Photochemical synthesis of 2,3,9,10-tetrabromopentacene: its unusual photodimerization†‡
Yuewei
Zhao
,
Xichen
Cai
,
Eugene
Danilov
,
Guifeng
Li
and
Douglas C.
Neckers
*
Center for Photochemical Sciences, Bowling Green State University, Bowling Green, OH 43403, USA. E-mail: neckers@photo.bgsu.edu; Fax: 1-419-372-0366; Tel: 1-419-372 2034
First published on 7th November 2008
Abstract
The photogeneration of 2,3,9,10-tetrabromopentacene from its precursor dione by photodecarbonylation was investigated. An unusual photodimer, 3,3′,9,9′,10,10′-hexabromo-2,2′-bipentacene, is produced.
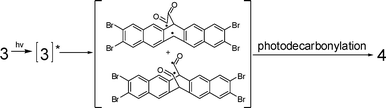
Pentacenes have been investigated extensively. The parent compound is used in electronic devices1 being the most promising organic p-type semiconductor material for plastic and disposable displays. TFT (thin film transistor) mobility above 5 cm2V−1 s−1 is comparable to amorphous silicon.2 Many functionalized pentacenes3 have been prepared because the parent is sparingly soluble in organic solvents, sensitive to oxygen and, as well, to light.4 Compared to the routine synthetic method, i.e., reduction of pentacene quinone and “homologation” involving ring extension,5 pentacenes are now most commonly obtained from photo or thermal precursors.6 If the precursors have good solubility and stability, one can produce pure pentacenes in situ by photochemically expelling CO from the appropriate dione.7 These precursors are easy to handle and chemically modify. Herein, we report the synthesis of 2,3,9,10-tetrabromopentacene (4) using the Strating–Zwanenburg photodecarbonylation8 of dione (3).9
The preparation of 4 by photodecarbonylation of 3, prepared from 1 by osmium tetroxide and modified Swern oxidations (Scheme 1), proceeds using either 395 nm light or light ≥450 nm. The intermediate, 1 is easily modified, and a diverse group of symmetrically substituted pentacenes obtained.7
 | ||
| Scheme 1 Reagents and conditions: i. water/acetone, MMO, Na2S2O4; ii. TFAA, DMSO/DCM, triethylamine, −78 °C; iii. 395 nm or ≥450 nm light. | ||
Yellow precursor 3 dissolves easily in chloroform, dichloromethane, benzene or toluene. When kept in the dark it is rather stable in solution and shows two ‘naphthalene like’ peaks at 323 (ε = 7700) and 337 nm (ε = 7385) as well as a characteristic, broad n–π* band at 465 nm (ε = 1423). Emission at 510 nm is observed from a toluene solution (quantum yield ϕ = 0.005) and at 536 nm from a PMMA matrix. The absorption does not change significantly while the emission exhibits about 26 nm red-shift in the PMMA ‘solid solution’. 3 is photosensitive and must be protected from light during and after preparation. It also exhibits different emission spectra when excited with different wavelengths of light (from 430 to 510 nm). The spectrum obtained when 3 is excited at 430 nm is that of dione 3; however the spectrum obtained when 3 is excited at 510 nm results mostly from photolysis product 4. The longer the excitation wavelength, the more prevalent the emission from the photolysis product, pentacene.
During emission measurement from a PMMA film, the disc holder was moved for every measurement in order that the excitation light hit fresh yellow 3 instead of blue 4 (Fig. 1a). From shielding the thin film plate with a photo mask with a line pattern during the irradiation (Fig. 1b) a yellow-blue-alternating pattern was obtained as a result of the transformation of 3 to 4.
 | ||
| Fig. 1 a). Thin film disc after fluorescence measurement; b). The striped regions were observed after 30 min irradiation under a cover. | ||
The photodecarbonylation from dione to pentacene either in degassed solution (Fig. 2) or in a PMMA matrix was followed with UV-vis spectroscopy. After 5 min irradiation, two new peaks assigned to the absorption of the photolysis product 4 appeared at 542 and 598 nm accompanied by a decrease in the n–π* absorption of 3. Further irradiation led to gradual precipitation of 4 accompanied by a baseline increase across the entire spectrum. Another long-wavelength peak was also observed (≈688 nm) after 30 min irradiation. This peak reached its maximum at 40 min and decreased with further irradiation. The decrease was triggered by formation of an obvious blue precipitate. Because the 688 nm peak was not formed simultaneously with the pentacene peaks and also its long-wavelength absorption, the mechanism for formation of what turned out to be a hand in hand 1:1 dimer, (Scheme 2) explained the phenomenon. The dimeric structure, 3,3′,9,9′,10,10′-hexabromo-2,2′-bipentacene, was confirmed by the MALDI-TOF. Once 4 formed, further irradiation yielded tribromopentacene radical, which produced the hand-in-hand dimer (5) via bimolecular combination. Neither 4 nor 5 are very soluble in common organic solvents, and precipitate together as a blue solid at the bottom of cuvette.
 | ||
| Scheme 2 The formation of hand-in-hand pentacene dimer. | ||
The phototransformation 3 to 4 was also carried out in a thin PMMA film. In contrast to the results in solution, 4 formed as the n–π* absorption of 3 decreased, but no dimer was observed. PMMA ‘solid solution’ blocked the free radical reaction.
