The synthesis of rare earthfluoride based nanoparticles
Paula
Rahman
and
Mark
Green
*
Department of Physics, King's College London, The Strand, London, WC2R 2LS, UK. E-mail: mark.a.green@kcl.ac.uk
First published on 13th August 2009
Abstract
The requirement for narrow emission profiles and systems that do not include metals perceived to be cytotoxic, has led to an increase in the reports on rare-earth (Ln) materials, with fluoride-based materials in particular attracting attention. This class of compounds includes rare-earthfluorides (LnF3), rare-earth oxyfluorides (LnOF) and alkali metalrare-earthfluorides (MLnF4, where M represents an alkali metal). Here we will describe the main reactions outlining key developments in roughly chronological order.
 Paula Rahman Paula Rahman | Paula Rahman graduated from Imperial College with a MSci in Chemistry in 2005. She then started her PhD in the synthesis of rare-earth nanomaterials in the Physics department at King’s College London. |
 Mark Green Mark Green | Mark Green obtained his BSc in chemistry from Manchester Metropolitan University in 1995 and his PhD in nanomaterials chemistry from Imperial College London in 1999. This was followed by post-doctoral appointments at Imperial and Oxford, before taking a position as senior scientist at Oxonica Ltd. He took up a lectureship in Physics at King’s College London in 2004, where he is currently a senior lecturer. |
Multiple precursor routes
Nanoparticle synthesis, as a major discipline in materials chemistry, has emerged over the last 16 years, and can, in part, be traced back to the seminal reports on the organometallic routes to high-quality semiconductor quantum dots.1 The main thrust of research has indeed been towards novel semiconducting nanostructures, where high quality materials are now routinely prepared by the thermolysis of inorganic and organometallic precursors in high-boiling-point solvents, with engineered ligands and surfactant systems optimised to control shape, size and optical quality. From this chemistry, further advances were made in the preparation of metals and metal oxides, to the point where now, almost all solid-state materials can be manufactured using chemistry that originated from the 1993 paper by Murray et al.1 One of the key driving forces in the preparation of, in particular, semiconductor quantum dots, was the development of biological labelling as a real and useful application that could be translated to biology labs. One major limitation in semiconductor quantum dots in biology is their perceived cytotoxicity, and other materials are being investigated as potential replacements—rare-earthfluorides appear to be one such material system.The rare-earthfluorides are expected to have the same properties as the rare-earth ions, as external ligand fields do not tend to affect the Ln3+ ion properties. These properties include very sharp emission lines when excited, and paramagnetic properties. No nanoscale effects change their emission characteristics but it is possible that their magnetic properties will change when the particles are small enough. Another useful property that rare-earthfluorides demonstrate is up-conversion or down-conversion when doped appropriately. Up-conversion is where a material can be excited by two low-energy photons and emit one high-energy photon, down-conversion is the opposite process where one high-energy photon is converted to two low-energy photons.
Rare earth fluoride compounds have been synthesised using most of the available wet chemical routes, with the hydrothermal method being the most investigated. A very basic hydrothermal reaction would involve mixing a rare-earth precursor with a fluoride precursor in aqueous solution, heating it and then separating out the precipitated product (often heating is not required). In order to obtain a more crystalline product, the reagents would be placed in an autoclave (often Teflon-lined to prevent fluoride ions attacking glass) and then sealed and heated.
The growth of the product can also be controlled by the addition of a ligand that binds to the surface of the growing nanoparticles, allowing slow and directed growth. Addition of a ligand often makes the products soluble in either polar or non-polar solvents, depending on the ligand. For example, the polyol method uses a long-chain alcohol as the solvent and as a ligand, the long chain allowing high reaction temperatures, and, as it coats the surface of the nanoparticles, imparts water-solubility.
Most groups, when using the hydrothermal method to synthesise Ln fluoridenanoparticles, use Ln nitrates as precursors, often synthesising the nitrate precursor in situ from oxides and nitric acid. Fewer groups used chlorides and fewer still the oxides directly. The choice of fluoride precursor varies much more than Ln precursors. Groups mainly use HF, NH4F and NH4HF2 when preparing fluorides of the type LnF3 and NaF or KF for fluorides of the type MLnF4. Other, less frequently used, precursors include NaBF4, KBF4 and 1-butyl, 2-methylimidazolium tetrafluoroborate (an ionic liquid). Occasionally, more than one of these precursors is used, especially when making MLnF4. The pH of these syntheses is often an important factor in determining the mode of growth and the final product. Groups often use NaOH, KCl, NH4OH and NH3 to adjust the pH appropriately. As with most chemical reactions, temperature is a key factor in determining the outcome of a hydrothermal synthesis of LnF3nanoparticles. Usually, higher temperatures lead to more crystalline particles, but also increase the rate of reaction. Another key factor, of course, is reaction time. Longer reactions tend to give larger nanoparticles and also provide conditions for Ostwald ripening. Some investigations into novel synthetic techniques have also been performed, including microwave irradiation, sonication and the use of ionic liquids. Several microemulsion methods will also be discussed briefly.
The first reported synthesis of a rare-earthfluoride nanomaterial was in 2001 by Zhang et al.2 The material was investigated as a potential additive for paraffin when used as a heavy duty lubricant, in which rare-earth-based complexes and micron-sized particles had already been investigated as additives. No heating was required and a ligand was added, resulting in a surface-coated nanoparticle. The nanoparticles produced were relatively polydispersed (Fig. 1) and a succinimide coating allowed the LaF3nanoparticles to be dissolved easily in paraffin .
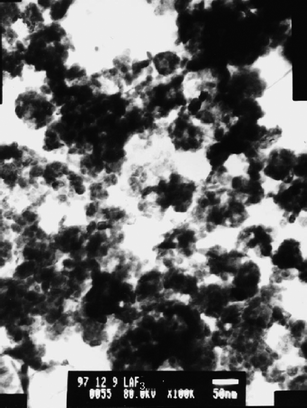 | ||
| Fig. 1 TEM image of LaF3 nanoclusters. Reproduced with permission from reference 2. Copyright Elsevier, 2001. | ||
A similar report on nanoparticles of LaF3 was made by Zhou et al. in 2001.3 The synthesis was similar to that of the Zhang et al.,2 although this time using pyridinium di-n-hexaoctyldithiophosphate. The group made small (ca. 6 nm) nanoparticles that were dispersible in organic solvents. The particles were in the hexagonal phase but poorly crystalline.
