y. C. Soh, F. Roddick and J. van Leeuwen, The future of water in Australia: The potential effects of climate change and ozone depletion on Australian water quality, quantity and treatability, Environmentalist, 2008, 28, 158–165 Search PubMed.
- A. Morel, B. Gentili, H. Claustre, M. Babin, A. Bricaud, R. J. Ras and F. Tieche, Optical properties of the “clearest” natural waters, Limnol. Oceanogr., 2007, 52, 217–229 CAS.
- C. G. Fichot, S. Sathyendranath and W. L. Miller, SeaUV and SeaUVC: Algorithms for the retrieval of UV/Visible diffuse attenuation coefficients from ocean color, Rem. Sens. Environ., 2008, 112, 1584–1602.
- I. E. Jokinen, E. S. Markkula, H. M. Salo, P. Kuhn, S. Nikoskelainen, M. T. Arts and H. I. Browman, Exposure to increased ambient ultraviolet B radiation has negative effects on growth, condition and immune function of juvenile Atlantic salmon (Salmo salar), Photochem. Photobiol., 2008, 84, 1265–1271 CrossRef CAS.
- S. E. Markkula, A. Karvonen, H. Salo, E. Tellervo Valtonen and I. E. Jokinen, Ultraviolet B irradiation affects resistance of rainbow trout (Oncorhynchus mykiss) against bacterium Yersinia ruckeri and trematode Diplostomum spathaceum, Photochem. Photobiol., 2007, 83, 1263–1269 CrossRef CAS.
- FAO, The State of World Fisheries and Aquaculture 2006, Food and Agriculture Organization of the United Nations, Rome, Italy, 2007, 162 pp. Search PubMed.
- World Resources Institute, World Resources 1998–99: Environmental Change and Human Health, World Resources Institute, Washington, DC, USA, 1998 Search PubMed.
- World Resources Institute, Ecosystems and Human Well-Being: Current State and Trends: Findings of the Condition and Trends Working Group, Island Press, Washington, DC, USA, 2005 Search PubMed.
- H. Thomas, A. E. F. Prowe, S. van Heuven, Y. Bozec, H. J. W. de Baar, L. S. Schiettecatte, K. Suykens, M. Kone, A. V. Borges, I. D. Lima and S. C. Doney, Rapid decline of the CO2 buffering capacity in the North Sea and implications for the North Atlantic Ocean, Global Biogeochem. Cycles, 2007, 21, 13.
- B. Schneider, L. Bopp, M. Gehlen, J. Segschneider, T. L. Frolicher, P. Cadule, P. Friedlingstein, S. C. Doney, M. J. Behrenfeld and F. Joos, Climate-induced interannual variability of marine primary and export production in three global coupled climate carbon cycle models, Biogeoscience, 2008, 5, 597–614 Search PubMed.
- R. G. Zepp, D. J. Erickson, N. D. Paul and B. Sulzberger, Interactive effects of solar UV radiation and climate change on biogeochemical cycling, Photochem. Photobiol. Sci., 2007, 6, 286–300 RSC.
- R. A. Betts, O. Boucher, M. Collins, P. M. Cox, P. D. Falloon, N. Gedney, D. L. Hemming, C. Huntingford, C. D. Jones, D. M. H. Sexton and M. J. Webb, Projected increase in continental runoff due to plant responses to increasing carbon dioxide, Nature, 2007, 448, 1037–1041 CrossRef CAS.
- M. Erlandsson, I. Buffam, J. Folster, H. Laudon, J. Temnerud, G. A. Weyhenmeyer and K. Bishop, Thirty-five years of synchrony in the organic matter concentrations of Swedish rivers explained by variation in flow and sulfate, Global Change Biol., 2008, 14, 1191–1198 CrossRef.
- J. Temnerud and G. A. Weyhenmeyer, Abrupt changes in air temperature and precipitation: Do they matter for water chemistry?, Global Biogeochem. Cycles, 2008, 22, 8.
- V. J. Fabry, B. A. Seibel, R. A. Feely and J. C. Orr, Impacts of ocean acidification on marine fauna and ecosystem processes, ICES J. Mar. Sci., 2008, 65, 414–432 CrossRef CAS.
