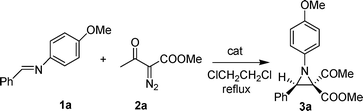
Catalyzed addition of diazoacetoacetates to imines: synthesis of highly functionalized aziridines†
Received
7th August 2008
, Accepted 14th October 2008
First published on 10th November 2008
1. Introduction
Aziridines are highly useful synthetic intermediates in organic synthesis and structural units of many natural products and drugs.1 Various synthetic methods of aziridines,2 including several asymmetric versions,3 have been reported. Among them, the addition of carbenes generated from diazo compounds to imines is highly attractive considering the anticipated good yield, high product stereoselectivity, and ease of procedure. Transition metal complexes,4 Lewis acids,5 Brønsted acids,6lithium perchlorate7 and ionic liquids8 have been found to promote efficiently the addition of diazoacetates to imines. The corresponding aziridinecarboxylates are prepared generally in good yields. In addition to diazoacetates, other diazo compounds such as TMSCHN2,9 diazophosphonates,10 phenyldiazoactetates and styryldiazoacetates11 were also successfully applied. On the other hand, addition of diazoacetoacetates to imines is also an attractive research subject and the reaction provides highly functionalized aziridines. They can be used to prepare β-hydroxy-α-amino acids, which are important synthetic intermediates for many natural products and drugs.12 In addition they are susceptible to ring opening reaction with various nucleophilic agents to provide a number of useful compounds.13 However, in a previous study the addition of diazoacetoacetates to imines failed to provide desired aziridines, probably due to their lower reactivity compared to diazoacetates.5g In this paper we report the rhodium-catalyzed reaction of diazoacetoacetates with imines derived from p-methoxyaniline. Highly functionalized aziridines are obtained in good yield and with excellent cis-selectivity.
2. Results and discussion
2.1 Study of catalysts and reaction conditions
The reaction of N-benzylidene-4-methoxyaniline (1a) with methyl diazoacetoacetate (2a) was studied in the presence of rhodium and copper catalysts. The results are summarized in Table 1. The use of Rh2(OAc)4 provided the expected aziridine product in good yield and excellent stereoselectivity. Only the cis-isomer 3a was observed and its relative configuration was determined by NOE experiment. The amount of diazo compound exerted a significant effect on the yield of 3a. When 1.0 equivalent, 1.5 equivalents and 2.0 equivalents of 2a were used, the yield of 3a increased from 69% to 87%, and to 92%, respectively (Table 1, entries 1–3). This result implied the partial loss of 2a under the reaction conditions. The higher reaction temperature of 1,2-dichloroethane (b.p. 81 °C) was favorable, since lower yield of 3a was obtained in dichloromethane (b.p. 39 °C) (Table 1, entry 4 vs. entry 1).
Table 1 Addition of 2a to 1a catalyzed by rhodium and copper catalysts.a
| Entry |
Catalyst
|
Time (h) |
Yield (%)b |
|
The reactions were carried out in refluxing 1,2-dichloroethane with 1a (1 mmol) and 2a (1.5 mmol).
Isolated yield based on 1a.
2 mmol 2a was used.
1mmol 2a was used.
CH2Cl2 was used as the reaction solvent.
|
| 1 |
Rh2(OAc)4 (1 mol%) |
2 |
87 |
| 2 |
Rh2(OAc)4 (1 mol%) |
2 |
92c |
| 3 |
Rh2(OAc)4 (1 mol%) |
2 |
69d |
| 4 |
Rh2(OAc)4 (1 mol%) |
2 |
35e |
| 5 |
Cu(hfacac)2 (3 mol%) |
2 |
30 |
| 6 |
Cu(acac)2 (3 mol%) |
2 |
30 |
| 7 |
CuPF6 (3 mol%) |
3 |
20 |
| 8 |
Cu(OTf)2 (3 mol%) |
6 |
∼0 |
Use of copper salts such as Cu(hfacac)2, Cu(acac)2 and CuPF6 gave low yields of 3a, but Cu(OTf)2 was totally ineffective in this reaction. Since Cu(OTf)2 is the strongest Lewis acid among the tested copper salts, the result suggested that the reaction did not proceed through the Lewis acid catalytic addition of the diazo compound to the imine. A metal carbene route is consistent with this information.14
2.2 The effect of electronic property of imines
Furthermore imines 1a–1c with different electronic properties were examined in the reaction with 2a, and the results are illustrated in Scheme 1. The electronic properties of imines show profound effects on the reaction. Imine 1c with a strong electron-withdrawing group (4-NO2) was completely inactive. In contrast imine 1a with an electron-donating group (4-MeO) provided aziridine 3a in good yield. Unsubstituted imine 1b gave 51% yield of aziridine 4. An additional advantage using imine 1a was that 4-MeO-phenyl could be removed readily by oxidization to provide 1-H aziridines in good yield (Scheme 2).11
 |
| | Scheme 1 The reaction of imines 1a–1c with 2a. | |
 |
| | Scheme 2 The reaction of 3a with cerium(IV) ammonium nitrate (CAN). | |
| Entry |
Imine
|
R1 |
Product (yield (%))b |
|
The reactions were carried out in refluxing 1,2-dichloroethane with 1 (1 mmol) and 2a (1.5 mmol).