In 2004 the Haase group developed a nanoparticle synthesis for NaLnF4nanoparticles,4 the main aim of which was to make efficient upconvertors that would be transparent when dispersed in solution. This synthesis used N-(2-hydroxyethyl)-ethylenediamine as the solvent and ligand, which also complexed to the rare-earth precursor, and 1-adamantanecarboxylic acid as a ligand. The resulting particles were around 15 nm and crystalline (Fig. 2); unfortunately the particles also had a large size distribution and had ill-defined shapes. The particles formed a transparent solution when dispersed in dimethylsulfoxide (Fig. 2). In 2007 a similar synthesis was performed5 but the nanoparticles were modified to be soluble in water. This was done by surface modification after the initial synthesis of the particles with 1-hydroxyethane 1,1-diphosphonic acid. The group found that this surface modification actually made up-conversion efficiency higher.
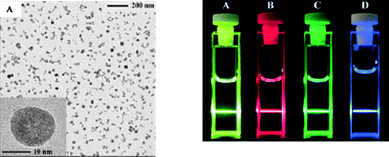 | ||
| Fig. 2 TEM image of doped NaYF4 (left picture) and photograph of the dispersed nanoparticles in DMSO illuminated under UV light (right picture). Reproduced with permission from reference 4. Copyright Wiley VCH, 2004. | ||
Karbowiak et al. investigated the synthesis of nanocrystalline NaGdF4 and KGdF4.6,7 They reported the first hydrothermal synthesis of an alkali metalrare-earthfluoride in 2004, which was doped with Eu3+ and the luminescence properties were investigated. The synthesis involved dissolving Ln oxides in concentrated HCl to which NaF or KF was added. The resulting precipitate was aged at 90 °C for up to 10 days. The report on KGdF4 shows no TEM images but shows data consistent with a nanocrystalline material as ascertained from the X-ray diffraction (XRD) patterns. The NaGdF4 results do have TEM images which show that the material consists of nanoparticles rather than a bulk material with nano-sized domains. The nanoparticles were uneven in size and shape and agglomerated, but were crystalline. The size and shape of the particles were ill-defined most likely because no ligand was added to the reaction to control growth rate and prevent agglomeration. The Karbowiak group reported more routes in 20058,9—the first report investigated various methods for the synthesis of NaGdF4 including a reverse micelle method and a solid-state reaction.9 The results showed that the co-precipitation method previously described was preferable for this type of material. Overall the method was relatively cheap and simple with no compromise in optical properties. The second report described doped KGdF4.8 The main investigation concentrated on the phase transitions of the nanoparticles when heated and the resulting changes in optical properties.
The Li group have looked in-depth into the synthesis of rare-earthfluoride materials including LnF3 and NaLnF4. The fluoride products obtained by Wang et al.10,11 were hollow structures (named inorganic fullerene-like particles by the group) rather than solid particles. This synthesis was a pH-dependent, hydrothermal synthesis where nitrate precursors were formed in situ from Ln2O3 and nitric acid. The fluoride source (F-source) was NH4F and hydroxides were used to change the pH of the solution. Temperatures varied from 80–180 °C and reaction times between 12–24 h. The results showed that an increase in temperature led to an increase in particle size, as expected. The fluoride particles only became hollow after heat treatment in an autoclave at pH between 4 and 5. No further mention of the effect of pH on the products was made and the group did not report a mechanism for the formation of the hollow particles. The products were non-uniform in size but were crystalline. The group developed a new synthetic method, mentioned first in 200512 and then in more detail in 200613 based on liquid-solid-solution (LSS) growth, which was employed to synthesise a range of rare-earthfluoride materials (Fig. 3). This hydrothermal method used sodium linoleate, linoleate acid and ethanol added to aqueous solutions of the precursors, rare-earthnitrates and NaF or NH4HF2. Reaction temperatures of 100–200 °C for about 8–10 h were used. The resulting nanoparticles varied in shape and size but were generally monodisperse and crystalline. The group discovered that nanoparticle shape depended on the ionic radius of the rare-earth ion which led to the theory that the growth mode changed with ionic size.
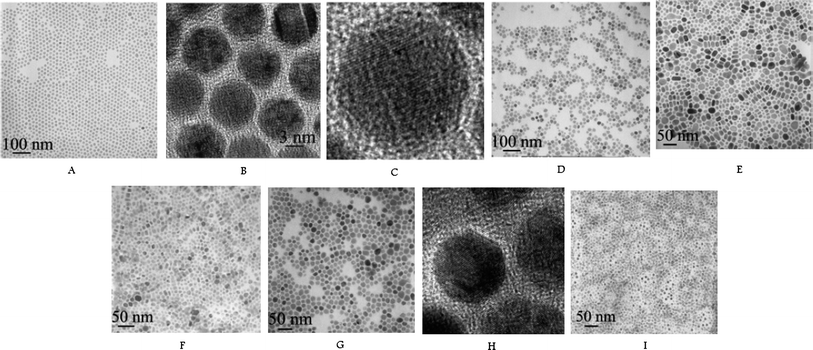 | ||
| Fig. 3 TEM images of NaYF4 (A, B, C), CeF3 (D), PrF3 (E), NdF3 (F), LaF3 (G, H), NaYb2F7 (I). Reproduced with permission from reference 13. Copyright American Chemical Society, 2006. | ||
Li carried extensive research into the synthesis of NaLnF4-type nanoparticles. This class of material is of some considerable interest as when doped appropriately, the materials can undergo either up-conversion or down-conversion. In 200514 the group used a hydrothermal process with cetyltrimethylammonium bromide (CTAB) and ethylenediaminetetraacetic acid (EDTA) as size- and shape-control agents for the synthesis of NaYF4. The particles exhibited various shapes and sizes depending on which ligand was added, for example, with CTAB, nanorods were formed, but with EDTA spherical shapes formed. The particles were relatively uniform and the XRD patterns suggest a high level of crystallinity. In 200615 attempts to make NaCeF4 using a similar synthesis showed that at lower temperatures (less than 180 °C) the formation of CeF3 was favoured, but at higher temperatures the target material was made. Other factors such as the pH of the reactant solution also affected the formation of all the rare-earthfluorides.
In another report16 the Li group used a solvothermal method to make NaYF4nanocrystals, where oleic acid and alcohol were added as co-solvents to water along with NaOH. The precursors were Ln nitrates and NaF. Temperatures and reaction times were similar to that of the 2003 syntheses of LnF3.10,11 The resulting particles were highly monodispersed and also highly crystalline. This work was further expanded to include a range of reaction parameters.17 The method yielded many different shapes and sizes for NaYF4, including nanorods, hexagonal nanoplates, rectangular particles and square particles, all of which were crystalline and monodisperse (Fig. 4). The mechanism of the formation of the differently shaped particles was also investigated and it was found that alcohol chain length, the ratio of F− to Y3+, reaction time and temperature were all factors in product shape and size.
 | ||
| Fig. 4 TEM images of NaYF4nanoparticles doped with 71.4% Eu3+ (A), 5% Eu3+ (B), 71.4% Tb3+ (C), 5% Tb3+ (D). Reproduced with permission from reference 17. Copyright American Chemical Society, 2007. | ||
Lezhnina et al. investigated two different types of syntheses,18 both based on previously reported methods. The first, which is based on Li's method,10 was modified by initially adjusting the pH to 7 followed by decreasing the pH after the reaction. The EuF3 products were highly crystalline and cuboidal in shape. This is in comparison to the original synthesis of PrF3 and LaF3 which also gave crystalline products, although roughly spherical particles. The second method is based on the method reported by Eiden-Assmann,19 but used NaF, ethylene glycol and water instead of KF, diethylene glycol and ethanol. The resultant EuF3 particles were non-spherical in shape, non-uniform in size but crystalline, which matched well with the original synthesis.
