Photodynamic therapy for non-resectable perihilar cholangiocarcinoma
Tobias
Kiesslich
a,
Gernot
Wolkersdörfer
a,
Daniel
Neureiter
b,
Hermann
Salmhofer
a and
Frieder
Berr
*a
aDepartment of Internal Medicine I, Paracelsus Medical University Salzburg, Muellner Hauptstrasse 48, 5020, Salzburg, Austria. E-mail: f.berr@salk.at; Tel: +43 (0)662/4482-2801; Fax: +43 (0)662/4482-2822
bInstitute of Pathology, University Hospital Salzburg, Paracelsus Medical University, Muellner Hauptstrasse 48, Salzburg, Austria
First published on 28th October 2008
Abstract
Photodynamic therapy (PDT) has emerged as a useful tool for palliative treatment of the otherwise difficult to treat perihilar cholangiocarcinoma (CC). PDT is a minimally invasive and effective technique for local tumour ablation with rare and predictable side effects. A modest number of studies and randomised trials using porfimer (Photofrin®) could demonstrate an improvement in quality of life and survival time. A novel approach to a priori non-resectable perihilar CC was proven in a pilot study using neoadjuvant porfimer-PDT for down-sizing of the tumour followed by R0 resection. These days, active phase II and phase III trials investigate if the tumouricidal activity can be increased using temoporfin (Foscan®) as an alternative photosensitiser with higher penetration capability and whether porfimer-based PDT plus stenting is superior to biliary stenting alone in terms of overall survival, respectively. The local tumour ablation and correction of obstructive cholestasis with PDT will allow for novel multimodal strategies to treat cholangiocarcinoma.
 Tobias Kiesslich Tobias Kiesslich | Tobias Kiesslich studied molecular genetics at the Paris Lodron University Salzburg and received his doctoral degree in biophysics/cell and tumour biology for experimental work on the cellular mechanisms of PDT-induced cell death carried out in the Department of Molecular Biology in the group of Prof. Barbara Krammer. Since 2007 he works at the Department of Internal Medicine I at the Paracelsus Medical University Salzburg as a Post-Doc researcher (research group Prof. Frieder Berr) with a focus on molecular mechanisms of cholangiocarcinogenesis. |
 Gernot Wolkersdörfer Gernot Wolkersdörfer | Gernot Wolkersdörfer (born 1968) completed his M.D. at the University of Leipzig and received his PhD on ‘Apoptosis in the human adrenal’ in 1995. 1998–2000 research visit at the National Institutes of Health, USA (Prof. S. R. Bornstein). 2000–2007 Medical doctor at Medical Department I, University of Technology, Dresden. Since 2005 consultant of Internal Medicine. Since 2008 senior physician at the Department of Internal Medicine I, Paracelsus Medical University, Salzburg. Currently, his main research interest is focused on photodynamic treatment and oncogenesis of cholangiocarcinoma. |
 Daniel Neureiter Daniel Neureiter | Daniel Neureiter was born in Jülich, Germany and completed his M.D. as well as his consultant of pathology at the University of Erlangen-Nuernberg and Institute of Pathology (Prof. Th. Kirchner). Here, main research projects dealt with the association of chronic inflammatory diseases and the extracellular matrix components. After changing to the Institute of Pathology at Salzburg (Prof. O. Dietze), he was promoted to an assistant professor at the Paracelsus Private Medical University Salzburg and is now chief senior consultant. His main research interest is the morphological and molecular embryonic differentiation patterning, especially of solid tumours in the gastrointestinal tract. |
 Hermann Salmhofer Hermann Salmhofer | Hermann Salmhofer graduated from Graz University Medical School in 1987 and did post-doctoral research on alcoholic liver disease at the Institute of Pathology, University of Graz, Austria, from 1988 to 1991. He started his clinical training in Graz in 1992, followed by a residency at the 2nd Medical Department, Technical University of Munich, Germany, since 1995. Specialist in Internal Medicine and Senior Physician 2002, Nephrologist 2003. Senior Physician at the 1st Medical Department, Paracelsus Medical University, Salzburg, Austria, since 2005. Clinical research interests: among other projects hepatobiliary interventional sonography. |
 Frieder Berr Frieder Berr | Frieder Berr (born 1950) graduated from Ludwig Maximilians University in Munich and qualified as a consultant for internal medicine and gastroenterology in Munich Grosshadern (Univ. Munich). 1981–1983 clinical and basic hepatobiliary research at Univ. Colorado, Denver, USA. 1994–2001 professor of medicine and director of Hepatology and Endoscopy programs at the University Clinic II in Leipzig (head Prof. J. Mössner). Since 2002 Head of the Department of Internal Medicine I of the Paracelsus Medical University Salzburg. Since 1995 he has conducted clinical and experimental work on diagnostics and photodynamic therapy of bile duct cancer and mechanisms of oncogenesis of cholangiocarcinoma. |
Introduction
Cholangiocarcinomas (CC) arising from biliary epithelium are classified according to their localisation in the biliary tree into intrahepatic (5–10% of all CC cases) and extrahepatic tumour types, with the latter being further subdivided into perihilar cholangiocarcinomas, approx. 70%, middle and distal bile duct carcinomas (see Fig. 1C).1 Tumours involving or extending to the bile duct bifurcation are also referred to as Klatskin tumours, i.e. perihilar CC; tumour extension along the biliary tree is surgically classified as type I–IV according to Bismuth and Corlette et al. (Fig. 1A).2 Separate clinical tumour entities represent intrahepatic cholangiocarcinomas which are staged in the TNM categories applied to hepatocellular carcinoma, as well as gall bladder and distal bile duct carcinomas, again with different TNM rules (Fig. 1B) and surgical strategies.3,4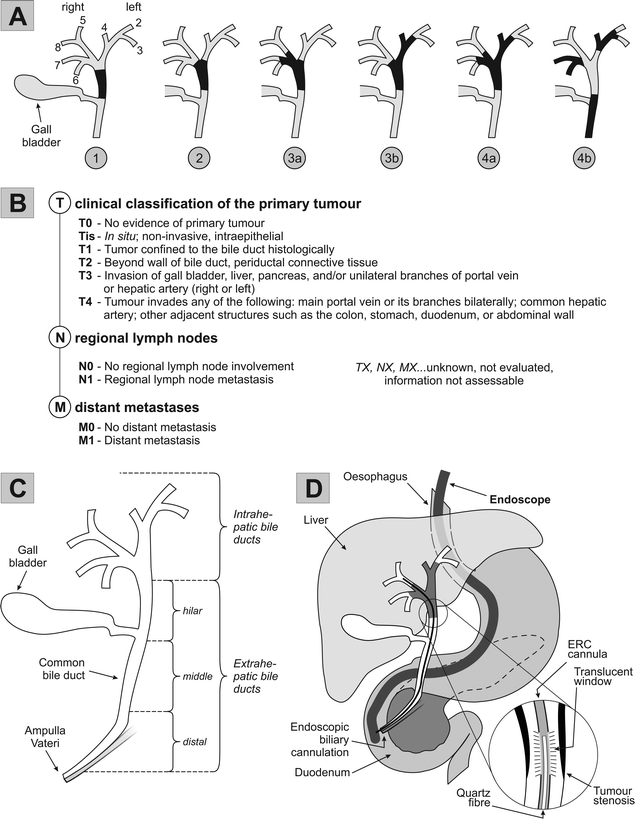 | ||