Isolated yield based on 1.
No reaction.
|
| 1 |
1a
|
Ph |
3a
(87) |
| 2 |
1d
|
p-MeO-Ph
|
3d (99) |
| 3 |
1e
|
o-MeO-Ph
|
3e
(99) |
| 4 |
1f
|
p-NO2-Ph |
—c |
| 5 |
1g
|
p-Cl-Ph
|
3g
(94) |
| 6 |
1h
|
o-Cl–Ph |
3h
(89) |
| 7 |
1i
|
2-naphthyl
|
3i
(97) |
| 8 |
1j
|
2-pyridinyl
|
—c |
2.4 The reaction of imine 1a with a variety of diazo compounds
Furthermore diazoacetoacetone (2b), 2-diazo-1-phenylbutane-1,3-dione (2c), dimethyl diazomalonate (2d) and diazoacetoacetate of chiral alcohols (2e–2i) were prepared (Scheme 3) and examined in the reaction with 1a. The results are summarized in Table 3. Both 2b and 2c gave the corresponding aziridines in good yield. 2d was inactive in the reaction. (1R)-endo-(+)-Fenchol-derived diazoacetoacetate 2e provided aziridine 6 in 77% yield and low diastereoselectivity (d.r.= 1.3:1). (R)-Pantolactone-derived diazoacetoacetate 2f afforded aziridine 7 in 69% yield and better diastereoselectivity (d.r.= 3:1). (L)-Menthol-derived diazoacetoacetate (2g) did not afford the corresponding aziridine product, instead an intramolecular C–H insertion product 10 was obtained in 73% yield (Scheme 4).15, which structure was confirmed by NMR and furthermore by X-Ray diffraction (Fig. 1)16 The chiral diazoacetoacetates 2h and 2i provided complex mixtures of products, from which no substantial amount of aziridines could be isolated. These results suggest that C–H insertion and other side reactions are competitive with the aziridination reaction for diazoacetoacetates derived from chiral alcohols. The structure of chiral alcohols has great effects on the reactivity of the corresponding diazoacetoacetates. The diazoacetoacetates with available C–H bonds to form insertion products of five-membered rings (2g, 2h) or with aromatic C–H bond (2i) failed to give aziridines. For 2e and 2f, no C–H bonds are available to form insertion products of five-membered rings, so good yields of aziridines were obtained. A detailed study of C–H insertion reaction of diazoacetoacetates had been reported by Doyle and coworkers.15
|
Diazo compound
|
R2 |
R3 |
Product (yield (%))b |
|
The reactions were carried out in refluxing dichloroethane with 1a (1 mmol) and 2a–2i (1.5 mmol).
Isolated yield based on 1a.
Mixture of cis/trans-aziridine (1/2.2).
Two diastereoisomers (1.3/1).
Two diastereoisomers (3/1).
|
|
2a
|
Me |
OMe
|
3a
(87) |
|
2b
|
Me |
Me |
6 (88) |
|
2c
|
Me |
Ph |
7 (84)c |
|
2d
|
MeO
|
MeO
|
(0) |
|
2e
|
Me |

|
8 (77)d |
|
2f
|
Me |

|
9 (69)e |
 |
| | Fig. 1 ORTEP drawing of 10. | |
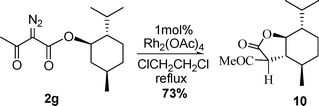 |
| | Scheme 4 Intramolecular C–H insertion of 2g. | |
2.4 Reaction mechanism
A proposed reaction mechanism is illustrated in Scheme 5. Methyl diazoacetoacetate is decomposed by rhodium acetate to generate a metal carbene, which reacts with the imine to provide azomethine ylide. The consequent intramolecular ring-closing step occurs via conformation A to provide the cis-aziridine stereoselectively.4e,11 Conformation B is unfavored due to large steric interactions between the ester group with N-aryl of imine.