Lemyre and Ritcey20 used an unusual method to make YF3nanoparticles; a microemulsion method based on previously reported syntheses for silica21 and Y2O322 which gave particles that were amorphous and ill-defined in shape. The original method used two separate solutions of the precursors; Lemyre and Ritcey however, adjusted the method to only use one solution containing YCl3 and NH4HF2 dissolved in water, igepal CO520 and cyclohexane, which were added together while stirring then sonicated. The resultant particles were highly crystalline, monodisperse and evenly shaped (Fig. 5). Lemyre applied this method to ErF3 and YbF3 and reported similar results showing promise as an effective room-temperature method for the synthesis of rare-earthfluorides.
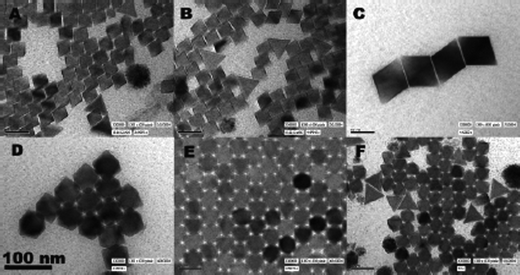 | ||
| Fig. 5 TEM images of YF3nanoparticles using the one-solution method. Reproduced with permission from reference 20. Copyright American Chemical Society, 2005. | ||
Zhang reported the synthesis of water-soluble LaF3, NaYF4 and NaGdF4nanoparticles in 2006.23 The group used a pH-dependent synthesis to make water-soluble particles. Chitosan, a naturally occurring biopolymer, made the particles soluble with functional groups that could be used to attach to other molecules. The method was a pH-dependent, hydrothermal synthesis which used NH4F as the F-source. Rare-earthchloride precursors were used and ammonia was used to adjust pH. The temperature and time were interesting, only 75 °C for 2 h—these conditions were relatively mild compared to similar syntheses.10 The particles were crystalline but were disperse in size and shape. Some quick studies into the toxicity of the nanoparticles in the presence of live cells of HT-29 and BSA were favourable, showing no more cell death than a control sample. Zhang's group also investigated the preparation of NaYF4nanoparticles and the up-conversion properties when doped.24 The group used polyvinylpyrrolidone (PVP) as a ligand to give the nanoparticles good water solubility as well as being a size-control agent. The synthesis also used ethylene glycol and water as solvents, NaCl and rare-earthnitrates as precursors and NH4F as the F-source. The temperature of the reaction was 160 °C and reaction time 2 h. The NaYF4 particles were monodisperse in size and crystalline but varied in shape. It was also found that coating the doped nanoparticles in a silica shell did not affect the luminescence properties. Another method for making water-soluble NaYF4nanoparticles with polyethylenimine (PEI) as the capping agent was also reported, utilising NaCl, NH4F and Ln chloride as precursors, and water and ethanol as solvents. The reaction time was 24 h and the temperature was 200 °C, significantly longer and higher than the previously described synthesis. The particles were more disperse in size and shape, however. A quick study was performed on the biocompatibility of the nanoparticles with human cells, HT-29, and found that the particles were not toxic. The same synthesis was employed for NaGdF4nanoparticles except the reaction time was significantly reduced from 24 to 5 h.25 The resulting particles were even in shape and size and also crystalline. Although NaGdF4nanoparticles have been doped previously, Fig. 6 shows their emissive properties.
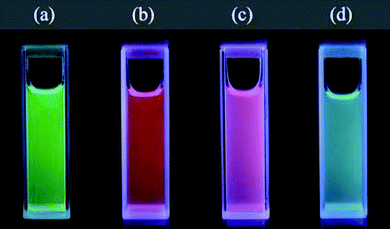 | ||
| Fig. 6 Photo of NaGdF4 particles dispersed in solution and illuminated under UV, the particles have been doped with Tb3+, Eu3+, Sm3+ and Dy3+ for (a), (b), (c) and (d) respectively. Reproduced with permission from reference 25. Copyright Institute of Physics Publishing, 2007. | ||
Two papers from the Zhu26,27 group reported a synthesis for EuF3 and LaF3. In this synthesis, rare-earthnitrates were first made in situ and then mixed with a solution of KBF4. The mixture was sonicated or stirred but not heated. This resulted in particles which were not quite nano-sized but were small enough to warrant interest. When stirred, disc-shaped particles were formed. The EuF3, when sonicated, resulted in a very interesting ‘flower’ shape, around 1 micron in diameter.
A similar reaction described in another paper by the same group28 was used to synthesise LaCeF3nanoparticles. In this case the F-source was changed to NH4F. The particles were not as interesting in morphology as the KBF4 reactions, however, as they formed approximately spherical shapes.
Lin's group reported two different syntheses for CeF3, a polyol method29 and a hydrothermal method.30 The polyol method used Ln nitrate precursors and NH4F in diethylene glycol (DEG) at 200 °C for 1 h. The hydrothermal method used chloride precursors with NaF. Trisodium citrate was added to form a complex with the Ln ion and acted as a ligand after the nanoparticles had formed. This solution was autoclaved at 180 °C for 24 h. The polyol method resulted in approximately spherical particles which were crystalline. The particles were very small, around 7 nm. The hydrothermal method yielded larger crystalline particles, all of which had flat plate-like structures. The nanoplates tended to have at least one dimension below 100 nm, with larger diameters. The trisodium citrate is the reason the plate-like shapes are formed as the ligand binds strongly to the (0001) surface slowing growth in that direction.
