Singlet oxygen generation using a porous monolithic polymer supported photosensitizer: potential application to the photodynamic destruction of melanoma cells†
M. Isabel
Burguete
a,
Francisco
Galindo
*a,
Raquel
Gavara
a,
Santiago V.
Luis
*a,
Miguel
Moreno
b,
Paul
Thomas
c and
David A.
Russell
*b
aDepartamento de Química Inorgánica y Orgánica/UAMOA; Universitat Jaume I/CSIC; Av. Sos Baynat, s/n, 12071, Castellón, Spain. E-mail: francisco.galindo@uji.es; luiss@uji.es
bSchool of Chemical Sciences and Pharmacy, University of East Anglia, Norwich, Norfolk, UK NR4 7TJ. E-mail: D.Russell@uea.ac.uk
cSchool of Biological Sciences, University of East Anglia, Norwich, Norfolk, UK NR4 7TJ
First published on 28th October 2008
Abstract
Photogeneration of singlet oxygen (1O2) by rose bengal is improved through the use of a porous monolithic polymer (PMP) as a support, as compared to a classic gel-type resin matrix. This type of monolithic polymeric matrix can be made at a multigram scale in quantitative yields enabling the preparation of large amounts of supported photosensitizer at low cost. The singlet oxygen induced oxidation of 9,10-diphenylanthracene has been used as a benchmark reaction, and a comparative study using rose bengal in solution, entrapped within gel-type derived polymer and entrapped within a porous monolithic polymer (PMP) has been performed. The enhanced photoreactivity of the PMP-rose bengal conjugates has been utilised for the successful photodynamic therapy (PDT) of melanoma cells.
Introduction
Oxidations are of broad interest, especially when performed using molecular oxygen (3O2) as a reactant. However the reactivity of 3O2 is limited, and a large research effort has been focused on the development of new oxidation methods.1 In this context, singlet oxygen (1O2), which can be obtained from 3O2, has been shown to be useful in diverse areas such as synthetic chemistry2 and biomedical sciences.3 The photochemical generation of singlet oxygen from 3O2 using a suitable photosensitizer can be achieved both in solution and in heterogeneous media. In the heterogeneous case, the pioneering work of Schaap and Neckers4 with rose bengal5 immobilized onto low crosslinked polystyrene beads (Merrifield resin) has shown the utility of the approach. In addition to polystyrene beads and rose bengal, other solid supports and photosensitizers have been used to generate singlet oxygen. Thus, recent representative examples of organic and inorganic matrices are polyacrylamine resins,6 polycyanoacrylates,7silica8 and gold9 nanoparticles, magnetic carriers,10and quantum dots.11 Even C60fullerene has been incorporated into zeolites12 in order to create a solid source of 1O2. Many other supports and photosensitizers have been used which have been reported in recent reviews.13,14 Specifically, the immobilization of rose bengal is covered in detail in the review by De Vos et al.14Some of the materials that have been used as supports for photosensitizers require complex and expensive synthetic routes or the reactions are unable to generate multigram quantities of the supported photosensitizer. One type of support, to the best of our knowledge not employed for the immobilization of singlet oxygen photosensitizers, are the so called “porous monolithic polymers” (PMP).15–17 Such polymers are obtained by unstirred mass polymerisation in the presence of a porogen. The resulting materials consist of a continuous polymer-phase with a high inner porosity. Various applications of these materials for the development of polymer-supported catalytic processes have been reported, in particular taking advantage of their excellent properties for flow processes.18
Here we show how immobilization of rose bengal on microparticles derived from a PMP can provide additional benefits for the generation of singlet oxygen, as compared to the rose bengal in solution and to rose bengal supported on the widely employed Merrifield-type resin (gel-type). The resultant photoactive materials, produced at a multigram scale and in a quantitative fashion, are shown here to be active for the generation of 1O2 as exemplified by two paradigmatic applications, one from the synthetic realm and another one from the biomedical field. Firstly, the microparticles have been found to induce effectively the photooxidation of 9,10-diphenylanthracene (DPA) to its endoperoxide. Secondly, the porous monolithic polymer supported photosensitizer has been used to induce the photodynamic destruction of melanoma cancer cells.