 |
| | Scheme 5 The proposed reaction mechanism. | |
3. Conclusion
In conclusion, highly functionalized aziridines were prepared in good yields and excellent stereoselectivities by catalyzed addition of diazoacetoacetates to imines derived from p-methoxyaniline. Dirhodium tetraacetate is a better catalyst than copper salts in this reaction. Diazoacetoacetates of chiral alcohols were also examined and moderate diastereoselectivity was achieved with (R)-pantolactone-derived diazoacetoacetate. The reported reaction provides a new synthetic method of aziridines from diazoacetoacetates and imines.
4. Experimental
General details
NMR spectra were recorded on a Varian Inova 400 MHz instrument and chemical shifts are reported in parts per million (ppm, δ) downfield from the internal standard. Me4Si (TMS, δ = 0) is used as the internal standard for 1H NMR spectra. 13C NMR spectral data are reported relative to the central line of the chloroform signal (δ = 77.00 ppm). High-resolution mass spectra were obtained on JEOL HX110A or GCT-TOF spectrometer. Low-resolution mass spectra were obtained on a Finnigan LC-MS spectrometer. Infrared spectra were recorded on Mattson Alpha-Centauri FT-IR spectrometer, either as a thin film, a nujol mull, or a solution on sodium chloride plates. The absorptions are reported in wave-numbers (cm−1). Element analyses were recorded on carlo-Erba EA1110 CNNO-S instrument. X-ray diffraction was recorded on Rigaku Mercury CCD spectrometer. Thin layer chromatography was performed on Merck Silica Gel 40 F254 glass-backed plates. The visualization was achieved with UV, iodine, or phosphomolybdic acid. Flash column chromatography was performed on 40–63 μm, 230–400 mesh, 60Å silica gel. Dichloromethane and 1,2-dichloroethane were distilled over calcium hydride. Other solvents were used as their commercial anhydrous grade. Chemicals were purchased form Aldrich or Acros chemical company. The imines 1a-1j were prepared by reflux of corresponding aldehydes (1 equivalent) and anilines (1 equivalent) in ethanol for 1–2 hours. Generally crystal imines in good purity were obtained after cooling of the reaction mixture. The diazo compounds 2a-2i were prepared via treatment of corresponding acetoacetates or 1,3-diketones with methanesulfonyl azide and triethylamine according to reported procedures.15
Representative experimental procedure for the catalyzed addition of diazoacetoacetates to imines
A solution of methyl diazoacetoacetate 2a (213 mg, 1.5 mmol) in ClCH2CH2Cl (4 mL) was added via a syringe pump over one hour to a refluxing solution of Rh2(OAc)4 4.4 mg (0.01 mmol) and benzylidene-p-methoxyaniline 1a (211 mg, 1.0 mmol) in ClCH2CH2Cl (8 mL). The resulting solution was allowed to reflux for another hour. The reaction mixture was filtered through a short silica plug, which was washed with ClCH2CH2Cl (10 mL). The solvent was removed and the crude product was purified by flash column chromatography on silica gel (hexane: ethyl acetate = 3: 1) afforded 3a as a colorless oil (284 mg, 87%).
cis-Methyl 1-(4-methoxyphenyl)-2-acetyl-3-phenyl-aziridine-2-carboxylate (3a).
IR (film, cm−1): 1744 (s), 1610 (m), 1511 (s), 1245 (m); 1H NMR (CDCl3, 400 MHz) δ 7.36-7.29 (m, 3 H), 7.28-7.24 (m, 4 H), 6.80 (d, J = 9.0 Hz, 2 H), 4.87 (s, 1 H), 3.73 (s, 3 H), 3.25 (s, 3 H), 1.78 (s, 3 H); 13C NMR (CDCl3, 100 Hz) δ 168.58, 163.49, 156.16, 133.42, 130.75, 128.65, 128.36, 126.60, 118.41, 114.22, 66.06, 65.84, 55.30, 51.66, 17.43; HRMS (FAB) calc. for C19H19NO4 (M+): 325.1314, found: 325.1330.
cis-Mehtyl 2-acetyl-1,3-bis (4-methoxyphenyl)-aziridine-2-carboxylate (3d).