- J. M. Hall-Spencer, R. Rodolfo-Metalpa, S. Martin, E. Ransome, M. Fine, S. M. Turner, S. J. Rowley, D. Tedesco and M. C. Buia, Volcanic carbon dioxide vents show ecosystem effects of ocean acidification, Nature, 2008, 454, 96–99 CrossRef CAS.
- A. V. Vähätalo and R. G. Wetzel, Long-term photochemical and microbial decomposition of wetland-derived dissolved organic matter with alteration of C-13:C-12 mass ratio, Limnol. Oceanogr., 2008, 53, 1387–1392 CAS.
- J. H. Butler, D. B. King, J. M. Lobert, S. A. Montzka, S. A. Yvon-Lewis, B. D. Hall, N. J. Warwick, D. J. Mondeel, M. Aydin and J. W. Elkins, Oceanic distributions and emissions of short-lived halocarbons, Global Biogeochem. Cycles, 2007, 21 Search PubMed.
- L. J. Carpenter, D. J. Wevill, C. J. Palmer and J. Michels, Depth profiles of volatile iodine and bromine-containing halocarbons in coastal Antarctic waters, Mar. Chem., 2007, 103, 227–236 CrossRef CAS.
- M. B. Williams, M. Aydin, C. Tatum and E. S. Saltzman, A 2000 year atmospheric history of methyl chloride from a South Pole ice core: Evidence for climate controlled variability, Geophys. Res. Lett., 2007, 34 DOI:07810.01029/02006GL029142.
- R. M. Moore, A photochemical source of methyl chloride in saline waters, Environ. Sci. Technol., 2008, 42, 1933–1937 CrossRef CAS.
- F. Keppler, J. T. G. Hamilton, W. C. McRoberts, I. Vigano, M. Brass and T. Rockmann, Methoxyl groups of plant pectin as a precursor of atmospheric methane: evidence from deuterium labelling studies, New Phytol., 2008, 178, 808–814 Search PubMed.
- D. J. Beerling, T. Gardiner, G. Leggett, A. McLeod and W. P. Quick, Missing methane emissions from leaves of terrestrial plants, Global Change Biol., 2008, 14, 1821–1826 CrossRef.
- T. A. Dueck, R. de Visser, H. Poorter, S. Persijn, A. Gorissen, W. de Visser, A. Schapendonk, J. Verhagen, J. Snel, F. J. M. Harren, A. K. Y. Ngai, F. Verstappen, H. Bouwmeester, L. Voesenek and A. von der Werf, No evidence for substantial aerobic methane emission by terrestrial plants: a C-13-labelling approach, New Phytol., 2007, 175, 29–35 Search PubMed.
- J. R. Evans, Resolving methane fluxes, New Phytol., 2007, 175, 1–4 Search PubMed.
- F. Keppler, J. T. G. Hamilton, M. Brass and T. Rockmann, Methane emissions from terrestrial plants under aerobic conditions, Nature, 2006, 439, 187–191 CrossRef CAS.
- P. Tiiva, R. Rinnan, P. Faubert, J. Rasanen, T. Holopainen, E. Kyro and J. K. Holopainen, Isoprene emission from a subarctic peatland under enhanced UV-B radiation, New Phytol., 2007, 176, 346–355 Search PubMed.
- R. Cropp, J. Norbury and R. Braddock, Dimethylsulfide, clouds, and phytoplankton: Insights from a simple plankton ecosystem feedback model, Global Biogeochem. Cycles, 2007, 21 Search PubMed.
- D. A. Toole, D. A. Siegel and S. C. Doney, A light-driven, one-dimensional dimethylsulfide biogeochemical cycling model for the Sargasso Sea, J. Geophys. Res. Biogeosci., 2008, 113, 20 Search PubMed.
- K. E. Bailey, D. A. Toole, B. Blomquist, R. G. Najjar, B. Huebert, D. J. Kieber, R. P. Kiene, R. Matrai, G. R. Westby and D. A. del Valle, Dimethylsulfide production in Sargasso Sea eddies, Deep-Sea Res. Part II-Top. Stud. Oceanogr., 2008, 55, 1491–1504 CrossRef.
- D. A. del Valle, D. J. Kieber, J. Bisgrove and R. P. Kiene, Light-stimulated production of dissolved DMSO by a particle-associated process in the Ross Sea, Antarctica, Limnol. Oceanogr., 2007, 52, 2456–2466 CAS.