Experimental
Materials and methods
p-Chloromethylstyrene (Aldrich, 90%), divinylbenzene (DVB, Fluka, ∼80% mixture of isomers; the residual is composed mainly of 1,3- and 1,4-ethylstyrene isomers), 2,2′-azobis(isobutyronitrile) (AIBN, Fluka, ≥98.0%), rose bengal sodium salt (Fluka), tetrabutylammonium hydroxide solution ∼25% in MeOH (∼0.8 M) (TBAOH solution, Fluka), 9,10-diphenylanthracene (DPA, Fluka, ≥98.0%), 1-dodecanol (Aldrich, 98%), tetrahydrofuran (Scharlau, synthesis grade), ethyl acetate (Scharlau, synthesis grade), ethanol (Scharlau, 96%), methanol (Scharlau, synthesis grade), methanol (Scharlau, spectroscopy grade), 1,4-dioxane (Scharlau, spectroscopy grade) were used as received. Toluene (Scharlau, synthesis grade) was dried over 4 Å molecular sieves. N,N′-dimethylformamide was treated previously with anhydrous MgSO4.To characterize the polymeric materials and to perform the photochemical assays the following techniques were used: Fourier-transform infrared spectroscopy (FT-IR) spectra were recorded using a Perkin Elmer System 2000 FT-IR spectrometer. FT-Raman spectra were recorded using a Perkin Elmer Spectrum 2000 NIR FT-Raman spectrometer. Diffuse reflectance UV-vis absorption spectra were recorded using a Perkin Elmer Lambda 19 spectrophotometer. UV-vis absorption spectra were recorded using a Hewlett-Packard 8453 spectrophotometer. Steady-state fluorescence spectra were acquired using a Spex Fluorolog 3-11 equipped with a 450 W Xenon lamp. Scanning electron micrographs were taken on a LEO 440I microscope equipped with a digital camera. The samples were placed on top of a tin plate and sputtered with Au/Pd in a Polaron SC7610 Sputter Coater from Fisons Instruments. The particle size distribution of the synthesized polymer was determined by means a MASTERSIZER 2000 (MALVERN) laser diffraction instrument. To perform the measurements the sample was suspended in MeOH. The data were analyzed with the software supplied with the instrument.
The polymer Pm (5.77 g, 7.5 mmol of –CH2Cl) and rose bengal sodium salt (8.81 g, 8.66 mmol) were mixed in a 500 ml round-bottom flask and stirred in 400 ml of DMF (treated previously with anhydrous MgSO4) at 80 °C for 8 h in a nitrogen atmosphere. Then, the reaction mixture was cooled to ambient temperature and filtered through a sintered-glass funnel. The obtained resin, Pm-RB, was washed with 250 ml portions of the following solvents: DMF, ethyl acetate, ethanol, ethanol:water 1:1, water, methanol:water 1:1 and methanol. Next, the polymer was extracted with methanol in a Soxhlet apparatus until no visible colour appeared in the solvent. Finally, the light pink polymeric particles were dried in a vacuum oven. M = 5.50 g. FTIR (KBr) 3020, 2925, 1603, 1266, 710 cm−1; FT-Raman 3055, 2909, 1631, 1611, 1512 (RB grafting, low intensity) 1266, 1000, 641 cm−1; RB loading (hydrolysis reaction) = 2 μmol RB g−1 of resin; UV-vis diffuse reflectance: λmax = 572 nm; Fluorescence emission: λmax = 593 nm (550 nm exc.).
Photochemical experiments
Biological evaluation
Results and discussion
A porous monolithic polymer containing reactive chloromethyl groups was synthesized by polymerization of a mixture of divinylbenzene and chloromethylstyrene (80:20 molar) in the presence of dodecanol:toluene (5:1, w:w) as the porogens (3:2 w:w, porogens:monomers ratio).15,18 This procedure led to a continuous rod of several cm length and ca. 1 cm width. This material (Pm) was then ground to obtain small-sized particles. Reaction of this powdered resin (1.3 mmol Cl g−1polymer) with rose bengal (disodium salt)4 in DMF at 80 °C for 8 h lead to the resin Pm-RB displaying a pink colour that was indicative of the anchoring of rose bengal to the polymer (see Scheme 1). It is worth noting that the Pm bound rose bengal was obtained in a multigram scale (5–6 g) in a four-step process, which represents an important advantage over other time-consuming methodologies that may lead to sophisticated matrices but which may be only available at the milligram, or lower, scale. Additionally, while many types of 1O2 generators have been developed to date, the well-known rose bengal is still used for the generation of 1O2 when anchored to solid supports,19 and hence we selected this dye as an appropriate photosensitizer for this study. However, the method presented herein should be compatible with any other photosensitizers of interest, if stable at the grafting conditions.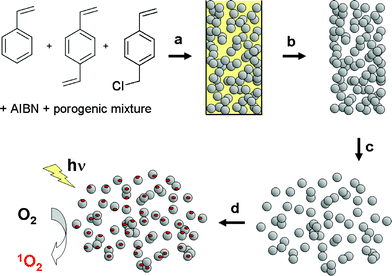 | ||
| Scheme 1 (a) Polymerisation of the vinyl monomers. (b) Unmoulding and extraction of the porogenic mixture. (c) Dissaggregation of the porous monolithic polymer rod. (d) Derivatization with rose bengal. | ||
Steady-state fluorescence and diffuse-reflectance UV-vis spectroscopies were used to confirm the presence of rose bengal attached to Pm which can be seen from the typical spectral signature of rose bengal (see ESI,† Figure S1). The recorded fluorescence and absorption maxima are located at: λem = 593 nm, λexc = 572 nm, λdr-uv = 572 nm respectively.