White solid. M. p. 100–102 °C; IR (KBr, cm−1): 1757 (s), 1614 (m), 1514 (s), 1457 (m), 1390 (m); 1H NMR (CDCl3, 400 MHz) δ 7.27 (d, J = 8.8 Hz, 2H), 7.19 (d, J = 8.8 Hz, 2H), 6.85 (d, J = 8.8 Hz, 2H), 6.81 (d, J = 8.8 Hz, 2H), 4.81 (s, 1H), 3.78 (s, 3H), 3.76 (s, 3H), 3.32 (s, 3H), 1.77 (s, 3H); 13C NMR (CDCl3, 100 Hz) δ 168.8, 163.8, 159.9, 156.2, 130.8, 128.0, 125.2, 118.5, 114.3, 113.9,66.0, 65.9, 55.4, 55.2, 51.8, 17.4; HRMS (EI) calc. for C20H21O5N1 (M+): 355.1414, found: 355.1413.
cis-Methyl 1-(4-methoxyphenyl)-2-acetyl-3-(2-methoxyphenyl)-aziridine-2-carboxylate (3e).
White solid. M. p. 104–105 °C; IR (KBr, cm−1): 1749 (m), 1637 (s), 1618 (s), 1514 (s), 1455 (m); 1H NMR (CDCl3, 400 MHz) δ 7.32-7.26 (m, 3H), 7.08 (dd, J = 1.6, 7.6 Hz, 1H), 6.85 (dd, J = 1.2, 8.0 Hz, 1H), 6.85-6.83 (m, 3H), 5.22 (s, 1H), 3.88 (s, 3H), 3.77 (s, 3H), 3.21 (s, 3H), 1.80 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 168.9, 164.2, 157.3, 156.1, 131.1, 129.3, 127.2, 122.1, 119.9, 118.5, 114.3, 110.3, 65.3, 61.1, 55.5, 55.4, 51.5, 17.0; HRMS (EI) calc. for C20H21O5N1 (M+): 355.1414, found: 355.1412.
cis-Mehtyl 1-(4-methoxyphenyl)-2-acetyl-3-(4-chlorophenyl)-aziridine-2-carboxylate (3g).
White solid. M. p. 140–142 °C; IR (KBr, cm−1): 1748 (s), 1639 (m), 1618 (m), 1511 (s); 1H NMR (CDCl3, 400 MHz) δ 7.30-7.22 (m, 6H), 6.82 (d, J = 9.2 Hz, 2H), 4.83 (s, 1H), 3.76 (s, 3H), 3.32 (s, 3H), 1.79 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 168.5, 163.3, 156.4, 134.7, 132.1, 130.5, 128.7, 128.1, 118.4, 114.4, 66.0, 65.5, 55.4, 51.9, 17.5; HRMS (EI) calc. for C19H18O4N1Cl (M+): 359.0919, found: 359.0917.
cis-Methyl 1-(4-methoxyphenyl)-2-acetyl-3-(2-chlorophenyl)-aziridine-2-carboxylate (3h).
White solid. M. p. 116–118 °C; IR (KBr, cm−1): 1749 (m), 1637 (s), 1618 (s), 1514 (m), 1455 (m); 1H NMR (CDCl3, 400 MHz) δ 7.43 (dd, J = 1.2, 8.0 Hz, 1H), 7.27-7.14 (m, 5H), 6.84 (d, J = 8.8 Hz, 2H), 5.32 (s, 1H), 3.77 (s, 3H), 3.26 (s, 3H), 1.89 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 168.3, 163.6, 156.4, 133.4, 131.5, 130.6, 129.7, 129.6, 128.1, 126.4, 118.4, 114.4, 65.7, 62.7, 55.4, 51.8, 17.5; HRMS (EI) calc. for C19H18O4N1Cl1 (M+): 359.0919, found: 359.0918.
cis-Methyl 1-(4-methoxyphenyl)-2-acetyl-3-(naphthalen-2-yl)-aziridine-2-carboxylate (3i).
White solid. M. p. 142–143 °C; IR (KBr, cm−1): 1753 (s), 1736 (s), 1602 (m), 1511 (s), 1446 (m), 1245 (s); 1H NMR (CDCl3, 400 MHz) δ 7.82-7.76 (m, 3H), 7.50-7.47 (m, 2H), 7.37 (dd, J = 2.0, 8.8 Hz, 2H), 7.30-7.26 (m, 2H), 6.79 (d, J = 9.2 Hz, 2H), 5.02 (s, 1H), 3.74 (s, 3H), 3.19 (s, 3H), 1.85 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 168.7, 163.7, 156.2, 133.3, 132.9, 131.1, 130.9, 128.3, 127.9, 127.7, 126.5, 126.4, 126.3, 123.9, 118.5, 114.3, 66.4, 66.1, 65.3, 51.8, 17.7; HRMS (EI) calc. for C23H21O4N1 (M+): 375.1465, found: 375.1464.
cis-methyl 2-acetyl-1,3-diphenyl-aziridine-2-carboxylate (4).