| Fig. 1 Anatomy, Bismuth/TNM classification, and PDT for hilar cholangiocarcinoma.2,6,12,74 A. Bismuth classification of cholangiocarcinoma: 1: Tumour is confined to the common hepatic duct. 2: Tumour also affects the confluence of the left and right hepatic ducts. 3a and 3b: Tumour occludes the common hepatic duct and the hepatic ducts. 4a: Tumour affects the biliary confluence and extends to both hepatic ducts. 4b: Multifocal bile duct tumour. B. TNM classification of extrahepatic bile duct tumours.3 C. Anatomy and nomenclature of the bile duct system. D. Schematic procedure of ERC-based PDT: following endoscopic passage of the upper gastrointestinal tract, endoscopic retrograde cholangiography (ERC) involves biliary cannulation with the laser light fibre accompanied by contrast medium injection and X-ray monitoring. A translucent ERC cannula is inserted into the tumour stenosis through which a 400 μm quartz fibre with a cylindrical light diffuser is placed along the tumour stenosis. | ||
With an incidence of about 3 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per year, bile duct cancers represent a rare disease accounting for about 2–3% of all malignant tumours.4,5 Risk factors are primary sclerosing cholangitis (PSC), extra- and intrahepatic bile duct cysts (choledochocele, Caroli's syndrome), hepatolithiasis, liver fluke infestation (Clonorchis, Opisthorchis).6,7 For example, 10–20% of the patients with PSC develop an extrahepatic cholangiocarcinoma.8 Chronic bacterial inflammation is common to all of these predispositions leading to production of secondary carcinogenic bile acids such as deoxycholic acid and lithocholic acid.9
000 per year, bile duct cancers represent a rare disease accounting for about 2–3% of all malignant tumours.4,5 Risk factors are primary sclerosing cholangitis (PSC), extra- and intrahepatic bile duct cysts (choledochocele, Caroli's syndrome), hepatolithiasis, liver fluke infestation (Clonorchis, Opisthorchis).6,7 For example, 10–20% of the patients with PSC develop an extrahepatic cholangiocarcinoma.8 Chronic bacterial inflammation is common to all of these predispositions leading to production of secondary carcinogenic bile acids such as deoxycholic acid and lithocholic acid.9
Prognosis for cholangiocarcinoma is poor. At the time of diagnosis only 30–50% of the patients with extrahepatic CC show local lymph node metastases and 10–20% show distant metastases (especially in liver and peritoneum). Nevertheless, 70–80% of perihilar tumours are not resectable due to tumour extension to other adjacent anatomical structures.10–12 Without treatment half of the patients die within three to four months due to the indirect consequences of local tumour progression, i.e. increasing bile duct obstruction, bacterial cholangitis, gallbladder empyema, liver abscesses, cholestasis and liver failure.13 Patients with extrahepatic CC usually present with painless icterus, pruritus, anorexia and rapid weight loss, or signs of cholangitis.14,15
So far, surgical removal of the tumour is the only curative approach. However, even after curative (R0) resection the 5-year survival rate is limited to 30–40%.6,16,17 In most cases (70–80%) resection is precluded by local tumour extension - especially owing to the distinct anatomical features of the liver hilus comprising three neighbouring vessel systems (arterial, portal venous and biliary ductal). Liver transplantation may be considered in exceptional cases, although non-resectable CCs do not represent an assured indication.18
Tumours of the bile duct often show poor response to combination chemotherapy13 with median overall survival time up to 15 months at best with gemcitabine/oxaliplatin (or cisplatin) or gemcitabine/capecitabine.19 External beam radiation therapy does not improve the prognosis;20–22 nevertheless, CCs appear to respond moderately to combination radiochemotherapy. However, many of the patients with CC are never fit for aggressive chemo- or radiochemotherapy because of tumour complications like obstructive cholestasis or cholangitis. Photodynamic therapy (PDT) is now gaining acceptance for alternative palliative or neoadjuvant management of CC (see below).
Further progress of palliative treatment strategies is important to improve quality of life and survival time. The main goal of palliation for non-resectable CC is abatement of obstructive cholestasis and associated morbidities such as pruritus and cholangitis. This can be achieved mainly via three strategies: i) surgically, via creation of a choledochojejunostomy or hepaticojejunostomy (surgical formation of a communication between the common bile duct and the jejunum or of the hepatic duct and the jejunum, respectively), ii) percutaneously, via percutaneous implantation of a transhepatic cholangiodrainage (PTCD), and, iii) endoscopically, by means of endoscopic retrograde cholangio(pancreatico)graphy (ERC(P)) and implantation of biliary stents (see Fig. 1D).15 Stent insertion can be successfully combined with photodynamic therapy or intraluminal brachytherapy (ILBT). The latter is performed by application of a 192Iridium radiation source directly along the malignant stenoses. Due to the close proximity to the tumour tissue energy doses in the range of 10–20 Gy are achieved. Although ILBT has been successfully used in some studies for palliation of non-resectable CC, its combination with external beam radiation resulted in increased risk of cholangitis (incidence 40–50%) and, furthermore, the survival benefit of this combined approach could not be demonstrated throughout all studies (see15 for further discussion). A new treatment option for local tumour ablation is given by high-intensity intraductal ultrasound (HIIUS) for which only pilot data exist23,24 that must be substantiated by further studies.15
During the past years, photodynamic therapy has been established for palliative treatment of perihilar cholangiocarcinoma and was also tested in pilot fashion for neoadjuvant downstaging of advanced CC prior to curative resection. The subsequent paragraphs deal with the use of PDT for cholangiocarcinoma highlighting the mode of operation, the experimental preclinical studies and the clinical data available thus far.