The basic hydrolysis of rose bengal from the polymeric support and the UV-vis measurement of the hydrolisate enabled determination of the content of the photosensitizer to be 2 μmol g−1 on the Pm-RB. Low efficiencies for the grafting procedure have been reported in such monolithic polymers and hence the low loading in Pm-RB could be due to the extensive crosslinking of this macroporous material.15,17
Optical and scanning electron microscopy (SEM, see ESI,† Figure S2 and S3 respectively) revealed that Pm-RB consisted of clusters of small microglobules, with each globule ca. 1 μm in diameter. The analysis of Pm-RB by wet laser diffraction afforded a size distribution with a maximum peak at 12.4 μm (see ESI,† Figure S4). The polymeric photosensitizer appears to be composed of three populations of large (ca. 12 μm), medium (ca. 3 μm) and small (ca. 0.5 μm) particles.
In order to demonstrate the ability of the Pm-RB photosensitizer to generate 1O2, the new material was used as a photocatalyst for the chemical oxidation of a well-known 1O2 acceptor, and as a photodynamic agent for the induction of cell death in a culture of melanoma cells. The oxidation of 9,10-diphenylanthracene (DPA) with 1O2 yields a transannular endoperoxide as shown in Scheme 2. This acceptor was chosen because of its high reaction rate constant20 with 1O2 and the fact that it does not absorb at the wavelength used for irradiation of the samples (556 ± 7 nm). Kinetic experiments were performed by monitoring the bleaching of the absorption band of DPA in methanol (350–400 nm) as a function of the irradiation time and at several concentrations of Pm-RB (Fig. 1). Rose bengal in solution, as its disodium salt, was used as a control.
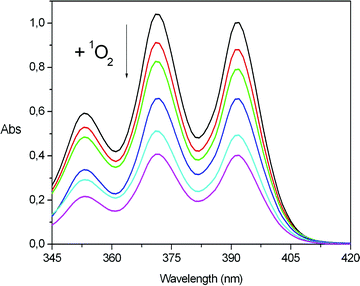 | ||
| Fig. 1 Representative kinetic experiment. Bleaching of the absorption bands of DPA (4 × 10−5 M initially, in MeOH) when irradiated using Pm-RB (6.65 mg ml−1) as a photosensitizer. The spectra correspond to the following irradiation times (from top to bottom): 0, 5, 10, 15, 20 and 25 min. | ||
 | ||
| Scheme 2 Singlet oxygen oxidation of DPA to its endoperoxide. | ||
Irradiation of all the samples was achieved by passing the light of a Xe lamp through a monochromator in order to select the 556 ± 7 nm window, as described in the experimental section. The resulting kinetic profiles were linear with time for both rose bengal and Pm-RB (Fig. 2 and 3 respectively). As shown in Fig. 4, the oxidation of DPA sensitized by increasing amounts of rose bengal in solution, attained a limiting observed constant of kobs∼ 7.5 × 10−3 min−1. However, when the oxidation of DPA was sensitized by the functional macroporous resin Pm-RB, the maximum kobs measured was 3.7 × 10−2 min−1. In order to eliminate the possibility that physical adsorption of DPA onto the polymer surface was the cause of the reduction of DPA from the solution, the corresponding dark experiments using Pm-RB were conducted, with no change in the DPA absorption band observed (Fig. 3a and b ). Additionally, it should be noted that no rose bengal was detected in the solution after irradiation of Pm-RB, which removes the possibility that sensitisation was induced by the leaching of the rose bengal photosensitizer from the matrix.