Colorless oil. 1H NMR (CDCl3, 400 MHz) δ 7.34-7.32 (m, 5H), 7.29-7.23 (m, 4H), 7.09 (t, J = 7.3 Hz, 1H), 4.90 (s, 1H), 3.26 (s, 3H), 1.80 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 168.56, 164.15, 137.31, 133.38, 129.09, 128.78, 128.49, 126.65, 124.26, 117.23, 66.14, 65.94, 51.81, 17.58; HRMS (FAB) calc. for C18H18NO3 (M+ + 1): 296.1287, found: 296.1285.
1-(4-Methoxyphenyl)-2,2-diacetyl-3-phenyl-aziridine (6).
Colorless oil. 1H NMR (CDCl3, 400 MHz) δ 7.45-7.39 (m, 2H), 7.36-7.29 (m, 3H), 7.12 (d, J = 9.0 Hz, 2H), 6.79 (d, J = 9.0 Hz, 2H), 6.42 (s, 1H), 3.72 (s, 3 H), 1.90 (s, 3H), 1.77 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 163.54, 158.58, 137.20, 132.68, 128.97, 128.27, 127.08, 126.82, 113.93, 106.65, 88.91, 55.22, 17.30, 10.24; HRMS (FAB+) calc. for C19H19NO3 (M+): 309.1365, found: 309.1367.
1-(4-Methoxyphenyl)-2-acetyl-2-benzoyl-3-phenyl-aziridine (7).
Colorless oil, cis-isomer. 1H NMR (CDCl3, 400 MHz) δ 7.56-7.49 (m, 2H), 7.43-7.36 (m, 3H), 7.31-7.18 (m, 5H), 7.14 (d, J = 9.0 Hz, 2H), 6.82 (d, J = 9.0 Hz, 2H), 6.59 (s, 1H), 3.75 (s, 3H), 1.84 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 162.23, 160.92, 157.70, 137.23, 133.35, 132.67, 130.85, 129.29, 128.52, 127.86, 127.23, 126.89, 114.87, 114.10, 90.33, 89.50, 55.42, 18.63; HRMS (FAB+) calc. for C24H21NO3 (M+): 371.1521, found: 371.1513.
trans-isomer. 1H NMR (CDCl3, 400 MHz) δ 7.55 (d, J = 8.4 Hz, 2H), 7.45-7.34 (m, 8 H), 7.19 (d, J = 9.0 Hz, 2H), 6.84 (d, J = 9.0 Hz, 2H), 6.60 (s, 1H), 3.76 (s, 3H), 1.95 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 164.50, 157.76, 156.55, 136.87, 133.05, 132.59, 130.04, 129.31, 129.15, 128.54, 128.11, 127.44, 126.84, 114.14, 107.97, 89.39, 55.39, 12.04; HRMS (FAB+) calc. for C24H21NO3 (M+): 371.1521, found: 371.1532.
1,3,3-Trimethylbicyclo[2.2.1]heptan-2-yl 1-(4-methoxyphenyl)-2-acetyl-3-phenyl-aziridine-2-carboxylate (8).
Major isomer. IR (KBr, cm−1): 1755 (s), 1716 (s), 1585 (m), 1512 (m), 1458 (m), 1396 (m), 1292 (s), 1176 (m), 1157 (m), 1030 (m), 956 (w), 694 (w); 1H NMR (CDCl3, 400 MHz) δ 7.31-7.26 (m, 5H), 7.24 (d, J = 8.4 Hz, 2H), 6.80 (d, J = 8.4 Hz, 2H), 4.86 (s, 1H), 3.99 (s, 1H), 3.75 (s, 3H), 1.82 (s, 3H), 1.69-1.42 (m, 2H), 1.39-1.26 (m, 3H), 1.08-0.96 (m, 2H), 0.96 (s, 3H), 0.92 (s, 3H), 0.55 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 164.4, 156.6, 129.3, 127.6, 127.4, 119.1, 114.7, 87.9, 67.0, 55.9, 48.7, 41.7, 39.9, 29.8, 26.8, 26.2, 23.2, 20.7, 19.4, 19.2, 18.7; HRMS (EI+) calc. for C28H33NO4 (M+): 447.2410, found: 447.2374; Anal. Calc. for C28H33NO4: C, 75.14; H, 7.43; N, 3.13, found: C, 75.14; H, 7.45; N, 3.13.