- J. D. Willey, R. J. Kieber, P. J. Seaton and C. Miller, Rainwater as a source of Fe(II)-stabilizing ligands to seawater, Limnol. Oceanogr., 2008, 53, 1678–1684 CAS.
- M. L. I. Witt, S. Skrabal, R. Kieber and J. Willey, Photochemistry of Cu complexed with chromophoric dissolved organic matter: implications for Cu speciation in rainwater, J. Atmos. Chem., 2007, 58, 89–109 CrossRef CAS.
- L. Whalin, E.-H. Kim and R. Mason, Factors influencing the oxidation, reduction, methylation and demethylation of mercury species in coastal waters, Mar. Chem., 2007, 107, 278–294 CrossRef CAS.
- H. Teyssedre, M. Michou, H. L. Clark, B. Josse, F. Karcher, D. Olivie, V. H. Peuch, D. Saint-Martin, D. Cariolle, J. L. Attie, P. Nedelec, P. Ricaud, V. Thouret, R. J. Van Der A, A. Volz-Thomas and F. Cheroux, A new tropospheric and stratospheric Chemistry and Transport Model MOCAGE-Climat for multi-year studies: evaluation of the present-day climatology and sensitivity to surface processes, Atmos. Chem. Phys., 2007, 7, 5815–5860 CAS.
- S. Brand, K. Dethloff and D. Handorf, Tropospheric circulation sensitivity to an interactive stratospheric ozone, Geophys. Res. Lett., 2008, 35, L05809 CrossRef.
- D. Helmig, S. J. Oltmans, D. Carlson, J. F. Lamarque, A. Jones, C. Labuschagne, K. Anlauf and K. Hayden, A review of surface ozone in the polar regions, Atmos. Environ., 2007, 41, 5138–5161 CrossRef CAS.
- D. Helmig, B. Johnson, S. J. Oltmans, W. Neff, F. Eisele and D. D. Davis, Elevated ozone in the boundary layer at South Pole, Atmos. Environ., 2008, 42, 2788–2803 CrossRef CAS.
- B. Meneghini, I. Simmonds and I. N. Smith, Association between Australian rainfall and the Southern Annular Mode, Int. J. Climatol., 2007, 27, 109–121 CrossRef.
- M. E. Jenkin, Trends in ozone concentration distributions in the UK since 1990: Local, regional and global influences, Atmos. Environ., 2008, 42, 5434–5445 CrossRef CAS.
- S. Wu, L. J. Mickley, E. M. Leibensperger, D. J. Jacob, D. Rind and D. G. Streets, Effects of 2000–2050 global change on ozone air quality in the United States, J. Geophys. Res. Atmos., 2008, 113, D06302 CrossRef.
- C. G. Nolte, A. B. Gilliland, C. Hogrefe and L. J. Mickley, Linking global to regional models to assess future climate impacts on surface ozone levels in the United States, J. Geophys. Res. Atmos., 2008, 113, D14307 CrossRef.
- J. J. West, A. M. Fiore, V. Naik, L. W. Horowitz, M. D. Schwarzkopf and D. L. Mauzerall, Ozone air quality and radiative forcing consequences of changes in ozone precursor emissions, Geophys. Res. Lett., 2007, 34, L06806 CrossRef.
- S. Li, J. Matthews and A. Sinha, Atmospheric hydroxyl radical production from electronically excited NO2 and H2O, Science, 2008, 319, 1657–1660 CrossRef CAS.
- J. N. Crowley and S. A. Carl, OH formation in the photoexcitation of NO2 beyond the dissociation threshold in the presence of water vapor, J. Phys. Chem. A, 1997, 101, 4178–4184 CrossRef CAS.
- P. O. Wennberg and D. Dabdub, Atmospheric chemistry - Rethinking ozone production, Science, 2008, 319, 1624–1625 CrossRef CAS.
- D. Cariolle, M. J. Evans, M. P. Chipperfield, N. Butkovskaya, A. Kukui and G. Le Bras, Impact of the new HNO3-forming channel of the HO2+NO reaction on tropospheric HNO3, NOx, HOx and ozone, Atmos. Chem. Phys., 2008, 8, 4061–4068 CAS.