The van Veggel group took the synthesis reported by Dang's group3 and improved it. In this report,31 particles that were polydisperse in size and shape, but crystalline, were reported. The synthesis was modified to include a ligand known to impart better solubility; ammonium di-n-octadecyldithiophosphate. The group also made doped LaF3nanoparticles and investigated the luminescence properties of LaF3 doped with various other Ln3+ ions. The same group also formulated a synthesis which rendered doped LaF3nanoparticles soluble in water.32 The synthesis was similar to the previous methods with the exception of the ligands; the group tried out several different ligands all with the general formula RO(PO3)2− where R was PEG-based or –NH2-based. These ligands acted as a coating on the nanoparticles which controlled growth during the reaction, imparting solubility and preventing agglomeration when in water whilst the particles remained luminescent. The group also tried another synthetic method for making doped LaF3nanoparticles—a pH dependent co-precipitation method.33 The ligand used was citrate from citric acid, and the Ln precursors were nitrates, the F-source was NaF and the solvent varied from water to a water–methanol combination. The pH was adjusted using NH4OH. The reaction conditions were mild; 75 °C and 2 h. From the AFM images reported, the particles were polydisperse in size but the XRD pattern suggested a high level of crystallinity. However, this synthesis was also used to make GdF3 particles,34 which were disperse in size and shape. The GdF3nanoparticles were investigated for use as NMR and MRI contrast agents with promising results. The group foresees GdF3nanoparticles in general use, as it is relatively easy to modify the surface ligands to contain a range of functional groups.
Huang et al. started investigating the synthesis of LnF3 in 2004.35 The first synthesis made wire-shaped LnF3, nanosize in two dimensions but several hundred nanometres long in the other. This method was interesting as the synthesis first made very long, thin wires of NH4LnF4 (Ln = Nd, Sm, Eu, Gd and Tb) which were then calcined to form LnF3. This was a solvothermal reaction in methanol with Ln nitrate precursors and NH4F as the F-source. The LnF3nanorods were not regular in shape, but were however crystalline. The same group also reported a room temperature synthesis of NaEuF4nanoparticles in the same year.36 The synthesis was very simple; europium chloride hydrate and NaF were dissolved and stirred in water. The ratio of europium to sodium and reaction time determined the product; either NaEuF4 or Na5Eu9F32. The resultant products were regular in size but were uneven in shape. High-resolution optical microscopy however suggests a high level of crystallinity.
The Chen group investigated this type of reaction more thoroughly. One paper reported the effect of F:Eu ratio when NaBF4 was used as the F-source.37 The shapes of the nanoparticles changed from nanodiscs, which self-aligned to nanospindles (prolate spheroids). The particles again were uneven but regular in size. A second paper38 investigated the effect of the F-source on the synthesis of EuF3. Changing the source changed not only the crystal phase of the product, but the shape as well. Potassium fluoride, for example, led to hexagonal EuF3 particles in crescent-moon shapes while CsF gave orthorhombic nanowires of EuF3. This reaction is one of the more versatile syntheses for rare-earthfluorides. The process required no heating, only two precursors, and water. Control over morphology could be changed by adjusting the precursor ratio or by using alternative F-sources.
Chen's group also looked at another water-based, room temperature synthesis of NaYF4.39 The group first complexed the rare-earth-chloride starting material with EDTA and injected the solution rapidly into a separate NaF solution. This, in theory, meant that nucleation occurred over a very small period of time and so limiting the initial size distribution. The resulting particles were uniform in shape and size, most being spherical in shape with diameters in the range 30–45 nm. The most interesting result of this investigation was the effect of the EDTA:Ln3+ ratio on particle size. As the relative amount of EDTA was increased, the resultant particles decreased in diameter. The group also found that annealing the samples partly changed the crystal structure from cubic to hexagonal, the amount of hexagonal phase increasing with annealing temperature. The same group looked at a related synthesis for NaYF4, which was similar to the organometallic-type reactions typically used in semiconductor quantum dot synthesis.40 The rare-earth precursor was made into an oleate complex, dissolved in 1-octadecene and mixed with NaF in octadecene. This reaction was performed at 260 °C rather than at room temperature like the previously reported synthesis. The resulting particles were hexagonal-shaped and in the hexagonal phase (Fig. 7) or spherical-shaped in the cubic phase depending on the reaction temperature. The cubic shape formed at the lower temperature of 210 °C and the hexagonal shape formed at 260 °C. The oleic acid bound to the complex also acted as a ligand which imparted solubility to the resultant particles, which did not occur with the EDTA-complexed particles.
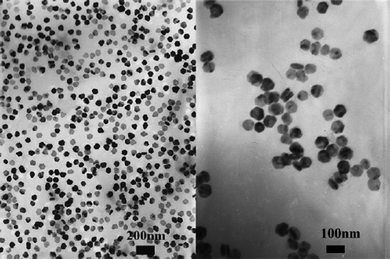 | ||
| Fig. 7 TEM images of hexagonal NaYF4:Yb, Er nanoplates formed at 260 °C. Reproduced with permission from reference 40. Copyright American Chemical Society, 2006. | ||
Fan et al. came up with a very simple reaction towards doped GdF3nanoparticles;41 Ln chlorides were dissolved in ethanol and then NH4F was added. The solution was heated using an autoclave at temperatures varying from 60–180 °C for 2 h. The particles were irregular in shape, regular in size and more crystalline at higher reaction temperatures. The researchers also investigated the up-conversion properties of GdF3 doped with Er3+ and Yb3+ and found that the intensity of emission was much lower than the equivalent bulk material.
Jacob et al. investigated the synthesis of metal fluoridenanoparticles,42 including LaF3 and YF3. This is one of the few reported syntheses that used ionic liquids and/or a microwave to heat the reaction. The ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate (BMIBF4), acted as the solvent and as the F-source. The synthesis was relatively simple; the rare-earthnitrate and ionic liquid were heated in a domestic microwave for just 5 min. The resulting products were highly crystalline but were disperse in shape and size and exhibited ‘needle’ morphologies.
Kumar et al. used another solvothermal method;43 a solution containing Ln nitrates in water was added drop-wise to an ethylene glycol and water solution of NH4F and then refluxed. The products were also heat treated after synthesis. The LaF3nanocrystals were highly crystalline but uneven in shape and size.
Ma et al. developed another microwave synthetic pathway, combined with a pH-adjusted hydrothermal method.44Rare-earthnitrates were formed in situ; NaF was added and the pH adjusted to between 4 and 5. The solution was then refluxed in a microwave for 20 min. The resulting particles were around 30 nm in diameter and hollow, approximately spherical and highly crystalline (Fig. 8). The group also analysed the resulting particles before the microwave heating and found that prior to heating, the particles were small, aggregated and solid (not hollow).
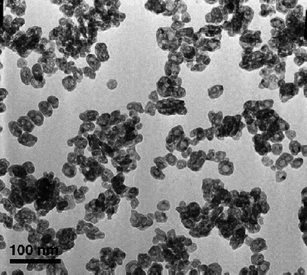 | ||
| Fig. 8 TEM image of PrF3nanoparticles after microwave heating. Reproduced with permission from reference 44. Copyright Elsevier, 2007. | ||
Miao et al. used an aqueous room-temperature reaction with Ln nitrate precursors and a NaBF4 F-source to synthesise EuF3.45 This reaction could be modified by changing the ratios of the precursors to give various morphologies such as discs, rods and rings. The rings were composed of aggregated smaller particles consistently throughout the sample. The same synthesis was used to make LaF3 nanobundles and spherical nanoparticles.