Minor isomer. 1H NMR (CDCl3, 400 MHz) δ 7.31-7.26 (m, 5H), 7.21 (d, J = 8.8 Hz, 2H), 6.78 (d, J = 8.8 Hz, 2H), 4.86 (s, 1H), 3.94 (s, 1H), 3.74 (s, 3H), 1.82 (s, 3H), 1.69-1.42 (m, 2 H), 1.39-1.26 (m, 3H), 1.08-0.96 (m, 2H), 0.98 (s, 3H), 0.67 (s, 3H), 0.43 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 164.4, 156.6, 129.3, 127.6, 127.4, 119.1, 114.7, 87.9, 67.0, 55.9, 48.7, 41.7, 39.9, 29.8, 26.8, 26.2, 23.2, 20.7, 19.4, 19.2, 18.7; HRMS (EI+) calc. for C28H33NO4 (M+): 447.2410; found: 447.2374.
Tetrahydro-4,4-dimethyl-2-oxofuran-3-yl 1-(4-methoxyphenyl)-2-acetyl-3-phenyl-aziridine-2-carboxylate (9).
Major isomer. IR (KBr, cm−1): 1763 (s), 1734 (s), 1609 (s), 1583 (m), 1510 (m), 1466 (m), 1373 (m), 1292 (m), 1246 (m), 1178 (w), 1161 (m), 823 (m), 594 (w); 1H NMR (CDCl3, 400 MHz) δ 7.51 (d, J = 6.4 Hz, 2H), 7.28 (d, J = 9.2 Hz, 3H), 6.92 (d, J = 9.2 Hz, 2H), 6.79 (d, J = 9.2 Hz, 2H), 4.99 (s, 1H), 4.58 (s, 1H), 4.21 (d, J = 9.6 Hz, 1H), 4.04 (d, J = 9.6 Hz, 1H), 3.72 (s, 3H), 2.0 (s, 3H), 1.39 (s, 3H), 1.23 (d, J = 8.4 Hz, 3 H); 13C NMR (CDCl3, 100 MHz) δ 196.9, 164.6, 155.04, 137.2, 133.9, 129.2, 118.8, 115.4, 114.6, 114.5, 83.7, 79.9, 65.5, 60.9, 55.9, 55.8, 40.0, 28.5, 21.7; HRMS (EI+) calc. for C24H25NO6 (M+): 423.1682, found: 423.1641; Anal. Calc. for C24H25NO6 : C, 68.07; H, 5.95; N, 3.31, found: C, 67.62; H, 6.01; N, 3.27.
Minor isomer. 1H NMR (CDCl3, 400 MHz) δ 7.59 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 11.4 Hz, 3H), 7.01 (d, J = 9.2 Hz, 2H), 6.79 (d, J = 9.2 Hz, 2H), 5.28 (s, 1H), 4.69 (s, 1H), 4.12 (d, J = 8.4 Hz, 1H), 3.99 (d, J = 8.4 Hz, 1H), 3.74 (s, 3H), 2.05 (s, 3H,), 1.35 (s, 3H), 1.26 (d, J = 8.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ 197.9, 164.6, 157.2, 138.2, 135.7, 129.5, 129.0, 128.9, 127.7, 126.0, 114.6, 90.7, 87.0, 86.9, 80.8, 69.5, 40.8, 27.1, 25.4, 20.8; HRMS (EI+) calc. for C24H25NO6 (M+): 423.1682, found: 423.1641.
(3S,3aR,4R,7S,7aS)-3-Acetyl-hexahydro-7-isopropyl-4-methylbenzofuran-2(3H)-one (10).
IR (KBr, cm−1): 1755 (s), 1725 (s), 1458 (m), 1385 (m), 1362 (m), 1289 (m), 1238 (m), 1154 (w), 992 (w); 1H NMR (CDCl3, 400 MHz) δ 3.72 (t, J = 7.2 Hz, 1H), 3.45 (d, J = 12.0 Hz, 1H), 2.42 (s, 3H), 2.37-2.27 (m, 1H), 1.96-1.92 (m, 1H), 1.78 (d, J = 9.6 Hz, 2H), 1.69 (t, J = 10.8 Hz, 1H), 1.47 (t, J = 5.6 Hz, 1H), 1.21-1.12 (m, 2H), 0.95 (d, J = 6.8 Hz, 3H), 0.9 (d, J = 7.2 Hz, 3H), 0.83 (d, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz) δ 202.6, 173.0, 84.9, 59.1, 52.5, 47.0, 35.8, 34.8, 31.3, 28.9, 25.3, 20.3, 20.2, 18.2; HRMS (EI+) calc. for C14H22O3 (M+): 238.1569, found: 238.1581; Anal. Calc. for C14H22O3: C, 70.56; H, 9.30, found: C, 70.64; H, 9.28.