Principles of photodynamic therapy
In several countries photodynamic therapy (PDT) has been approved for treatment of various tumours and precancers, such as Barrett's oesophagus with dysplasia (sodium porfimer, Photofrin®), actinic keratosis (ALA, δ-aminolevulinic acid; Metvix®, Levulan®), solid tumours such as endobronchial, oesophagus, gastric and bladder carcinoma (porfimer, Photofrin®), basal cell carcinoma (ALA, Metvix®) and for head and neck tumours (temoporfin, Foscan®).25,26Photodynamic therapy is based on the administration of a per se non-toxic photosensitising agent (photosensitiser, PS), its accumulation in the target tumour tissue and subsequent irradiation with (laser) light in the visible spectral range according to the appropriate absorption maximum of the PS (see Fig. 2). Light-induced electronic excitation is followed by either radiation-free dissipation of energy/fluorescence emission or conversion to a long-living activated state (triplet state T1). Primary photochemical reactions (transfer of electrons/protons or energy to adjacent molecules, type-I or type-II photochemical reaction, respectively) and subsequent secondary reactions produce reactive oxygen compounds (collectively referred to as reactive oxygen species, ROS) that oxidise cellular molecules and induce cell damage.25–28 Depending on the light energy (J cm−2) applied and the amount of PS (concentration), the cellular response ranges from repair and survival, induction of apoptosis to necrosis.27,29–32
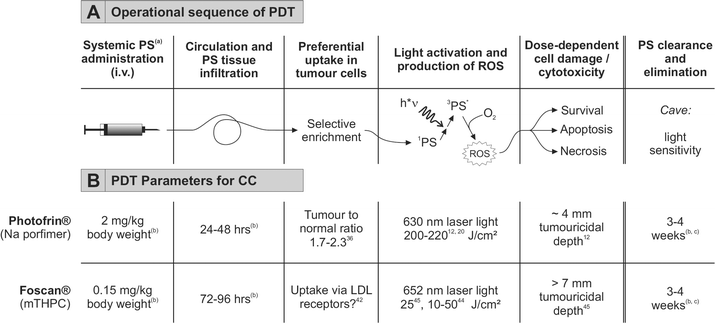 | ||
| Fig. 2 Principle of photodynamic therapy for cholangiocarcinoma. A. General operational sequence of PDT. Modified from.27 B. Established parameters for PDT using Photofrin® or Foscan®. (a) Abbreviations: PS, photosensitiser; 1PS, singlet PS; 3PS*, activated triplet PS; ROS, reactive oxygen species. (b) According to manufacturer's instructions. (c) Time required for systematic bleaching of all skin areas by increasing doses of light until exposure to bright sunlight is possible. | ||
Several advantages of PDT over radio- or chemotherapy can be named: i) both, the PS distribution and the light application allow for tumour selective treatment, ii) PDT induces a cellular stress response which is effective also in the case of tumours resistant to chemotherapy, iii) only minor and predictable side effects such as general photosensitivity, and, iv) PDT allows combination with other therapies, e.g. surgery, chemo- and radiation therapy.27 It is worth mentioning that minimisation of the side effects as well as optimisation of the treatment require validation of the PS and light doses for the particular tumour type and tumour localisation of the photosensitiser accompanied, where applicable, with dosimetric analyses in the target tissue.33 PDT is characterised by tumour selectivity - first, by confining the light beam to the tumour tissue and, second, by selective enrichment of many photosensitisers in tumour tissue. Diverse reasons account for this phenomenon such as augmented tumour vessel permeability, increased LDL receptor-based PS uptake or reduced lymphatic drainage.34,35
Effective photosensitisers for PDT of cholangiocarcinoma
Several preclinical studies demonstrate a significant efficiency of PDT against cholangiocarcinoma. Selective PS accumulation could be shown by Pahernik et al.36 by means of quantitative fluorescence microscopy of biopsies. At 24 and 48 hours post i.v. administration the concentration of Photofrin® increased in CC and normal tissue at different rates. This resulted in fluorescence ratios between tumour and normal biliary epithelia tissue of 1.7 and 2.3 at 24 and 48 hours post administration, respectively.36Photofrin® is excited at 630 nm and is characterised by small tumouricidal depth of only 4 mm.37,38 An alternative PS, THP (tetrakis-pyridyl-tetrahydroporphyrin tosylate), was studied by Oertel et al. with a cholangiocarcinoma model.39THP absorbs at 763 nm thus allowing for a greater tumouricidal penetration depth and this bacteriochlorine showed a good in vitro efficiency on two CC cell lines and effective induction of tumour necrosis up to 8 mm in the mouse xenograft tumours.39 A similar study was published by Wong Kee Song et al.40 using mono-L-aspartyl chlorin e6 (NPe6, λmax = 664 nm) as a photosensitiser compared to HpD (hematoporphyrin derivative). In this study, NPe6 was superior to HpD regarding reduction in tumour volume, cutaneous photosensitisation, and effective tumouricidal depth (as measured by reduction of the vertical tumour extension within two days following PDT).40
Verteporfin (benzoporphyrin derivative, Visudyne®) was studied in a recent publication by Park et al.41 in combination with the known radiosensitiser paclitaxel for enhancement of the cytotoxic response by combination of PDT and paclitaxel in a gastric and a biliary cancer cell line. The authors found that the PDT-induced cytotoxic effects were increased by pre-treatment with paclitaxel, mediated by an augmented apoptotic response and conclude that the radiosensitiser paclitaxel should be investigated in subsequent (pre-)clinical studies with the aim to overcome sublethal photodamage due to improper light application or photosensitiser uptake.41
We recently investigated the use of mTHPC (meta-tetrahydroxyphenyl chlorine, temoporfin) for treatment of cholangiocarcinoma cell lines in vitro.42Temoporfin is available under the trade name Foscan® as a solvent-based formulation and gained approval for PDT of several tumour types.25,26mTHPC as the photoactive substance has the advantage of activation at higher wavelengths (652 nm) and greater tumouricidal depth of up to 10 mm, as shown for pancreatic cancer.43 The mentioned study42 compared the lipophilic mTHPC in the solvent-based formulation (Foscan®) with a recently developed liposome-based water-soluble drug, Foslip®. In the presence of foetal calf serum (FCS) cellular uptake kinetics and PDT-induced cytotoxicity were found to be equally effective for both PS formulations. These data demonstrate a high in vitro efficiency of mTHPC-based PDT for CC cells and suggest good effectiveness in the clinical application42, which was recently confirmed by initial clinical pilot studies.44,45
PDT for cholangiocarcinoma - clinical experience