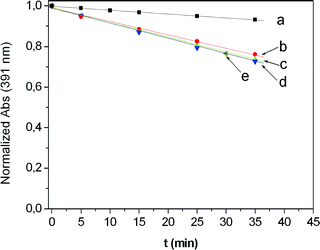 | ||
| Fig. 2 Irradiation of DPA (4 × 10−5M, MeOH) in the presence of rose bengal in solution at several concentrations of sensitizer. (a) 1.67 × 10−6 M; (b) 6.64 × 10−6 M; (c) 1.44 × 10−5 M; (d) 2.93 × 10−5 M; (e) 5.86 × 10−5 M. | ||
 | ||
| Fig. 3 Irradiation of DPA (4 × 10−5M, MeOH) in the presence of Pm-RB at several concentrations, including two dark controls (a) and (b). (a) 1.33 mg ml−1 (2.66 × 10−6 M equiv. RB); (b) 4.00 mg ml−1 (8.00 × 10−6 M equiv. RB); (c) 0.67 mg ml−1 (1.34 × 10−6 M equiv. RB); (d) 1.33 mg ml−1 (2.66 × 10−6 M equiv. RB); (e) 2.67 mg ml−1 (5.34 × 10−6 M equiv. RB); (f) 4.00 mg ml−1 (8.00 × 10−6 M equiv. RB); (g) 6.65 mg ml−1 (1.33 × 10−5 M equiv. RB); (h) 13.30 mg ml−1 (2.66 × 10−5 M equiv. RB). | ||
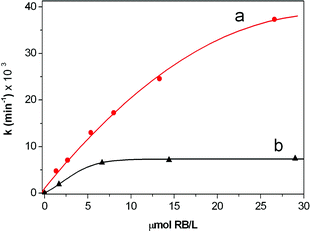 | ||
| Fig. 4 Kinetics of the photooxidation of DPA in methanol. kobsvs. concentration of rose bengal (which was estimated in the case of supported RB, according to the loading of rose bengal of P-RB and to the added amount of resin in each experiment.) (a) Pm-RB; (b) rose bengal in solution. | ||
An increase in the 1O2 quantum yield generation by Pm-RB relative to rose bengal in solution is not expected since rose bengal in methanol is known to possess a rather high efficiency for the generation of this reactive species (ϕΔ = 0.76).5 Thus the differences in kobs cannot be directly translated into differences in ϕΔ, since the obtained value for Pm-RB would be higher than unity. The heterogeneous nature of Pm-RB together with its morphology could account for the apparent increased efficiency of oxidation. Most likely, light scattering by the microparticles plays a significant role which may explain the results. On the other hand a higher local concentration of reactive species at the surface of the polymeric sensitizer (reversible and transient surfacial adsorption of DPA onto the microspheres, or longer lifetime of 1O2 close to the hydrophobic polymer matrix), could have also some effect.
A rose bengal coated gel-type resin (Pg-RB) was also prepared, characterized and used for the oxidation of DPA. The beads of this kind of polymer have a significantly greater size (in our case 40–70 μm diameter) than the Pm-RB (12.4 μm diameter) and also a lower permanent specific surface, and hence it would not be expected to produce the enhanced singlet oxygen production through the effects of light scattering and/or transient surfacial adsorption. The maximum observed rate for the oxidation of DPA promoted by Pg-RB was of 6 × 10−3 min−1 (see ESI,† Figure S5). In order to illustrate the size of the gel-type resin, a SEM micrograph of Pg-RB is shown in Figure S3,† which can be compared to Figure S2.† In Table 1 the kinetic constants measured for the rose bengal in solution and both the gel-type polymer and the porous monolithic polymer supports are shown.
| Photosensitizer | Conc. Polymer/mg ml−1 | Conc. RB/Ma | k/10−3 min−1 |
|---|---|---|---|
| a Calculated in the case of supported RB according to the loading of RB and to the added amount of resin in each experiment. | |||
| RB | — | 1.67 × 10−6 | 1.9 |
| — | 6.64 × 10−6 | 6.6 | |
| — | 1.44 × 10−5 | 7.2 | |
| — | 2.93 × 10−5 | 7.5 | |
| — | 5.86 × 10−5 | 7.4 | |
| Pm-RB | 0.67 | 1.34 × 10−6 | 4.8 |
| 1.33 | 2.66 × 10−6 | 7.1 | |
| 2.67 | 5.34 × 10−6 | 13.0 | |
| 4.00 | 8.00 × 10−6 | 17.3 | |
| 6.65 | 1.33 × 10−5 | 24.6 | |
| 13.30 | 2.66 × 10−5 | 37.3 | |
| Pg-RB | 4.33 | 6.90 × 10−4 | 2.9 |
| 8.33 | 1.33 × 10−3 | 4.2 | |
| 16.67 | 2.67 × 10−3 | 6.3 | |
| 33.33 | 5.33 × 10−3 | 5.8 |
Since singlet oxygen is important for biomedical applications, the utility of the newly developed polymeric material was tested for the destruction of cancerous cells. Photodynamic therapy (PDT)3 of cancer involves the uptake of non-toxic photosensitizers in tumour tissues and subsequent activation by light to generate cytotoxic 1O2. Particles of Pm-RB were incubated with a culture of melanoma (B16/F10) cells. The uptake of the particles by the cells following 16 h incubation was established by confocal fluorescence microscopy taking advantage of the intrinsic fluorescence of the photosensitizer (ϕF = 0.08 in MeOH).5 The combined differential interference contrast (DIC) and fluorescence microscopy images show the presence of the Pm-RB particles distributed within, or perhaps on the membrane surface, of the melanoma cells (Fig. 5). From this figure it appears that only the smaller sized particles have entered the cells.