Reaction of aziridine 3a with cerium(IV) ammonium nitrate
To a solution of 3a (168 mg, 0.5 mmol) in acetonitrile (7 mL) under an ice-bath was added a solution of cerium(IV) ammonium nitrate (685 mg, 1.25 mmol) in 4 mL of water. The reaction mixture was stirred for 2 hours and 5% aqueous NaHCO3 solution was added at 0 °C until pH of the solution was almost neutral. The resulting mixture was allowed to warm up to room temperature. The solid sodium sulfite was added portionwise until a brown slurry was formed. After AcOEt (10 mL) was added, the organic layer was separated and dried over anhydrous sodium sulfate. The solvent was removed under vacuum and the residue was purified by flash column chromatography on silica gel (hexane: ethyl acetate = 3: 1) to afford cis-methyl 2-acetyl-3-phenyl-aziridine-2-carboxylate (5) as a white solid (68 mg, 62% yield). M. p. 128–130 °C; IR (KBr, cm−1): 1741 (s), 1600 (m), 1453 (m), 1250 (m); 1H NMR (CDCl3, 400 MHz) δ 7.32-7.26 (m, 5H), 4.63 (s, 1H), 3.24 (s, 3H), 1.76 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 168.8, 167.8, 135.6, 128.5, 128.3, 126.1, 67.5, 63.0, 51.7, 17.2; HRMS (FAB) calc. for C12H13O3N1 (M+): 219.0890, found: 219.0886.
Acknowledgements
We thank the National Natural Science Foundation of China (No. 20472061, 20772160), NCET plan of Ministry of Education of China, Guangzhou Bureau of Science and Technology for financial support of this study. We also thank Prof. Michael P. Doyle for his help and suggestions.
References
-
(a) T. Kametani and T. Honda, Advances in Heterocyclic Chemistry, 1986, 39, 181 Search PubMed;
(b)
W. H. Pearson, B. W. Lian, and S. C. Bergmeier, Comprehensive Heterocyclic Chemistry II, vol. 1A, ed. A. Padwa, Pergamon Press, Oxford, 1996, pp 1-60 Search PubMed;
(c)
K. M. L. Rai, and A. Hassner, Comprehensive Heterocyclic Chemistry II, vol. 1A; A. Padwa, Ed.; Pergamon Press: Oxford, 1996, pp 61-96 Search PubMed.
-
(a) J. B. Sweeney, Aziridines and Epoxides in Organic Synthesis, 2006, 117 Search PubMed;
(b) D. Huang, M. Yan and Q. Shen, Chin. J. Org. Chem., 2004, 24, 1200 CAS;
(c) J. Royer, M. Bonin and L. Micouin, Chem. Rev., 2004, 104, 2311 CrossRef CAS.
-
(a) D. Tanner, Angew. Chem. Int. Ed. Engl., 1994, 33, 599 CrossRef;
(b) G. Cardillo, L. Gentilucci and A. Tolomelli, Aldrichimica Acta, 2003, 36, 39 CAS.
-
(a) K. G. Rasmussen and K. A. Jorgensen, J. Chem. Soc. Chem. Commun., 1995, 1401 RSC;
(b) Y. Li, P. W. H. Chan, N. Y. Zhu, C. M. Che and H. L. Kwong, Organometallics, 2004, 23, 54 CrossRef CAS;
(c) D. Morales, J. Perez, L. Riera, V. Riera, R. Corzo-Suarez, S. Garcia-Granda and D. Miguel, Organometallics, 2002, 21, 1540 CrossRef CAS;
(d) J. M. Mohan, B. S. Uphade, V. R. Choudhary, T. Ravindranathan and A. Sudalai, Chem. Commun., 1997, 1429 Search PubMed;
(e) M. Moran, G. Bernardinelli and P. Muller, Helv. Chim. Acta, 1995, 78, 2048 CrossRef CAS.