Pentacenes easily undergo a [4 + 4] Diels–Alder cycloadditionvia the center rings forming a “butterfly” dimer,1,11 but this is the first ‘hand in hand’ dimer of pentacene reported. An unusual side-to-face dimerization for 2,3,9,10-tetrachloropentacene has also been observed.11 Each of the dimers has photophysical characteristics similar to naphthalene because of a reduction in pentacene conjugation. The latter becomes crucial when pentacenes are used as semiconductive devices. Conversely, dimer 5 extends the conjugation, which means a lower energy level gap.
1H NMR was also used to follow the transformation from 3 to 4 or 5 by monitoring the signal evolution of an illuminated solution of 3 in a Norell Young valve NMR tube which was degassed with freeze-pump-thaw cycles before irradiation. Unfortunately nothing was observed except that the proton signals of 3 decreased gradually and eventually disappeared. This was due to the complete deposition of photolyzed products on the tube from all solvents. The solid blue photolyzed products are stable in the dark and, if protected from light, show no change even after half a year. Because of its poor solubility in organic solvents, 4 could not be purified even by sublimation where it decomposed nor could it be characterized by NMR.9
2,3,9,10-Tetrabromopentacene, 4, is oxygen-sensitive and forms endoperoxide in solution. A new peak appeared at 405 nm as the n–π* absorption for the dione decreased as the oxygen-saturated toluene solution of 3 was irradiated. The 405 nm peak is assigned to the absorption of the endoperoxide which is only partially soluble in toluene. The solubility is sufficient in C6D6 or CDCl3 however such that the 6,13-position protons of the endoperoxide can be easily observed at 6.25 ppm δ or 5.62 ppm δ in either C6D6 or CDCl3 (Fig. 3).7 MALDI-TOF analysis also proved the formation of endoperoxide in O2-saturated solution. No peaks of 4 were found during the process indicating the pentacene was oxidized immediately.
 | ||
| Fig. 3 The 1H NMR proof for the formation of endoperoxide of 4 in O2-saturated C6D6. | ||
Mondal et al. reported femtosecond (fs) pump–probe studies of anthracene, hexacene and heptacene diones and postulated a possible mechanism for their decarbonylation.12 Herein we report the fs transient UV-vis analysis of 3. During the measurement, the photolyzed products precipitate on the wall of the flow cell as blue spots at the point where the beam from the pump laser hits the sample. This decreases the signal-to-noise ratio greatly. In order to avoid the strong noise due to the blue precipitate gradually-increasing the step size along with quick measurement was employed. The transient absorption spectra at different times (Fig. 4) used a 475 nm laser to pump the n–π* absorption band of 3 and white light (450–800 nm) detection.
 | ||
| Fig. 4 a). Absorption difference spectra obtained from pump–probe spectrometry of 3 in toluene, inset: kinetic trace monitored at 582 nm; b). kinetic trace monitored at 743 nm. | ||
The transient absorption band at wavelengths shorter than 700 nm decays more slowly than the band at wavelengths longer than 700 nm. Both obey strictly mono-exponential kinetic decay when monitored at all wavelengths and the former has a lifetime ≈ 55 ps while the latter is ≈47 ps. The former decays to a nonzero baseline most obviously at 582 nm with a maximum y0 value of about 0.0036. The latter decayed to the baseline with no residual absorbance. That means an additional long-lifetime transient species too long to be determined on this pump–probe experiment time scale is produced during the decay of the former. Considering singlet lifetimes of 218.5 and 29.4 ps for anthracene dione and hexacene dione, we assign the 55 and 47 ps lifetimes to the decay of the singlet state of 3. In view of the overlap of the short and long lived species below 700 nm, we submit that the 55 ps lifetime is a bit long and the 47 ps measured a real lifetime for the singlet state of 3. The undetermined long lived species, detected around 582 nm, is ascribed either to the triplet state of 3 or free radical intermediates formed during the photodecarbonylation (Scheme 3).12
 | ||
| Scheme 3 Possible mechanism of photodecarbonylation of 3. | ||
A 490 nm pump laser and consecutive probe light was used in combination to measure the transient signal arising during the photodecarbonylation in a wider window (360–750 nm). Analysis of these transient spectra is similar to that when pumped at 475 nm and reveals a lifetime 52 ps for the singlet of 3 as well as an additional undetermined long lifetime. As reported in reference 10, no explicit evidence provides proof for which of the two free radical intermediates precede the photodecarbonylation to 4. Given theoretical calculations though, one bond dissociation occurring between the carbonyl and methylene carbons is likely. Ultrafast transient IR studies, and other methods by means of which the intermediates might be trapped, are under way.
In summary, we report the photogeneration of 2,3,9,10-tetrabromopentacene (4) from its photoprecursor dione 3 and first observations of its unusual photodimerization. The photodecarbonylation of 3 to yield 4 was investigated by femtosecond ultrafast pump–probe spectrometry.