 | ||
| Fig. 5 Confocal laser scanning microscopy images of melanoma cells with microparticles of Pm-RB. Left hand side; DIC (differential interference contrast) images. Centre; fluorescence images. Right hand side; the combined DIC and fluorescence images. Scale bars = 10 μm. | ||
A sample of the Pm-RB incubated cells was irradiated in order to test the ability of the microparticles to induce cell-death. Parameters for PDT were fixed based on our previously reported results using phthalocyanine stabilised gold nanoparticles.9 A final concentration of 10 ng ml−1 of Pm-RB was incubated for ca. 16 hours and a period of irradiation of the cells of 10 min using the 488 (Ar) and 543 nm (HeNe) lasers at full power. The efficiency of PDT was demonstrated by imaging cells before (Fig. 6A) and after (Fig. 6B) treatment and studying the viability as a function of the cell morphology. Hence, 90 min after PDT, the morphology of the melanoma cells changed dramatically. Cells began to show the presence of blebs (black arrows in Fig. 6B) and vacuoles (white arrows in Fig. 6B) as well as condensation of the nucleus (red arrow in Fig. 6B). These cellular features are not present in ‘healthy’ melanoma cells before treatment and are indicative of cell mortality induced by photodynamic action.
 | ||
| Fig. 6 Combined confocal fluorescence and DIC (differential interference contrast) images of: (A) a pre-irradiation image of Pm-RB particles (10 ng ml−1) incubated with melanoma (B16/F10) cells for 16 h and (B) 90 min post-irradiation image of B16/F10 melanoma cells. The cellular morphology in (B) shows the presence of blebs and vacuoles (white arrows), structures which are absent in the pre-PDT treated cells shown in (A). Scale bar = 10 μm. | ||
Kochevar et al. have shown that activation of rose bengal by visible light produces predominately singlet oxygen and consequently observed extensive apoptosis in HL-60 cells.21 A cell undergoing apoptosis shows a characteristic morphology, the cell membrane shows irregular buds (blebs), the cytoplasm appears dense, chromatin undergoes condensation into compact patches and the organelle appear tightly packed.22 The cellular features, which were all observed (Fig. 6B) under our experimental conditions, suggest that mortality of the melanoma cells was induced by apoptosis, although other mechanisms cannot be disregarded.23
Control experiments were performed using Pm, the matrix used to synthesize Pm-RB but without grafted rose bengal. The parameters used were the same as above and the combined DIC and fluorescence microscopy images (see ESI,† Figure S6) clearly show the presence of non-fluorescent particles (white arrows) within the cells. Following irradiation the cellular morphology did not change (Figure S6), indicating that the melanoma cell remains viable. Melanoma cells were also subjected to the same irradiation treatment but with no particles at all. Combined DIC and fluorescence microscopy images showed the absence of changes in the cellular morphology following laser irradiation. These two control experiments confirm the induction of photodynamic mortality of the melanoma cells by the porous monolithic polymer bound photosensitizer.24
Conclusion
In summary, we have shown that porous monolithic polymers can be used as an insoluble support for photooxidation reactions through the grafting of a prototypical 1O2 generator such as rose bengal. In this way, excellent photocatalytic particles of Pm-RB can be prepared. These PMP photosensitizers are able to generate 1O2 efficiently as shown with the oxidation of DPA and subsequently, the photodynamic destruction of melanoma cells. The effect of particle size, crosslinking degree, oxygen solubility in the matrix (both 3O2 and 1O2) and adsorption of reactants on the surface of the photosensitizer could contribute to the photocatalytic efficiency. Low cost and multigram scale of photosensitizer production are two advantages of PMPs over other classes of supports for oxidations with 1O2. In comparison with the classical rose bengal-coated gel-type resin, and to rose bengal in solution, the PMP matrix displays a significantly higher oxidation efficiency of DPA in methanol. While gel-type rose bengal-loaded resin and rose bengal in solution attained a limiting value for their rate constants of 6 × 10−3 min−1 and 7.5 × 10−3 min−1, respectively, for the oxidation of DPA, the same process using Pm-RB can be carried out with a rate constant of up to 3.7 × 10−2 min−1. The singlet oxygen induced destruction of melanoma cells using the polymer supported rose bengal (Pm-RB) has shown the utility of such porous monolithic polymers as a suitable support for the delivery of photosensitizer agents for photodynamic therapy.Acknowledgements
Financial support from the Spanish MEC (CTQ2005-08016-003), GVA (projects GV/2007/277, ARVIV/2007/079, ARVIV/2007/081) and F. Caixa Castelló-UJI (project P1·1A2007-05) is acknowledged. D.A.R. acknowledges financial support from Cancer Research UK (Grant number C22031/A7097). F.G. thanks the support from MEC (Ramón y Cajal Program). R.G. thanks the support from MEC (FPI). The technical support from SCIC (UJI) is also acknowledged.Notes and references
- L. Alaerts, J. Wahlen, P. A. Jacobs and D. E. De Vos, Recent progress in the immobilization of catalysts for selective oxidation in the liquid phase, Chem. Commun., 2008, 1727–1737 RSC.