-
(a) J. S. Yadav, B. V. S. Reddy, P. N. Reddy and M. S. Rao, Synthesis, 2003, 1387 CrossRef CAS;
(b) T. Akiyama, S. Ogi and K. Fuchibe, Tetrahedron Lett., 2003, 44, 4011 CrossRef CAS;
(c) M. F. Mayer, Q. W. Wang and M. M. Hossain, J. Organomet. Chem., 2001, 630, 78 CrossRef CAS;
(d) B. Crousse, S. Narizuka, D. Bonnet-Delpon and J. P. Begue, Synlett, 2001, 679 CrossRef CAS;
(e) S. Sengupta and S. Mondal, Tetrahedron Lett., 2000, 41, 6245 CrossRef CAS;
(f) J. C. Antilla and W. D. Wulff, Angew. Chem. In. Ed., 2000, 39, 4518 CrossRef CAS;
(g) W. H. Xie, J. W. Fang, J. Li and P. G. Wang, Tetrahedron, 1999, 55, 12929 CrossRef CAS;
(h) J. C. Antilla and W. D. Wulff, J. Am. Chem. Soc., 1999, 121, 5099 CrossRef CAS;
(i) K. G. Rasmussen, K. Juhl, R. G. Hazell and K. A. Jorgensen, J. Chem. Soc. Perkin Trans. 2, 1998, 1347 RSC;
(j) K. G. Rasmussen, R. G. Hazell and K. A. Jorgensen, Acta Chem. Scand., 1998, 52, 1056 CrossRef CAS;
(k) S. Nagayama and S. Kobayashi, Chem. Lett., 1998, 685 CrossRef CAS;
(l) K. G. Rasmussen and K. A. Jorgensen, J. Chem. Soc. Perkin Trans. 1, 1997, 1287 RSC;
(m) K. G. Rasmussen, R. G. Hazell and K. A. Jorgensen, Chem. Commun., 1997, 1103 RSC;
(n) L. Casarrubios, J. A. Perez, M. Brookhart and J. L. Templeton, J. Org. Chem., 1996, 61, 8358 CrossRef CAS.
- A. L. Williams and J. N. Johnston, J. Am. Chem. Soc., 2004, 126, 1612 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, M. S. Rao and P. N. Reddy, Tetrahedron Lett., 2003, 44, 5275 CrossRef CAS.
- W. Sun, C. G. Xia and H. W. Wang, Tetrahedron Lett., 2003, 44, 2409 CrossRef CAS.
-
(a) V. K. Aggarwal and M. Ferrara, Org. Lett., 2000, 2, 4107 CrossRef CAS;
(b) K. Juhl, R. G. Hazell and K. A. Jorgensen, J. Chem. Soc. Perkin Trans.1, 1999, 2293 RSC;
(c) R. Hori, T. Aoyama and T. Shioiri, Tetrahedron Lett., 2000, 41, 9455 CrossRef CAS.
- R. Pellicciari, L. Amori, N. Kuznetsova, S. Zlotsky and A. Gioiello, Tetrahedron Lett., 2007, 48, 4911 CrossRef CAS.
- M. P. Doyle, W. H. Hu and D. J. Timmons, Org. Lett., 2001, 3, 933 CrossRef CAS.
-
(a) J. P. Genet, Pure Appl. Chem., 1996, 68, 593 CrossRef CAS;
(b) B. liev, A. Linden, R. Kunz and H. Heimgartner, Tetrahedron, 2006, 62, 1079 CrossRef CAS.
-
(a) G. Righi and C. Bonini, Rec. Res. Dev. Org. Chem., 1999, 3, 343 Search PubMed;
(b) W. McCoull and F. A. Davis, Synthesis, 2000, 1347 CrossRef CAS.
- M. P. Doyle, M. Yan, W. H. Hu and L. S. Gronenberg, J. Am. Chem. Soc., 2003, 125, 4692 CrossRef CAS.
-
10 was obtained in a previous study of intramolecular C-H insertion of diazoacetoacetates, see: M. P. Doyle, L. J. Westrum, W. N. E. Wolthuis, M. M. See, W. P. Boone, V. Bagheri and M. M. Pearson, J. Am. Chem. Soc., 1993, 115, 958 Search PubMed.
- CCDC-682854 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge viawww.ccdc.cam.ac.uk/conts/retrieving.html [or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB21EZ, UK; fax (t44) 1223-336-033; or E-mail: deposit@ccdc.cam.ac.uk].
|
| This journal is © The Royal Society of Chemistry 2009 |
Click here to see how this site uses Cookies. View our privacy policy here.