Nuñez and Ocaña46 used a very similar ionic liquid to Jacob et al.,421-butyl, 2-methylimidazolium tetrafluoroborate (BMIMBF4) to make EuF3, TbF3 and YF3nanoparticles. The ionic liquid was also used as a solvent as well as F-source. Three different forms of Ln precursor were used, nitrate, acetate (OAc) and acetylacetonate (acac). The solvent system was ethylene glycol, the reaction temperature was 120 °C and the reaction time was 15 h. The ytterbium precursors were investigated to find which was most effective. The group found that Y(acac)3 and Y(OAc)3 yielded particles which were monodisperse and crystalline. The nitrate precursor gave particles which were irregular in shape and size. Both acac and OAc precursors resulted in flat, rhombohedral-shaped nanoparticles but the acac particles were larger. The acac precursors were used for Eu and Tb and gave approximately spherical and rhombohedral shapes respectively. These particles were less crystalline, although this is more likely due to their small size.
A paper by Fan Zhang et al.47 described the synthesis of NaYF4 particles, and although most results obtained are not small enough to be considered nanoparticles the morphologies are very different from any other reported synthesis. The synthesis used oleic acid, water and ethanol as solvents and NaF and Y(NO3)3 as precursors. NaOH was used to create basic conditions. Reactant solutions were heated in an autoclave at temperatures between 130–230 °C for up to 24 h. Different shapes and sizes were obtained by adjusting relative amounts of the reactants and solvents, (Fig. 9) for the unusual flower-decorated hexagonal discs formed in this synthesis.
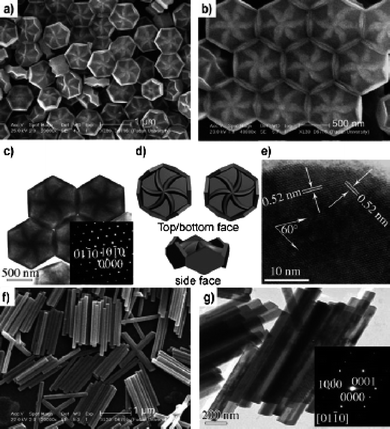 | ||
| Fig. 9 Electron micrographs and electron diffraction patterns of NaYF4 flower-decorated hexagonal discs and hexagonal rods. Reproduced with permission from reference 47. Copyright Wiley VCH, 2007. | ||
Zhang et al. developed another microwave method, using a microemulsion technique.48 This method was performed by the preparation of two separate solutions of HF and YCl3 in cetyltrimethylammonium bromide, cyclohexane and n-pentanol, combining the two and microwaving. The resulting YF3 particles were large and in the shape of bundles, each strand being itself on the nanoscale. The heating was performed in a domestic microwave for only 3 s as the products required annealing in order to become well-crystallised.
Ansari et al. used a solvothermal method with pyridine as a ligand to synthesise TbF3nanoparticles.49 The precursors, sodium fluoride and terbium nitrate were dissolved in water and ethanol and mixed; the combined solution was kept at 70 °C for 2 h. The particles were approximately rod-shaped but uneven in size and aggregated into small bundles. The TbF3nanorods showed strong green emission under UV excitation.
The trifluoroacetate precursor method
One interesting route to rare-earthfluorides is based on the decomposition of rare-earth trifluoroacetate salts (LnTFA). In the following section the main reports using trifluoroacetate (TFA) precursors to synthesise rare-earthfluorides alkali rare-earthfluoride and rare-earth oxyfluorides (LnOF) are summarised.Roberts published a paper in 196150 detailing the preparation of lanthanum and neodymium trifluoroacetate salts, their properties and importantly, the decomposition pathway. After heating, the solid residues were analysed using elemental analysis and the dehydrated forms of rare-earth trifluorides were identified. The decomposition pathway was also investigated by analysing the gaseous products given off when heated, and a reaction was suggested as shown in Fig. 10.
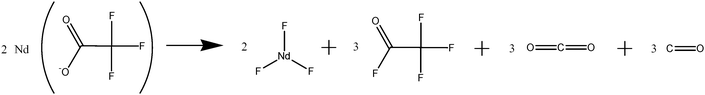 | ||
| Fig. 10 The decomposition products of neodymium trifluoroacetate. | ||
Roberts found that the trifluoroacetate salts slowly decomposed into the fluoride of the metal when heated above 110 °C and rapidly decomposed when heated to between 250 and 300 °C. If further heating continued to 700 °C the metal oxyfluoride formed. This report suggested that the trifluoroacetate salts of metals could potentially be used as a single source precursor for metal fluorides or metal oxyfluorides.
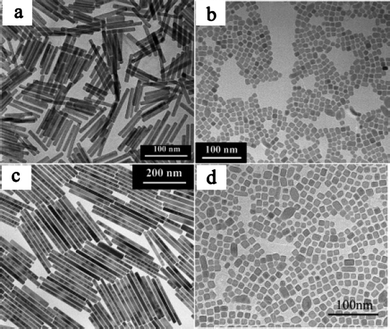
To the best of our knowledge the first group to use the trifluoroacetate-based precursor to synthesis rare-earthfluoridenanoparticles was Yan's group.51 Using lanthanum trifluoroacetate and a mixture of oleic acid and octadecene as solvent, the group made trigonal LaF3 triangular nanoplates which were highly monodisperse and had the tendency to self-align both side-down and face-down (Fig. 11). The nanoparticles were also highly crystalline and dispersible in organic solvents. The reaction temperature and time were 280 °C and 1 h. Not long after Yan's group published the use of trifluoroacetate salts as precursors for rare-earthfluoridenanoparticles, another group from Moscow State University published the synthesis of europium fluoride.52,53 This synthesis used trioctylphosphine oxide (TOPO) as the solvent and capping ligand and so gave different morphologies to Yan's method. These particles were small and were approximately spherical in shape, although not as crystalline as those produced by Yan's group.
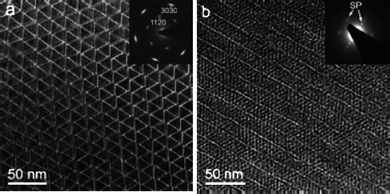 | ||
| Fig. 11 TEM images of self-aligned LaF3 nanoplates. Reproduced with permission from reference 51. Copyright American Chemical Society, 2005. | ||
The reaction temperatures of both syntheses were different, in fact the TOPO experiment had a higher temperature (310 °C in comparison to 280 °C). It would be expected that the higher reaction temperature would lead to a more crystalline product, but in these reactions this is not the case. TOPO, it seems, is not a good choice of solvent for the growth of rare-earth particles from the degradation of trifluoroacetate precursors (however a paper by Shan et al. in 200754 also used TOPO, but with more favourable results).