- E. L. Clennan and A. Pace, Advances in singlet oxygen chemistry, Tetrahedron, 2005, 61, 6665–6691 CrossRef CAS; M. C. DeRosa and R. J. Crutchley, Photosensitized singlet oxygen and its applications, Coord. Chem. Rev., 2002, 233, 351–371 CrossRef.
- Nanomaterials for Cancer Therapy, ed. C. S. S. R. Kumar, Wiley-VCH, Weinheim, 2006 Search PubMed; A. Juzeniene, Q. Peng and J. Moan, Milestones in the development of photodynamic therapy and fluorescence diagnosis, Photochem. Photobiol. Sci., 2007, 6, 1234–1245 Search PubMed; I. J. Macdonald and T. J. Dougherty, Basic principles of photodynamic therapy, J. Porph. Phthal., 2001, 5, 105–129 RSC.
- E. C. Bloosey, D. C. Neckers, A. L. Thayer and A. P. Schaap, Polymer-based sensitizers for photooxidations, J. Am. Chem. Soc., 1973, 95, 5820–5822 CrossRef CAS; A. P. Schaap, A. L. Thayer, E. C. Blossey and D. C. Neckers, Polymer-based sensitizers for photooxidations. II, J. Am. Chem. Soc., 1975, 97, 3741–3745 CrossRef CAS; J. Paczkowski and D. C. Neckers, Polymer-based sensitizers for the formation of singlet oxygen. New studies of polymeric derivatives of rose bengal, Macromolecules, 1985, 18, 1245–1253 CrossRef CAS; J. Paczkowski and D. C. Neckers, Photochemical properties of rose bengal. 11. Fundamental studies in heterogeneous energy transfer, Macromolecules, 1985, 18, 2412–2418 CrossRef CAS; A. P. Schaap, A. L. Thayer, K. A. Zaklika and P. C. Valenti, Photooxygenations in aqueous solution with a hydrophilic polymer-immobilized photosensitizer, J. Am. Chem. Soc., 1979, 101, 4016–4017 CrossRef CAS.
- D. C. Neckers, Rose Bengal, J. Photochem. Photobiol. A: Chem., 1989, 47, 1–29 CrossRef CAS; C. R. Lambert and I. E. Kochevar, Does rose bengal triplet generate superoxide anion?, J. Am. Chem. Soc., 1996, 118, 3297–3298 CrossRef CAS.
- M. J. Moreno, E. Monson, R. G. Reddy, A. Rehemtulla, B. D. Ross, M. Philbert, R. J. Schneider and R. Kopelman, Production of singlet oxygen by Ru(dpp(SO3)2)3 incorporated in polyacrylamide PEBBLES, Sens. Actuators, B, 2003, 90, 82–89 CrossRef; W. Tang, H. Xu, R. Kopelman and M. A. Philbert, Photodynamic characterization and in vitro application of methylene blue-containing nanoparticle platforms, Photochem. Photobiol., 2005, 81, 242–249 CrossRef CAS; D. Gao, H. Xu, M. A. Philbert and R. Kopelman, Ultrafine hydrogel nanoparticles: synthetic approach and therapeutic application in living cells, Angew. Chem. Int. Ed., 2007, 46, 2224–2227 CrossRef CAS.
- A. Labib, V. Lenaerts, F. Chouinard, J. C. Leroux, R. Ouellet and J. E. van Lier, Biodegradable nanospheres containing phthalocyanines and naphthalocyanines for targeted photodynamic tumor therapy, Pharm. Res., 1991, 8, 1027–1031 CrossRef CAS; E. Allemann, J. Rousseau, N. Brasseur, V. S. Kudrevich, K. Lewis and J. E. van Lier, Photodymamic therapy of tumours with hexadecafluoro zinc phthalocyanine formulated in peg-coated poly(lactic acid) nanoparticles, Int. J. Cancer, 1996, 66, 821–824 CrossRef.