Notes and references
- M. Bendikov, F. Wudl and D. F. Perepichka, Chem. Rev., 2004, 104, 4891 CrossRef CAS; J. E. Anthony, Chem. Rev., 2006, 106, 5028 CrossRef CAS; A. R. Murphy and J. M. J. Frechet, Chem. Rev., 2007, 107, 1066 CrossRef CAS.
- C. D. Dimitrakopoulos, S. Pursushothaman, J. Kymissis, A. Callegari and J. M. Shaw, Science, 1999, 283, 822 CrossRef CAS; T. W. Kelley, D. V. Muyres, P. F. Baude, T. P. Smith and T. D. Jones, Mater. Res. Soc. Symp. Proc., 2003, 771, L 6.5.1; L. Zhou, A. Wanga, S. C. Wu, J. Sun, S. Park and T. N. Jackson, Appl. Phys. Lett., 2006, 88, 083502 CrossRef.
- C. F. H. Allen and A. Bell, J. Am. Chem. Soc., 1942, 64, 1253 CrossRef CAS; M. M. Payne, J. H. Delcamp, S. R. Parkin and J. E. Anthony, Org. Lett., 2004, 6, 1609 CrossRef CAS; M. M. Payne, S. R. Parkin and J. E. Anthony, J. Am. Chem. Soc., 2005, 127, 8028 CrossRef CAS; Q. Miao, X. Chi, S. Xiao, R. Zeis, M. Lefenfeld, T. Siegrist, M. L. Steigerwald and C. Nuckolls, J. Am. Chem. Soc., 2006, 128, 1340 CrossRef CAS; K. Kobayashi, R. Shimaoka, M. Kawahata, M. Yamanaka and K. Yamaguchi, Org. Lett., 2006, 8, 2385 CrossRef CAS.
- A. Maliakal, K. Raghavachari, H. Katz, E. Chandross and T. Secrist, Chem. Mater., 2004, 16, 4690.
- T. Takahashi, S. Li, W. Huang, F. Kong, K. Nakajima, B. Shen, T. Ohe and K. -I. Kanno, J. Org. Chem., 2006, 71, 7967 CrossRef CAS; M. T. Stone and H. L. Anderson, J. Org. Chem., 2007, 72, 9776 CrossRef CAS.
- P. T. Herwig and K. Müllen, Adv. Mater., 1999, 11, 480 CrossRef; A. Afzali, C. D. Dimitrakopoulos and T. Breen, J. Am. Chem. Soc., 2002, 124, 8812 CrossRef CAS; K. P. Weidkamp, A. Afzali, R. M. Tromp and R. J. Hamers, J. Am. Chem. Soc., 2004, 126, 12740 CrossRef CAS.
- H. Yamada, Y. Yamashita, M. Kikuchi, H. Watanabe, T. Okaujima, H. Uno, T. Ogawa, K. Ohara and N. Ono, Chem. Eur. J., 2005, 11, 6212 CrossRef CAS; K. Chen, H. Hsieh, C. Wu, J. Hwang and T. J. Chow, Chem. Commun., 2007, 1065 RSC; T. Chuang, H. Hsieh, C. Chen, C. Wu, C. Lin, P. Chou, T. Chao and T. J. Chow, Org. Lett., 2008, 10, 2869 CrossRef CAS; Y. Zhao, R. Mondal and D. C. Neckers, J. Org. Chem., 2008, 73, 5506 CrossRef CAS.
- J. Strating, B. Zwanenburg, A. Wagenaar and A. C. Udding, Tetrahedron Lett., 1969, 3, 125 CrossRef.
- T. Okamoto, M. L. Senatore, M. Ling, A. B. Mallik, M. L. Tang and Z. Bao, Adv. Mater., 2007, 19, 3381 CrossRef CAS.
- S. H. Chan, H. K. Lee, Y. M. Wang, N. Y. Fu, X. M. Chen, Z. W. Cai and H. N. C. Wong, Chem. Commun., 2005, 66 RSC; C. P. Benard, Z. Geng, M. A. Heuft, K. VanCrey and A. G. Fallis, J. Org. Chem., 2007, 72, 7229 CrossRef CAS.
- D. F. Perepichka, M. Bendikov, H. Meng and F. Wudl, J. Am. Chem. Soc., 2003, 125, 10190 CrossRef CAS.
- R. Mondal, A. N. Okhrimenko, B. K. Shah and D. C. Neckers, J. Phys. Chem. B, 2008, 112, 11 CrossRef CAS.
Footnotes |
| † Contribution No. 672 from the Center for Photochemical Sciences. |
| ‡ Electronic supplementary information (ESI) available: The general compound preparation and measurement procedures, absorption, fluorescence, mass, IR and NMR spectra of 3 and 4 as well as their evolutions, transient absorption spectra and kinetic traces at different wavelengths and pump laser. See DOI: 10.1039/b814986k |
| This journal is © The Royal Society of Chemistry and Owner Societies 2009 |