Yan's group went on to develop and explore the use of metal TFA salts as precursors to rare-earthfluorides (LnF3), rare-earth oxyfluorides (LnOF) and sodium rare-earthfluorides (NaLnF4). A report published in 200655 thoroughly described the conditions needed to make NaLnF4 (Ln = Pr–Lu, Y). Using two precursors; sodium trifluoroacetate and rare-earth trifluoroacetate and a combination of three solvents; oleic acid, oleylamine (coordinating solvents) and octadecene (non-coordinating), the group obtained many different shapes and sizes (Fig. 12). By exploring the results when the reaction parameters were changed, the group managed to neatly summarise the conditions needed to synthesise the cubic and hexagonal phases of NaLnF4 in terms of free energy (Fig. 13). It is worth noting that the group have shown that the cubic phase of NaLnF4 is stable in the nanoscale regime and by allowing nanocrystals to grow, the change from cubic to hexagonal phases was observed.
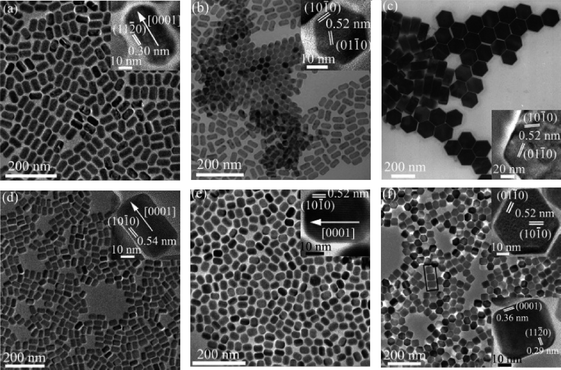 | ||
| Fig. 12 TEM and HRTEM (insets) images of NaYF4nanorods (a) and (b), NaYF4 nanoplates (c), NaNdF4nanorods (d), NaEuF4nanorods (e), NaHoF4 nanoplates (f). Reproduced with permission from reference 55. Copyright American Chemical Society, 2005. | ||
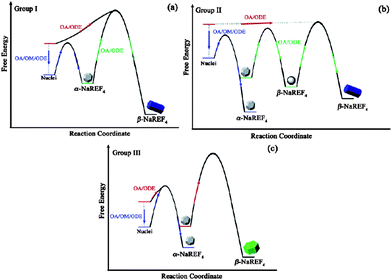 | ||
| Fig. 13 Schematic diagrams of the free energy for the synthesis of NaLaF4nanoparticles. Where Group I are Pr to Nd, II are SM to Tb and III are Dy to Lu, Y. Reproduced with permission from reference 55. Copyright American Chemical Society, 2005. | ||
The mechanism of the reaction was also examined and it was found that the nucleation time occurred over less than 1 min for α-NaYF4, which partly explains why the final products are so monodisperse.56 The up-conversion properties of doped NaYF4 were also investigated57 as a factor of crystal phase, surface ligand and surface coating and the results showed that phase change can result in different up-conversion pathways. The cubic/α-phase doped with Yb3+ and Er3+, favours green, which is a two-photon process and the hexagonal/β-phase favours red, which is a three-photon process.
Yan's group also explored, using the same detailed approach, the synthetic conditions needed to make LnOF and LnF3 (Ln = La to Lu, Y) using LnTFA and the three solvents already mentioned.58 They found that the solvent combination was important when it came to the synthesis of LnF3 or LnOF; the most important conclusion being that oleylamine is a fluorophile and encourages formation of LnOF rather than LnF3. The group again demonstrated excellent control over morphology of the nanoparticles and the ability of the product to self-assemble over a long range, Fig. 14, demonstrates the range of shapes and self-alignment possibilities. The group looked further into the synthesis of the oxyfluorides,59 specifically doped LaOF and GdOF, using TFA precursors. The group detailed the conditions needed for different shapes and sizes and also investigated the optical properties of the particles. The group found that LaOF is a better host for green emission (Ce3+ and Tb3+ doped) but GdOF is better for red emission (Eu3+ doped).
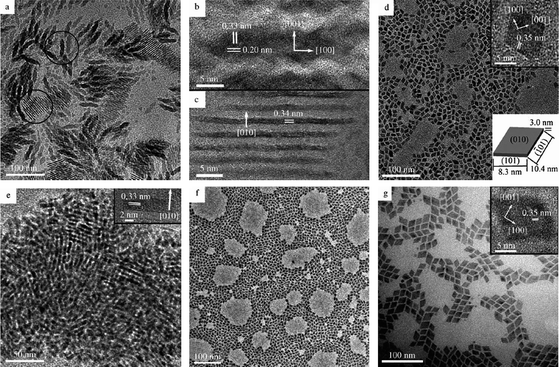 | ||
| Fig. 14 TEM and HRTEM (insets) images of GdF3 (a), (b) and (c), YF3 (d) and (e), HoF3 (f) and YbF3 (g) nanoparticles. Reproduced with permission from reference 58. Copyright Wiley VCH, 2007. | ||
At the same time as Yan's group, another group reported the synthesis of doped NaYF4 using TFA precursors in oleylamine at 330 °C.60 The group made the more efficient up-converting hexagonal phase and found oleylamine enables the phase transition to the hexagonal form. The particles were also relatively small, which had been difficult to achieve before even when using other synthetic approaches. As pointed out by Yi and Chow,60 this is much better for use in biological applications, where particles smaller than 5.5 nm are preferred for renal clearance.61 The synthesis did not require the cubic phase of NaYF4 as a starting material, just the appropriate TFA salts. The group also found that the up-conversion efficiency was around one order of magnitude less than that of the bulk. Additionally the particles were made water soluble by exchanging ligand with a PEG diacid.
Yi and Chow also investigated the effect of growing a shell of NaYF4 on doped NaYF4nanoparticles and found a significant improvement in up-conversion emission.62 The group also developed another method to make the particles water-soluble by attaching a poly(acrylic acid) in addition to the shell, which had the advantage of offering the ability to attach to biomolecules. Further work investigated the synthesis of alkali metalrare-earthfluorides with alternatives to sodium.63 The paper describes the synthesis of LiYF4 and BaYF4 using TFA precursors, except for the barium source which was barium acetoacetonate (barium trifluoroacetate was not soluble in the solvents). The synthesis of LiYF4 also involved adding trifluoroacetic acid in addition to the TFA precursors. The products exhibited good up-conversion emission when doped, and the particles themselves were monodisperse and crystalline. A brief study showed that the trifluoroacetic acid concentration and reaction temperatures were the more important influences on the outcome of the reactions, for example, the presence of trifluoroacetic acid and the relatively high temperature of 340 °C were necessary for the formation of pure LiYF4, otherwise YOF was formed as a side product.