Precise clinical staging of perihilar CC is required for treatment decisions, in particular to decide whether a curative approach or an only palliative strategy is feasible.12 For this, the tumour localisation and extension are defined by MRI (magnetic resonance imaging) and ERC (endoscopic retrograde cholangiography) (or PTC (percutaneous transhepatic cholangiography)) and malignancy proven with biopsies from the biliary tree stenosis. It is additionally important to visualise the intramural tumour and its proximal and distal extension in the biliary tree which is achieved with intraluminal IDUS (intraductal ultrasound) using 15 or 20 MHz miniprobes given that both ends of the tumour stenosis are accessible to the IDUS probe.46 Evidence for CC in presence of a perihilar biliary tree stenosis is supplemented by FDG PET (2-deoxy-[18F]fluoro-D-glucose positron emissions tomography) with diagnostic sensitivity of >90% and specificity of at least 80%.47,48As shown in Table 1, most clinical studies on PDT for cholangiocarcinoma employed the photosensitiser hematoporphyrin derivative, pure fraction sodium porfimer (Photofrin®): 24–48 hours post i.v. administration of 2 mg Photofrin® per kg body weight, laser light is applied during ERC by a quartz fibre with a cylindrical diffuser tip at λmax 630 nm and a power of 100 mW and an average light dose of 200–220 J.cm−1 of the stenosis (see Fig. 1D, Fig. 2).12,46 With Photofrin® complete tumour eradication is not possible due to the limited tumouricidal penetration of only 4 mm in presence of a tumour infiltration up to 8 mm in most cases.37,49 Repeated PDT applications (e.g. every 6 months) as compared with the first PDT show similar high response rates of about 75% as defined by re-opening of occluded biliary tree segments.50 After neoadjuvant porfimer-PDT resected CC specimens showed complete necrosis of all tumour tissue and residues of degraded porfimer pigment within the inner 4 mm thick layer of the bile duct wall, but viable tumour cell nests and nil degraded porfimer pigment deeper (5–10 mm) in the wall.46 Currently, an ongoing multicentre randomised phase III trial investigates whether porfimer-based PDT plus stenting is superior over stenting alone with respect to overall survival, progression-free survival, toxicity and quality of life (EUDRACT-2005-001173-96).51
| PS | Year | N a | Study type/aim | Results | Ref. |
|---|---|---|---|---|---|
| a Abbreviations: DHE, dihematoporphyrin ether; HpD, haematoporphyrin derivative; N, number of patients; PF, Photofrin®; Ps3, Photosan-3®; PTBD, percutaneous transhepatic biliary drainage. b n = 60: surgery following neoadjuvant PDT, n = 68: PDT plus stents, n = 56: stenting alone. | |||||
| PF | 2008 | 19 | Comparison of patients with non-resectable CC undergoing ERCP with stenting or stenting alone | 16.2 vs 7.4 months survival in PDT-stent group (n= 19) and stent group (n= 29), respectively. Similar decrease in bilirubin, adverse effect of skin phototoxicity in 3 patients | 59 |
| Foscan® | 2008 | 11 | Results from stage I of a phase-II study on mTHPC for non-resectable CC | Increase of tumouricidal depth and similar adverse effects compared to PF-based PDT. | 45 |
| Foscan® | 2007 | 9 | mTHPC PDT for non-resectable malignant biliary strictures and recurrent stent occlusion | Induction of tumour necrosis and recanalisation of blocked stents; median survival 8 months from PDT and increased stent patency | 44 |
| PF | 2007 | 25 | Analysis of factors associated with increased survival after PDT | Presence of a visible tumour mass during imaging and increasing time between diagnosis and PDT predicted a poorer survival rate. → Importance of early diagnosis and (PDT) treatment | 60 |
| HpD | 2007 | 14 | IL-6 as a tumour marker for monitoring of the PDT procedure | Reduction of the IL-6 serum concentration after PDT (282.1 ± 121.8 → 38.2 ± 9.9 pg/mL−1); IL-6 as a specific tumour marker for CC | 61 |
| PF | 2006 | 184b | Analysis of the surgical/palliative treatment of CC over 10 years | PDT plus stenting is superior to sole stenting with respect to survival (12 vs. 6,4 months), performance and bilirubin levels | 46 |
| Ps3 | 2005 | 32 | Palliative PDT for non-resectable bile duct tumours | Effective reduction of the malignant bile duct stenosis; improved survival (21 vs. 7 months) | 56 |
| HpD | 2005 | 24 | PDT by percutaneous cholangioscopy for advanced hilar CC | Improved survival (median 558 ± 178.8 days) and quality of life (Karnofsky: 39.1 ± 11.36 → 58.2 ± 22.72) | 62 |
| PF | 2005 | 8 | Pilot study on endoscopic PDT for non-resectable CC | Prolongation of survival (Median = 276 days) | 63 |
| PF | 2004 | 8 | Post-operative, adjuvant PDT for bile duct carcinoma | Reduction of stenosis caused by tumour progression; improved survival for adjuvant post-operative PDT | 64 |
| PF | 2004 | 4 | PDT + PTBD for treatment of bile duct carcinoma | Improved quality of life and performance status | 65 |
| 2004 | 27 | PDT + bile duct endoprotheses (B) versus“stenting alone” (A) for advanced hilar CC | Improved life expectancy (B, n= 27: 558 days vs. A, n= 20: 288 days); similar reduction in serum bilirubin | 66 | |
| PF | 2004 | 23 | 5-Year follow-up study on patients with non-resectable hilar CC | Median survival: 11,2 (M0) and 9,3 months (M0 + M1); improved cholestasis, performance, and quality of life–no prevention of progression of the disease | 67 |
| PF | 2003 | 7 | Neoadjuvant preoperative PDT for hilar CC | Reduction of cholestasis following PDT; R0 resection in all Patients; 1-year recurrence-free survival rate: 83% | 49 |
| PF | 2003 | 24 | Palliative PDT for non-resectable hilar CC | Stable quality of life and reduced serum bilirubin level; small, but not significantly improved survival rate | 68 |
| PF | 2003 | 20 | Randomised-prospective study on PDT for CC | Survival for group A (PDT + stenting, n= 20): 493 days; group B (stenting alone, n= 19): 98 days; improved biliary drainage and quality of life | 55 |
| PF | 2001 | 6 | PDT for advanced CC | Reduction of serum bilirubin; application and optimisation of the endoscopic technique | 69 |
| Ps3 | 2001 | 8 | Palliative PDT for non-resectable CC via diode laser system | Reduction of the bile duct stenoses (bilirubin: 5.8 mg/dl (2.0–10.1) → 1.0 mg/dl (0.8–4.4)) | 70 |
| PF | 2001 | 21 | PDT as a treatment accessory to endoscopic biliary drainage | Good clinical effects on bilirubin level, quality of life and survival (6-month survival rate: 95%) | 71 |
| PF | 2000 | 1 | Neoadjuvant PDT for hilar CC | Effective induction of necrosis within the superficial 4 mm; no tumour recurrence within 18 months following curative resection | 37 |