- K. A. Read, A. S. Mahajan, L. J. Carpenter, M. J. Evans, B. V. E. Faria, D. E. Heard, J. R. Hopkins, J. D. Lee, S. J. Moller, A. C. Lewis, L. Mendes, J. B. McQuaid, H. Oetjen, A. Saiz-Lopez, M. J. Pilling and J. M. C. Plane, Extensive halogen-mediated ozone destruction over the tropical Atlantic Ocean, Nature, 2008, 453, 1232–1235 CrossRef CAS.
- R. von Glasow, Atmospheric chemistry - Sun, sea and ozone destruction, Nature, 2008, 453, 1195–1196 CrossRef CAS.
- A. Schönhardt, A. Richter, F. Wittrock, H. Kirk, H. Oetjen, H. K. Roscoe and J. P. Burrows, Observations of iodine monoxide columns from satellite, Atmos. Chem. Phys., 2008, 8, 637–653.
- N. M. Stark and L. M. Matuana, Characterization of weathered wood-plastic composite surfaces using FTIR spectroscopy, contact angle and XPS, Polym. Degrad. Stab., 2007, 92, 1883–1889 CrossRef CAS.
- F. P. La Mantia and M. Morreale, Accelerated weathering of polypropylene/wood flour composites, Polym. Degrad. Stab., 2008, 93, 1252–1258 CrossRef CAS.
- J. S. Fabiyi, A. G. McDonald, M. P. Wolcott and P. R. Griffiths, Wood plastic composites weathering: visual appearance and chemical changes, Polym. Degrad. Stab., 2008, 93, 1405–1414 CrossRef CAS.
- D. Burgard, C. Hegedus, D. Lindermuth and F. Pepe, Zinc oxide nanoparticle dispersions as unique additives for coatings, J. Coat. Tech., April 2008, pp. 1–3 Search PubMed.
- K. Shaefer, M. Moeller, B. Foelliner, M. Vennes and J. Bohnen, in International Textile Conference, Aachen-Dresden, 2007, pp. 402–409 Search PubMed.
- S. Bocchini, S. Morlat-Therias, J. L. Gardetle and G. Camino, Influence of nanodispersed boehmite on polypropylene photooxidation, Polym. Degrad. Stab., 2007, 92, 1842–1856.
- G. Martin, R. A. J and D. Warsley, Effect of phthalate and sulfonic acid ester plasticizers on TiO2 catalyzed photodegradation of PVC, Mar. Sci. Tech., 2008, 24, 427–434 Search PubMed.
- W. J. Fa, Z. Ling, C. Gong and P. C. Zong, Solid-phase photocatalytic degradation of waste plastic with TiO2 as photocatalyst, Prog. Chem., 2008, 20, 19–25 Search PubMed.
- C. Saron, M. I. Felisberti, F. Zulli and M. Giorando, Influence of diazo pigment on polycarbonate photodegradation, J. Appl. Polym. Sci., 2008, 107, 1071–1079 CrossRef CAS.
- Z. Ahmadi, M. H. Kish, H. Freeman, K. R and A. A. Katlab, Photostability of isotactic polypropylene containing monoazo pigment, J. Appl. Polym. Sci., 2008, 108, 2950–2957 CrossRef CAS.
Footnotes |
| † This publication should be cited as follows: Andrady et al., Environmental effects of ozone depletion and its interactions with climate change: Progress report, 2008, United Nations Environment Programme, Environmental Effects Assessment Panel, Photochem. Photobiol. Sci., 8, 2009, DOI:10.1039/b820432m |
| ‡ List of contributing authors in alphabetical order: Anthony Andrady, Pieter J. Aucamp, Alkiviadis F. Bais, Carlos L. Ballaré, Lars Olof Björn, Janet F. Bornman (Co-Chair), Martyn Caldwell, Anthony P. Cullen, David J. Erickson, Frank R. de Gruijl, Donat-P. Häder, Mohammad Ilyas, G. Kulandaivelu, H. D. Kumar, Janice Longstreth, Richard L. McKenzie, Mary Norval, Nigel Paul, Halim Hamid Redhwi, Raymond C. Smith, Keith R. Solomon (Secretary), Barbara Sulzberger, Yukio Takizawa, Xiaoyan Tang (Co-Chair), Alan H. Teramura, Ayako Torikai, Jan C. van der Leun (Co-Chair), Stephen R. Wilson, Robert C. Worrest and Richard G. Zepp. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2009 |