In a communication, Capobianco reported using TFA precursors for the synthesis of doped, cubic NaYF464 based on the report by Zhang et al.51 The particles themselves were polydisperse in size but crystalline and displayed red, green and blue up-conversion luminescence when doped. The group extended the synthesis64 and modified it to yield NaYF4 particles that were highly monodisperse, self-assembled and crystalline (Fig. 15).65 The change to the synthesis simply modified the method of delivery of the Ln precursors, which were first dissolved in the appropriate solvents and added slowly through a cannula into another solution at the required reaction temperature containing the rest of the precursors. Furthermore, Capobianco's group showed66 that coatingNaGdF4 (doped with Ce3+ and Tb3+) in a NaYF4 shell prevents the oxidation of the Ce3+ to Ce4+ and improved luminescence from Tb3+. In this system, NaGdF4 was the host material and the Ce3+ acted as a sensitiser which absorbed UV light and transferred the energy to Tb3+ allowing the particles to emit green fluorescence. The synthetic method itself was not however novel, but yielded high-quality particles.
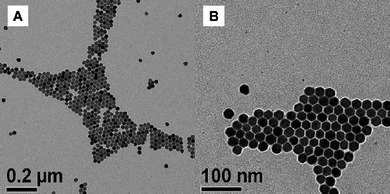 | ||
| Fig. 15 TEM images of NaYF4:Er, Yb nanoparticles. Reproduced with permission from reference 65. Copyright American Chemical Society, 2007. | ||
Liu and Chen tried a different approach to making rare-earthfluorides using TFA precursors. The group developed67 a solvothermal method to synthesise LaF3nanoparticles in the hexagonal phase. The experiment was based on a versatile general approach described by Wang et al.12 and involved adding the TFA precursors dissolved in water to a mixture of sodium oleate, oleylamine and ethanol, mixing, and then heating in an autoclave for 24 h at 190 °C. While this experiment was carried out at much lower temperatures than the conventional approach for this type of synthesis, it had a significantly longer reaction time. The products themselves were crystalline and were soluble in organic solvents, but tended to be relatively large and asymmetric in shape.
In 2007, another group attempted to use TOPO as the solvent when synthesising NaYF4 using TFA precursors.54 The resulting particles were not only crystalline and monodisperse but also in the hexagonal phase. The particles were also small enough to be used as biological labels (around 11 nm). TOPO has a higher boiling point than the usual three solvents used in TFA syntheses so it has the potential to produce the hexagonal phases of this class of material with much more ease.
In conclusion, the various techniques to synthesise rare-earthfluoridenanoparticles have all demonstrated control over shape and size. The methods using trifluoroacetate precursors in particular have demonstrated ease of control over the morphology of the Ln fluorides using three key solvents (oleylamine, oleic acid and octadecene), whilst other factors such as temperature and precursor concentrations also allow control over size and shape. The desire for nanoparticles with no heavy metals, narrow emission profiles and a narrow size distribution has lead to a wealth of research in this materials system,68,69 with many more applications expected in the up-coming years.
Acknowledgements
We acknowledge the EPSRC for support into rare-earth nanomaterials (EP/D501563). We thank the ACS, Wiley VCH, IoP publishing and Elsevier for kind use of the figures.References
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706 CrossRef CAS.
- Z. F. Zhang, L. G. Yu, W. M. Liu and Q. J. Xue, Tribol. Int., 2001, 34, 83 CrossRef CAS.
- J. F. Zhou, Z. S. Wu, Z. J. Zhang, W. M. Liu and H. X. Dang, Wear, 2001, 249, 333 CrossRef CAS.
- S. Heer, K. Kompe, H. U. Gudel and M. Haase, Adv. Mater., 2004, 16, 2102 CrossRef CAS.
- H. Schäfer, P. Ptacek, K. Kömpe and M. Haase, Chem. Mater., 2007, 19, 1396 CrossRef.
- M. Karbowiak, A. Mech, A. Bednarkiewicz and W. Strek, J. Alloys Compd., 2004, 380, 321 CrossRef CAS.
- A. Mech, M. Karbowiak, L. Kepinski, A. Bednarkiewicz and W. Strek, J. Alloys Compd., 2004, 380, 315 CrossRef CAS.
- M. Karbowiak, A. Mech, L. Kepinski, W. Mielcarek and S. Hubert, J. Alloys Compd., 2005, 400, 67 CrossRef CAS.
- M. Karbowiak, A. Mech, A. Bednarkiewicz, W. Strek and L. Kepinski, J. Phys. Chem. Solids, 2005, 66, 1008 CrossRef CAS.
- X. Wang and Y. D. Li, Chem.–Eur. J., 2003, 9, 5627 CrossRef CAS.
- X. Wang and Y. D. Li, Angew. Chem., Int. Ed., 2003, 42, 3497 CrossRef CAS.
- X. Wang, J. Zhuang, Q. Peng and Y. D. Li, Nature, 2005, 437, 121 CrossRef CAS.
- X. Wang, J. Zhuang, Q. Peng and Y. D. Li, Inorg. Chem., 2006, 45, 6661 CrossRef CAS.
- J. H. Zeng, J. Su, Z. H. Li, R. X. Yan and Y. D. Li, Adv. Mater., 2005, 17, 2119 CrossRef CAS.
- J. H. Zeng, Z. H. Li, J. Su, L. Wang, R. Yan and Y. Li, Nanotechnology, 2006, 17, 3549 CrossRef CAS.
- L. Y. Wang and Y. D. Li, Nano Lett., 2006, 6, 1645 CrossRef CAS.
- L. Y. Wang and Y. D. Li, Chem. Mater., 2007, 19, 727 CrossRef CAS.
- M. M. Lezhnina, T. Justel, H. Katker, D. U. Wiechert and U. H. Kynast, Adv. Funct. Mater., 2006, 16, 935 CrossRef CAS.
- S. Eiden-Assmann and G. Maret, Mater. Res. Bull., 2004, 39, 21 CrossRef CAS.
- J. L. Lemyre and A. M. Ritcey, Chem. Mater., 2005, 17, 3040 CrossRef CAS.
- F. J. Arriagada and K. Osseo-Asare, J. Colloid Interface Sci., 1999, 211, 210 CrossRef CAS.
- M. H. Lee, S. G. Oh and S. C. Yi, J. Colloid Interface Sci., 2000, 226, 65 CrossRef CAS.
- F. Wang, Y. Zhang, X. P. Fan and M. Q. Wang, Nanotechnology, 2006, 17, 1527 CrossRef CAS.