| PF | 2000 | 23 | Single-arm phase-II study | 6-month survival rate: 91% post diagnosis/74% post first PDT; reduction of bile duct stenoses and improved survival | 50 |
| HpD | 1998 | 9 | Palliative PDT for non-resectable Bismuth type III and IV CC | Bilirubin: 318 ± 72 → 103 ± 35 μmol L−1, improved Karnofsky index: 32.2% ± 8.1% → 68.9% ± 6.1%; mean survival: 439 days | 72 |
| DHE | 1991 | 1 | First clinical application of PDT for CC | PDT treatment over 4 years resulted in stable condition: no icterus, Karnofsky index: 70 | 73 |
With the intention of improving the tumouricidal penetration depth we use temoporfin (Foscan®) within a prospective study for non-resectable hilar cholangiocarcinoma, allowing for a secondary resection two months post neoadjuvant PDT (Phase II trial, EUDRA CT Nr. 2005-004866-17).45Temoporfin is intravenously applied at 0.15 mg kg−1 body weight and after 72 or 96 hours, PDT is performed during ERC at 652 nm wavelength and a power of 30 J cm−1 tumour extension (Fig. 2). Contraindications for PDT are porphyria, severe liver or kidney dysfunction, sepsis, and concomitant use of radio-/chemotherapy or of other photosensitising or dermatotoxic drugs.12
Recent review articles12,15,20,52–54 on palliative use of Photofrin® PDT for non-resectable cholangiocarcinoma predominantly attest this procedure positive results as regards biliary drainage, quality of life and prolonged survival time. Table 1 gives an up-to-date overview of the original articles published on PDT for cholangiocarcinoma.
A reduction of the bilirubin level, improvement of the Karnofsky index (quantitative assessment of the patient's performance status; 100% = normal, 10% = moribund, rapidly progressive fatal disease) and a prolongation of life expectancy are regularly reported for PDT-based treatment of cholangiocarcinoma as compared with biliary stenting (Table 1).
The first randomised-controlled clinical study55 describes treatment of 39 patients with non-resectable CC, randomised either for PDT with subsequent stenting (group A, n = 20) or for stenting only (group B, n = 19). The median survival was 493 days for the PDT group (vs. 98 days for group B); PDT was well tolerated and only this group showed significant improvement of the overall situation (Karnofsky index and performance). A related study using a similar hematoporphyrin derivative (Photosan-3®) as photosensitiser confirms the improved survival time with palliative PDT.56
According to the study with the hitherto greatest number of patients (184)46 only complete resection including hepatic resection (R0) guarantees long-term survival of patients with hilar cholangiocarcinoma. A combination of PDT and subsequent stenting was superior to treatment with only biliary endoprotheses with respect to survival (12 vs. 6.4 months), lowest bilirubin concentration (ranging from 1.4 to 6.0 mg dL−1) and Karnofsky performance status. Palliative PDT was comparable with incomplete (R1 (microscopic residual tumour) and R2 (macroscopic residual tumour)) resection, however, reduced the rate of complications and the burden for the patients. Palliative PDT allows controlling cholestasis and cholangitis and renders the patients fit for other anti-tumour therapies.46
First pilot data on preoperative neoadjuvant PDT with Photofrin® demonstrated that this procedure allows selective tumour eradication within the tumouricidal depth of 4 mm accompanied by low risk of complications.37,49 Based on these data a prospective evaluation whether neoadjuvant PDT can reduce the threat of tumour recurrence following potential curative resection seems promising.
Conclusion and outlook
Photodynamic therapy has been established as a powerful procedure for treatment of cholangiocarcinoma. Besides maintenance and improvement of the quality of life the goal of palliative measures consists in sustaining effective biliary drainage from all liver segments which usually allows reduction of the risks of tumour complications, especially liver abscesses and sepsis. PDT combined with biliary stenting has proven its advantage over the sole use of biliary endoprotheses and prolongs median overall survival time by approximately 12 months compared to the natural course.Based on a literature survey a C1 recommendation was given for the use of PDT for non-resectable bile duct cancer (‘The clinical action may be considered although there is a lack of high-level scientific evidence for its use. May be useful.’).57 In a recent editorial58, PDT is described as the only treatment to date where there is evidence to support an improvement of survival over plastic stent placement alone for advanced CC. It was concluded that for the question of whether PDT should be considered standard of care for palliation of cholangiocarcinoma, “the answer is a qualified yes”.58
Nevertheless, additional efforts are required to investigate alternative photosensitisers with better tumouricidal depth, shorter duration of phototoxicity and more rapid onset (i.e. a reduced drug-to-light interval).58 Most importantly, larger comparative trials are needed to prove the advantages of PDT over chemoradiation.57,58 Within this context two ongoing phase II and III trials aim at improving the tumouricidal depth45 and to provide additional data on the efficiency of PDT plus biliary stenting compared to stenting alone for overall survival,51 respectively.
Taken together, further improvement of local tumour ablation with PDT will allow treatment of the patients with neoadjuvant protocols aiming at higher rates of definitive cure or allow multimodal protocols combined with radio- or chemotherapy in the palliative situation. Given the emerging additional indications for PDT and successful proof of the benefit for the patients suffering from perihilar CC, PDT will find a broader use beyond specialised centres.
Acknowledgements
Tobias Kiesslich was supported by a research grant of the Oesterreichische Nationalbank (project number 12677).References
- A. Nakeeb, H. A. Pitt, T. A. Sohn, J. Coleman, R. A. Abrams, S. Piantadosi, R. H. Hruban, K. D. Lillemoe, C. J. Yeo and J. L. Cameron, Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors, Ann. Surg., 1996, 224, 463–473 CrossRef CAS
; discussion 473–465.
- H. Bismuth, R. Nakache and T. Diamond, Management strategies in resection for hilar cholangiocarcinoma, Ann. Surg., 1992, 215, 31–38 CAS
.