- F. Yan and R. Kopelman, The embedding of meta-tetra(hydroxyphenyl)-chlorin into silica nanoparticle platforms for photodynamic therapy and their singlet oxygen production and pH-dependent optical properties, Photochem. Photobiol., 2003, 78, 587–591 CrossRef CAS; I. Roy, T. Y. Ohulchanskyy, H. E. Pudavar, E. J. Bergey, A. R. Oseroff, J. Morgan, T. J. Dougherty and P. N. Prasad, Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: a novel drug-carrier system for photodynamic therapy, J. Am. Chem. Soc., 2003, 125, 7860–7865 CrossRef CAS; T. Y. Ohulchanskyy, I. Roy, L. N. Goswami, Y. Chen, E. J. Bergey, R. K. Pandey, A. R. Oseroff and P. N. Prasad, Organically modified silica nanoparticles with covalently incorporated photosensitizer for photodynamic therapy of cancer, Nano Lett., 2007, 7, 2835–2842 CrossRef CAS; S. Kim, T. Y. Ohulchanskyy, H. E. Pudavar, R. K. Pandey and P. N. Prasad, Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy, J. Am. Chem. Soc., 2007, 129, 2669–2675 CrossRef CAS.
- D. C. Hone, P. I. Walker, R. Evans-Gowing, S. FitzGerald, A. Beeby, I. Chambrier, M. J. Cook and D. A. Russell, Generation of cytotoxic singlet oxygen via phthalocyanine-stabilized gold nanoparticles: a potential delivery vehicle for photodynamic therapy, Langmuir, 2002, 8, 2985–2987 CrossRef CAS; M. E. Wieder, D. C. Hone, M. J. Cook, M. M. Handsley, J. Gavrilovic and D. A. Russell, Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: cancer therapy using a ‘Trojan horse’, Photochem. Photobiol. Sci., 2006, 5, 727–734 RSC.
- D. B. Tada, L. L. R. Vono, E. L. Duarte, R. Itri, P. K. Kiyohara, M. S. Baptista and Liane M. Rossi, Methylene blue-containing silica-coated magnetic particles: a potential magnetic carrier for photodynamic therapy, Langmuir, 2007, 23, 8194–8199 CrossRef CAS.
- L. Shi, B. Hernandez and M. Selke, Singlet oxygen generation from water-soluble quantum dot-organic dye nanocomposites, J. Am. Chem. Soc., 2006, 128, 6278–6279 CrossRef CAS; A. C. S. Samia, X. Chen and C. Burda, Semiconductor quantum dots for photodynamic therapy, J. Am. Chem. Soc., 2003, 125, 15736–15737 CrossRef CAS.
- M. S. Galletero, H. García and J. L. Bourdelande, Dramatic persistence (minutes) of the triplet excited state and efficient singlet oxygen generation for C60 incorporated in Y zeolite and MCM-41 silicate, Chem. Phys. Lett., 2003, 370, 829–833 CrossRef CAS.
- S. Wang, R. Gao, F. Zhou and M. Selke, Nanomaterials and singlet oxygen photosensitizers: potential applications in photodynamic therapy, J. Mater. Chem., 2004, 14, 487–493 RSC.
- J. Wahlen, D. E. De Vos, P. A. Jacobs and P. L. Alsters, Solid materials as sources for synthetically useful singlet oxygen, Adv. Synth. Catal., 2004, 346, 152–164 CrossRef CAS.
- F. Svec and J. M. J. Fréchet, New designs of macroporous polymers and supports: from separation to biocatalysis, Science, 1996, 273, 205–211 CrossRef CAS; D. C. Sherrignton, Preparation, structure and morphology of polymer supports, Chem. Commun., 1998, 2275–2286 RSC; Synthesis and separations using functional polymers, ed. D. C. Sherrington and P. Hodge, John Wiley & Sons Ltd, Chichester, 1988 Search PubMed; Polymeric materials in organic synthesis and catalysis, ed. M. R. Buchmeiser, Wiley-VCH, Weinheim, 2003 Search PubMed.
- A. Chighine, G. Sechi and M. Bradley, Tools for efficient high-throughput synthesis, Drug Disc. Today, 2007, 12, 459–464 CrossRef CAS; A. Solinas and M. Taddei, Solid-supported reagents and catch-and-release techniques in organic synthesis, Synthesis-Stuttgart, 2007, 2409–2453 Search PubMed; B. M. L. Dioos, I. F. J. Vankelecom and P. A. Jacobs, Aspects of immobilisation of catalysts on polymeric supports, Adv. Synth. Catal., 2006, 348, 1413–1446 CrossRef CAS; A. Chesney, Selected highlights in the application of ion-exchangers: as supports for reagents in organic synthesis, Green Chem., 1999, 1, 209–219 RSC.