- Z. Li and Y. Zhang, Angew. Chem., Int. Ed., 2006, 45, 7732 CrossRef CAS.
- F. Wang, X. P. Fan, M. Q. Wang and Y. Zhang, Nanotechnology, 2007, 18, 025701 CrossRef.
- L. Zhu, X. M. Liu, J. Meng and X. Q. Cao, Cryst. Growth Des., 2007, 7, 2505 CrossRef CAS.
- L. Zhu, I. Meng and X. Q. Cao, Eur. J. Inorg. Chem., 2007, 3863 CrossRef CAS.
- L. Zhu, J. Meng and X. Q. Cao, J. Nanopart. Res., 2008, 10, 383 CrossRef CAS.
- Z. L. Wang, Z. W. Quan, P. Y. Jia, C. K. Lin, Y. Luo, Y. Chen, J. Fang, W. Zhou, C. J. O'Connor and J. Lin, Chem. Mater., 2006, 18, 2030 CrossRef CAS.
- C. Li, X. Liu, P. Yang, C. Zhang, H. Lian and J. Lin, J. Phys. Chem. C, 2008, 112, 2904 CrossRef CAS.
- J. W. Stouwdam and F. van Veggel, Nano Lett., 2002, 2, 733 CrossRef CAS.
- P. R. Diamente and F. van Veggel, J. Fluoresc., 2005, 15, 543 CrossRef CAS.
- V. Sudarsan, S. Sivakumar, F. van Veggel and M. Raudsepp, Chem. Mater., 2005, 17, 4736 CrossRef CAS.
- F. Evanics, P. R. Diamente, F. van Veggel, G. J. Stanisz and R. S. Prosser, Chem. Mater., 2006, 18, 2499 CrossRef CAS.
- B. Huang, Z. Liu, J. M. Hong, X. T. Chen, Z. L. Xue and X. Z. You, J. Cryst. Growth, 2005, 276, 613 CrossRef CAS.
- M. Wang, Q. L. Huang, J. M. Hong, W. H. Wu, Z. Yu, X. T. Chen and Z. L. Xue, Solid State Commun., 2005, 136, 210 CrossRef CAS.
- M. Wang, Q. L. Huang, J. M. Hong, X. T. Chen and Z. L. Xue, Cryst. Growth Des., 2006, 6, 1972 CrossRef CAS.
- M. Wang, Q. L. Huang, J. M. Hong, X. T. Chen and Z. L. Xue, Cryst. Growth Des., 2006, 6, 2169 CrossRef CAS.
- G. S. Yi, H. C. Lu, S. Y. Zhao, G. Yue, W. J. Yang, D. P. Chen and L. H. Guo, Nano Lett., 2004, 4, 2191 CrossRef CAS.
- Y. Wei, F. Q. Lu, X. R. Zhang and D. P. Chen, Chem. Mater., 2006, 18, 5733 CrossRef CAS.
- X. P. Fan, D. B. Pi, F. Wang, J. R. Qiu and M. Q. Wang, IEEE Trans. Nanotechnol., 2006, 5, 123 CrossRef.
- D. S. Jacob, L. Bitton, J. Grinblat, I. Felner, Y. Koltypin and A. Gedanken, Chem. Mater., 2006, 18, 3162 CrossRef.
- G. A. Kumar, C. W. Chen, J. Ballato and R. E. Riman, Chem. Mater., 2007, 19, 1523 CrossRef CAS.
- L. Ma, W. X. Chen, Y. F. Zheng, J. Zhao and Z. D. Xu, Mater. Lett., 2007, 61, 2765 CrossRef CAS.
- Z. J. Miao, Z. M. Liu, K. L. Ding, B. X. Han, S. D. Miao and G. M. An, Nanotechnology, 2007, 18, 125605 CrossRef.
- N. O. Nuñez and M. Ocaña, Nanotechnology, 2007, 18, 455606 CrossRef.
- F. Zhang, Y. Wan, T. Yu, F. Q. Zhang, Y. F. Shi, S. H. Xie, Y. G. Li, L. Xu, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2007, 46, 7976 CrossRef CAS.
- J. S. Zhang, W. P. Qin, J. S. Zhang, Y. Wan, C. Y. Cao, Y. Jin, G. D. Wei, G. F. Wang and L. L. Wang, Chem. Res. Chin. Univ., 2007, 23, 733 Search PubMed.
- A. A. Ansari, N. Singh and S. P. Singh, J. Nanopart. Res., 2008, 10, 703 CrossRef CAS.
- J. E. Roberts, J. Am. Chem. Soc., 1961, 83, 1087 CrossRef CAS.
- Y. W. Zhang, X. Sun, R. Si, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2005, 127, 3260 CrossRef CAS.
- N. G. Zhuravleva, A. A. Eliseev, N. A. Sapoletova, A. V. Lukashin, U. Kynast and Y. D. Tretyakov, Mater. Sci. Eng., C, 2005, 25, 549 CrossRef.
- N. G. Zhuravleva, N. A. Sapoletova, A. A. Eliseev, A. V. Lukashin, Y. D. Tretyakov and U. Kynast, Opt. Mater., 2006, 28, 606 CrossRef CAS.
- J. Shan, X. Qin, N. Yao and Y. Ju, Nanotechnology, 2007, 18, 445607 CrossRef.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13730 CrossRef CAS.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13721 CrossRef CAS.
- X. Sun, Y. W. Zhang, Y. P. Du, Z. G. Yan, R. Si, L. P. You and C. H. Yan, Chem.–Eur. J., 2007, 13, 2320 CrossRef CAS.
- Y. P. Du, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2008, 112, 405 CrossRef CAS.
- G. S. Yi and G. M. Chow, Adv. Funct. Mater., 2006, 16, 2324 CrossRef CAS.
- H. Soo Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165 CrossRef CAS.
- G. S. Yi and G. M. Chow, Chem. Mater., 2007, 19, 341 CrossRef CAS.
- G. S. Yi, W. B. Lee and G. M. Chow, J. Nanosci. Nanotechnol., 2007, 7, 2790 CrossRef CAS.
- J. C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444 CrossRef CAS.
- J. C. Boyer, L. A. Cuccia and J. A. Capobianco, Nano Lett., 2007, 7, 847 CrossRef CAS.
- J. C. Boyer, J. Gagnon, L. A. Cuccia and J. A. Capobianco, Chem. Mater., 2007, 19, 3358 CrossRef CAS.
- C. H. Liu and D. P. Chen, J. Mater. Chem., 2007, 17, 3875 RSC.
- Z. G. Yan and C. H. Yan, J. Mater. Chem., 2008, 18, 5046 RSC.
- J. Shen, L. D. Sun and C. H. Yua, Dalton Trans., 2008, 5687 RSC.
| This journal is © The Royal Society of Chemistry 2009 |