- Collaborative Staging Manual and Coding Instructions, Part II, Version 01.03.00, Collaborative Staging Task Force of the American Joint Committee on Cancer; U.S. Department, of Health and Human Services/National Institutes of Health/National Cancer Institute, Chicago, IL, USA, http://www.cancerstaging.org/.
- A. Tannapfel and C. Wittekind, Gallbladder and bile duct carcinoma. Biology and pathology, Internist (Berl), 2004, 45, 33–41 Search PubMed
.
- Y. Shaib and H. B. El-Serag, The epidemiology of cholangiocarcinoma, Semin. Liver Dis., 2004, 24, 115–125 CrossRef
.
- K. N. Lazaridis and G. J. Gores, Cholangiocarcinoma, Gastroenterology, 2005, 128, 1655–1667 CrossRef
.
- S. A. Khan, H. C. Thomas, B. R. Davidson and S. D. Taylor-Robinson, Cholangiocarcinoma, Lancet, 2005, 366, 1303–1314 CrossRef
.
- A. Tannapfel and C. Wittekind, Anatomy and pathology of intrahepatic and extrahepatic bile duct tumors, Pathologe, 2001, 22, 114–123 CrossRef CAS
.
- G. Fava, M. Marzioni, A. Benedetti, S. Glaser, S. DeMorrow, H. Francis and G. Alpini, Molecular pathology of biliary tract cancers, Cancer Lett., 2007, 250, 155–167 CrossRef CAS
.
- D. E. Henson, J. Albores-Saavedra and D. Corle, Carcinoma of the extrahepatic bile ducts. Histologic types, stage of disease, grade, and survival rates, Cancer, 1992, 70, 1498–1501 CrossRef CAS
.
- W. Kimura, H. Nagai, Y. Atomi, A. Kuroda, T. Muto, M. Yamashiro and Y. Esaki, Clinicopathological characteristics of hepatic hilar bile duct carcinoma, Hepatogastroenterology, 1993, 40, 21–27 Search PubMed
.
- F. Berr, Photodynamic therapy for cholangiocarcinoma, Semin. Liver Dis., 2004, 24, 177–187 CrossRef
.
- T. Zopf and J. F. Riemann, Therapy of pancreatic and bile duct tumors: value of radiotherapy and photodynamic therapy, Schweizerische Rundschau fur Medizin Praxis = Revue suisse de medecine Praxis, 2000, 89, 1293–1298 Search PubMed
.
- P. C. de Groen, G. J. Gores, N. F. LaRusso, L. L. Gunderson and D. M. Nagorney, Biliary tract cancers, New Engl. J. Med., 1999, 341, 1368–1378 CrossRef CAS
.
- P. Chahal and T. H. Baron, Endoscopic palliation of cholangiocarcinoma, Curr. Opin. Gastroenterol., 2006, 22, 551–560
.
- W. R. Jarnagin and M. Shoup, Surgical management of cholangiocarcinoma, Semin. Liver Dis., 2004, 24, 189–199 CrossRef
.
- P. Neuhaus, S. Jonas, W. O. Bechstein, R. Lohmann, C. Radke, N. Kling, C. Wex, H. Lobeck and R. Hintze, Extended resections for hilar cholangiocarcinoma, Ann. Surg., 1999, 230, 808–818 CrossRef CAS
; discussion 819.
- R. Robles, J. Figueras, V. S. Turrion, C. Margarit, A. Moya, E. Varo, J. Calleja, A. Valdivieso, J. C. Valdecasas, P. Lopez, M. Gomez, E. de Vicente, C. Loinaz, J. Santoyo, M. Fleitas, A. Bernardos, L. Llado, P. Ramirez, F. S. Bueno, E. Jaurrieta and P. Parrilla, Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma, Ann. Surg., 2004, 239, 265–271 CrossRef
.
- S. Thongprasert, The role of chemotherapy in cholangiocarcinoma, Ann. Oncol., 2005, 16 Suppl 2, ii93–96 CrossRef
.
- L. Ayaru, S. G. Bown and S. P. Pereira, Photodynamic therapy for pancreatic and biliary tract carcinoma, Int. J. Gastrointest. Cancer, 2005, 35, 1–13 Search PubMed
.
- M. Hejna, M. Pruckmayer and M. Raderer, The role of chemotherapy and radiation in the management of biliary cancer: a review of the literature, Eur. J. Cancer, 1998, 34, 977–986 CrossRef CAS
.
- S. A. Khan, B. R. Davidson, R. Goldin, S. P. Pereira, W. M. Rosenberg, S. D. Taylor-Robinson, A. V. Thillainayagam, H. C. Thomas, M. R. Thursz and H. Wasan, Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document, Gut, 2002, 51 Suppl 6, VI1–9 CrossRef
.
- F. Prat, C. Lafon, D. M. De Lima, Y. Theilliere, J. Fritsch, G. Pelletier, C. Buffet and D. Cathignol, Endoscopic treatment of cholangiocarcinoma and carcinoma of the duodenal papilla by intraductal high-intensity US: Results of a pilot study, Gastrointest. Endosc., 2002, 56, 909–915 CrossRef
.
- F. Prat, C. Lafon, J. Y. Theilliere, J. Fritsch, A. D. Choury, I. Lorand and D. Cathignol, Destruction of a bile duct carcinoma by intraductal high intensity ultrasound during ERCP, Gastrointest. Endosc., 2001, 53, 797–800 CrossRef CAS
.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Photodynamic therapy for cancer, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS
.
- M. Triesscheijn, P. Baas, J. H. Schellens and F. A. Stewart, Photodynamic therapy in oncology, Oncologist, 2006, 11, 1034–1044 CrossRef CAS
.
- T. Kiesslich, B. Krammer and K. Plaetzer, Cellular mechanisms and prospective applications of hypericin in photodynamic therapy, Curr. Med. Chem., 2006, 13, 2189–2204 CrossRef CAS
.
- K. Plaetzer, J. Berlanda, F. Berr, B. Krammer and T. Kiesslich, Photophysics and Photochemistry of Photodynamic Therapy: Fundamental Aspects, Lasers Med. Sci., in press, DOI:10.1007/s10103-008-0539-1.
- J. Berlanda, T. Kiesslich, C. B. Oberdanner, F. J. Obermair, B. Krammer and K. Plaetzer, Characterization of apoptosis induced by photodynamic treatment with hypericin in A431 human epidermoid carcinoma cells, J. Environ. Pathol. Toxicol. Oncol., 2006, 25, 173–188 Search PubMed
.