- F. Svec and J. M. J. Fréchet, Continuous rods of macroporous polymer as high-performance liquid chromatography separation media, Anal. Chem., 1992, 64, 820–822 CrossRef CAS; C. Vicklund, F. Svec, J. M. J. Fréchet and K. Irgum, Monolithic, “molded”, porous materials with high flow characteristics for separations, catalysis, or solid-phase chemistry: control of porous properties during polymerization, Chem. Mater., 1996, 8, 744–750 CrossRef CAS; E. C. Peters, F. Svec and J. M. J. Fréchet, Rigid Macroporous Polymer Monoliths, Adv. Mater., 1999, 11, 1169–1181 CrossRef CAS; M. R. Buchmeiser, Polymeric monolithic materials: syntheses, properties, functionalization and applications, Polymer, 2007, 48, 2187–2198 CrossRef CAS.
- M. I. Burguete, A. Cornejo, E. García-Verdugo, J. García, M. J. Gil, S. V. Luis, V. Martinez-Merino, J. A. Mayoral and M. Sokolova, Bisoxazoline-functionalised enantioselective monolithic mini-flow-reactors: development of efficient processes from batch to flow conditions, Green Chem., 2007, 9, 1091–1096 RSC; M. I. Burguete, A. Cornejo, E. García-Verdugo, M. J. Gil, S. V. Luis, J. A. Mayoral, V. Martinez-Merino and M. Sokolova, Pybox monolithic miniflow reactors for continuous asymmetric cyclopropanation reaction under conventional and supercritical conditions, J. Org. Chem., 2007, 72, 4344–4350 CrossRef CAS; N. Karbass, V. Sans, E. García-Verdugo, M. I. Burguete and S. V. Luis, Pd(0) supported onto monolithic polymers containing IL-like moieties. Continuous flow catalysis for the Heck reaction in near-critical EtOH, Chem. Commun., 2006, 3095–3097 RSC; G. Jas and A. Kirschning, Continuous flow techniques in organic synthesis, Chem. Eur. J., 2003, 9, 5708–5723 CrossRef CAS; M. I. Burguete, E. García-Verdugo, M. J. Vicent, S. V. Luis, H. Pennemann, N. Graf, von Keyserling and J. Martens, New Supported β-Amino Alcohols as Efficient Catalysts for the Enantioselective Addition of Diethylzinc to Benzaldehyde under Flow Conditions, Org. Lett., 2002, 4, 3947–3950 CrossRef CAS; B. Altava, M. I. Burguete, J. M. Fraile, J. I. Garcia, S. V. Luis, J. A. Mayoral and S. V. Luis, How important is the inert matrix of supported enantiomeric catalysts? Reversal of topicity with two polystyrene backbones, Angew. Chem. Int. Ed., 2000, 39, 1503–1506 CrossRef CAS.
- J. M. Tsay, M. Trzoss, L. Shi, X. Kong, M. Selke, M. E. Jung and S. Weiss, Singlet oxygen production by peptide-coated quantum dot-photosensitizer conjugates, J. Am. Chem. Soc., 2007, 129, 6865–6871 CrossRef CAS.
- W. Fudickar and T. Linker, Remote substituent effects on the photooxygenation of 9,10-diarylanthracenes: strong evidence for polar intermediates, Chem. Commun., 2008, 1771–1773 RSC; B. M. Monroe, Rates of reaction of singlet oxygen with olefins, J. Phys. Chem., 1978, 82, 15–18 CrossRef CAS.
- I. E. Kochevar, M. C. Lynch, S. G. Zhuang and C. R. Lambert, Singlet oxygen, but not oxidizing radicals, induces apoptosis in HL-60 cells, Photochem. Photobiol., 2000, 72, 548–553 CAS.
- G. Hacker, The morphology of apoptosis, Cell Tissue Res., 2000, 301, 5–17 CrossRef CAS.
- G. Majno and I. Joris, Apoptosis, oncosis and necrosis. An overview of cell death, Am. J. Pathol., 1995, 146, 3–15 Search PubMed.
- The reported experimental results were qualitatively reproducible. In order to achieve a quantative assessment of the phototoxicity induced by the new materials, a cell viability assay, such as the MTT assay (9b), should be used.
Footnote |
| † Electronic supplementary information (ESI) available: Fig S1–S6. See DOI: 10.1039/b810921d |
| This journal is © The Royal Society of Chemistry and Owner Societies 2009 |