- K. Plaetzer, T. Kiesslich, B. Krammer and P. Hammerl, Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS4-PDT, Photochem. Photobiol. Sci., 2002, 1, 172–177 RSC
.
- K. Plaetzer, T. Kiesslich, C. B. Oberdanner and B. Krammer, Apoptosis following photodynamic tumor therapy: induction, mechanisms and detection, Curr. Pharm. Des., 2005, 11, 1151–1165 CrossRef CAS
.
- K. Plaetzer, T. Kiesslich, T. Verwanger and B. Krammer, The Modes of Cell Death Induced by PDT: An Overview, Med. Laser Appl., 2003, 18, 7–19 CrossRef
.
- R. R. Allison, G. H. Downie, R. Cuenca, X.-H. Hu, C. J. Childs and C. H. Sibata, Photosensitizers in clinical PDT, Photodiagn. Photodyn. Ther., 2004, 1, 27–42 CrossRef CAS
.
- A. Juzeniene, K. P. Nielsen and J. Moan, Biophysical aspects of photodynamic therapy, J. Environ. Pathol. Toxicol. Oncol., 2006, 25, 7–28 Search PubMed
.
- M. R. Hamblin and E. L. Newman, On the mechanism of the tumour-localising effect in photodynamic therapy, J. Photochem. Photobiol., B, 1994, 23, 3–8 CrossRef CAS
.
- S. A. Pahernik, M. Dellian, F. Berr, A. Tannapfel, C. Wittekind and A. E. Goetz, Distribution and pharmacokinetics of Photofrin in human bile duct cancer, J. Photochem. Photobiol., B, 1998, 47, 58–62 CrossRef CAS
.
- F. Berr, A. Tannapfel, P. Lamesch, S. Pahernik, M. Wiedmann, U. Halm, A. E. Goetz, J. Mossner and J. Hauss, Neoadjuvant photodynamic therapy before curative resection of proximal bile duct carcinoma, J. Hepatol., 2000, 32, 352–357 CrossRef CAS
.
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, Photodynamic therapy, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS
.
- M. Oertel, S. I. Schastak, A. Tannapfel, R. Hermann, U. Sack, J. Mossner and F. Berr, Novel bacteriochlorine for high tissue-penetration: photodynamic properties in human biliary tract cancer cells in vitro and in a mouse tumour model, J. Photochem. Photobiol., B, 2003, 71, 1–10 CrossRef CAS
.
- L. M. Wong, Kee Song, K. K. Wang and A. R. Zinsmeister, Mono-L-aspartyl chlorin e6 (NPe6) and hematoporphyrin derivative (HpD) in photodynamic therapy administered to a human cholangiocarcinoma model, Cancer, 1998, 82, 421–427 CrossRef
.
- S. Park, S. P. Hong, T. Y. Oh, S. Bang, J. B. Chung and S. Y. Song, Paclitaxel augments cytotoxic effect of photodynamic therapy using verteporfin in gastric and bile duct cancer cells, Photochem. Photobiol. Sci., 2008, 7, 769–774 RSC
.
- T. Kiesslich, J. Berlanda, K. Plaetzer, B. Krammer and F. Berr, Comparative characterization of the efficiency and cellular pharmacokinetics of Foscan- and Foslip-based photodynamic treatment in human biliary tract cancer cell lines, Photochem. Photobiol. Sci, .2007, 6, 619–627 Search PubMed
.
- S. G. Bown, A. Z. Rogowska, D. E. Whitelaw, W. R. Lees, L. B. Lovat, P. Ripley, L. Jones, P. Wyld, A. Gillams and A. W. Hatfield, Photodynamic therapy for cancer of the pancreas, Gut, 2002, 50, 549–557 CrossRef CAS
.
- S. P. Pereira, L. Ayaru, A. Rogowska, A. Mosse, A. R. Hatfield and S. G. Bown, Photodynamic therapy of malignant biliary strictures using meso-tetrahydroxyphenylchlorin, Eur. J. Gastroenterol. Hepatol., 2007, 19, 479–485 CrossRef CAS
.
- G. Wolkersdoerfer, K. Emmanuel, U. Denzer, A. Puespoek, D. Neureiter, T. Kiesslich, A. Lohse and F. Berr, Temoporfin improves tumoricidal efficacy of photodynamic therapy (PDT) for bile duct cancer. 33rd Congress of the European Society for Medical Oncology, Stockholm, Sweden, Ann. Oncol., 2008, 19(Suppl. 8), 176
.
- H. Witzigmann, F. Berr, U. Ringel, K. Caca, D. Uhlmann, K. Schoppmeyer, A. Tannapfel, C. Wittekind, J. Mossner, J. Hauss and M. Wiedmann, Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection, Ann. Surg., 2006, 244, 230–239 CrossRef
.
- R. Kluge, F. Schmidt, K. Caca, H. Barthel, S. Hesse, P. Georgi, A. Seese, D. Huster and F. Berr, Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer, Hepatology, 2001, 33, 1029–1035 CrossRef CAS
.
- H. Wakabayashi, S. Akamoto, S. Yachida, K. Okano, K. Izuishi, Y. Nishiyama and H. Maeta, Significance of fluorodeoxyglucose PET imaging in the diagnosis of malignancies in patients with biliary stricture, Eur. J. Surg. Oncol., 2005, 31, 1175–1179 CrossRef CAS
.
- M. Wiedmann, K. Caca, F. Berr, I. Schiefke, A. Tannapfel, C. Wittekind, J. Mossner, J. Hauss and H. Witzigmann, Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study, Cancer, 2003, 97, 2783–2790 CrossRef
.
- F. Berr, M. Wiedmann, A. Tannapfel, U. Halm, K. R. Kohlhaw, F. Schmidt, C. Wittekind, J. Hauss and J. Mossner, Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival, Hepatology, 2000, 31, 291–298 CrossRef CAS
.
- Trial information: “Phase III Randomized Study of Biliary Stenting With Versus Without Photodynamic Therapy Using Porfimer Sodium in Patients With Unresectable Locally Advanced, Recurrent, or Metastatic Cholangiocarcinoma and Other Biliary Tract Tumors”http://www.cancer.gov/clinicaltrials/CRUK-PHOTOSTENT-02.
- F. L. Dumoulin, E. Horst, T. Sauerbruch and T. Gerhardt, Palliative locoregional therapy for hilar cholangiocarcinoma: photodynamic therapy and brachytherapy, Zentralbl Chir, 2007, 132, 336–341 CrossRef CAS
.
- M. A. Ortner, Photodynamic therapy in cholangiocarcinomas, Best Pract. Res. Clin. Gastroenterol., 2004, 18, 147–154 Search PubMed
.
- H. C. Wolfsen, Uses of photodynamic therapy in premalignant and malignant lesions of the gastrointestinal tract beyond the esophagus, J. Clin. Gastroenterol., 2005, 39, 653–664 CrossRef CAS
.
- M. E. Ortner, K. Caca, F. Berr, J. Liebetruth, U. Mansmann, D. Huster, W. Voderholzer, G. Schachschal, J. Mossner and H. Lochs, Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study, Gastroenterology, 2003, 125, 1355–1363 CrossRef
.
- T. Zoepf, R. Jakobs, J. C. Arnold, D. Apel and J. F. Riemann, Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy, Am. J. Gastroenterol., 2005, 100, 2426–2430 CrossRef CAS
.
- H. Saito, T. Takada, M. Miyazaki, S. Miyakawa, K. Tsukada, M. Nagino, S. Kondo, J. Furuse, T. Tsuyuguchi, F. Kimura, H. Yoshitomi, S. Nozawa, M. Yoshida, K. Wada, H. Amano and F. Miura, Radiation therapy and photodynamic therapy for biliary tract and ampullary carcinomas, J. Hepatobiliary Pancreat. Surg., 2008, 15, 63–68 CrossRef
.
- T. H. Baron, Photodynamic therapy: standard of care for palliation of cholangiocarcinoma?, Clin. Gastroenterol. Hepatol., 2008, 6, 266–267 CrossRef
.
- M. Kahaleh, R. Mishra, V. M. Shami, P. G. Northup, C. L. Berg, P. Bashlor, P. Jones, K. Ellen, G. R. Weiss, C. M. Brenin, B. E. Kurth, T. A. Rich, R. B. Adams and P. Yeaton, Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy, Clin. Gastroenterol. Hepatol., 2008, 6, 290–297 CrossRef
.
- G. A. Prasad, K. K. Wang, T. H. Baron, N. S. Buttar, L. M. Wongkeesong, L. R. Roberts, A. J. LeRoy, L. S. Lutzke and L. S. Borkenhagen, Factors associated with increased survival after photodynamic therapy for cholangiocarcinoma, Clin. Gastroenterol. Hepatol., 2007, 5, 743–748 CrossRef
.
- Y. K. Cheon, Y. D. Cho, J. H. Moon, J. Y. Jang, Y. S. Kim, M. S. Lee, J. S. Lee and C. S. Shim, Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy, Am. J. Gastroenterol., 2007, 102, 2164–2170 CrossRef CAS
.
- C. S. Shim, Y. K. Cheon, S. W. Cha, S. Bhandari, J. H. Moon, Y. D. Cho, Y. S. Kim, L. S. Lee, M. S. Lee and B. S. Kim, Prospective study of the effectiveness of percutaneous transhepatic
photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment, Endoscopy, 2005, 37, 425–433 CrossRef CAS
.
- G. C. Harewood, T. H. Baron, A. Rumalla, K. K. Wang, G. J. Gores, L. M. Stadheim and P. C. de Groen, Pilot study to assess patient outcomes following endoscopic application of photodynamic therapy for advanced cholangiocarcinoma, J. Gastroenterol. Hepatol., 2005, 20, 415–420 CrossRef
.
- A. Nanashima, H. Yamaguchi, S. Shibasaki, N. Ide, T. Sawai, T. Tsuji, S. Hidaka, Y. Sumida, T. Nakagoe and T. Nagayasu, Adjuvant photodynamic therapy for bile duct carcinoma after surgery: a preliminary study, J. Gastroenterol., 2004, 39, 1095–1101 CrossRef
.
- S. Suzuki, K. Inaba, Y. Yokoi, K. Ohata, S. Ota, M. Azuma, T. Tanaka, H. Konno, S. Baba, T. Hirano and S. Nakamura, Photodynamic therapy for malignant biliary obstruction: a case series., Endoscopy, 2004, 36, 83–87 CrossRef CAS
.
- Y. K. Cheon, Y. D. Cho, S. H. Baek, S. W. Cha, J. H. Moon, Y. S. Kim, J. S. Lee, M. S. Lee, C. S. Shim and B. S. Kim, Comparison of survival of advanced hilar cholangiocarcinoma after biliary drainage alone versus photodynamic therapy with external drainage, Korean J. Gastroenterol., 2004, 44, 280–287 Search PubMed
.
- M. Wiedmann, F. Berr, I. Schiefke, H. Witzigmann, K. Kohlhaw, J. Mossner and K. Caca, Photodynamic therapy in patients with non-resectable hilar cholangiocarcinoma: 5-year follow-up of a prospective phase II study, Gastrointest. Endosc., 2004, 60, 68–75 CrossRef
.
- F. L. Dumoulin, T. Gerhardt, S. Fuchs, C. Scheurlen, M. Neubrand, G. Layer and T. Sauerbruch, Phase II study of photodynamic therapy and metal stent as palliative treatment for nonresectable hilar cholangiocarcinoma, Gastrointest. Endosc., 2003, 57, 860–867 CrossRef
.
- A. Rumalla, T. H. Baron, K. K. Wang, G. J. Gores, L. M. Stadheim and P. C. de Groen, Endoscopic application of photodynamic therapy for cholangiocarcinoma, Gastrointest. Endosc., 2001, 53, 500–504 CrossRef CAS
.
- T. Zoepf, R. Jakobs, J. C. Arnold, D. Apel, A. Rosenbaum and J. F. Riemann, Photodynamic therapy for palliation of nonresectable bile duct cancer - preliminary results with a new diode laser system, Am. J. Gastroenterol., 2001, 96, 2093–2097 CAS
.
- M. Ortner, Photodynamic therapy for cholangiocarcinoma, J. Hepatobiliary Pancreat. Surg., 2001, 8, 137–139 CrossRef CAS
.
- M. A. Ortner, J. Liebetruth, S. Schreiber, M. Hanft, U. Wruck, V. Fusco, J. M. Muller, H. Hortnagl and H. Lochs, Photodynamic therapy of nonresectable cholangiocarcinoma, Gastroenterology, 1998, 114, 536–542 CrossRef CAS
.
- J. S. McCaughan, Jr., B. F. Mertens, C. Cho, R. D. Barabash and H. W. Payton, Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report, Arch. Surg., 1991, 126, 111–113 Search PubMed
.
- H. Malhi and G. J. Gores, Cholangiocarcinoma: modern advances in understanding a deadly old disease, J. Hepatol., 2006, 45, 856–867 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry and Owner Societies 2009 |
