Dendritic structures within dendritic structures: dendrimer-induced formation and self-assembly of nanoparticle networks
Grégory
Franc
ab,
Elena
Badetti
c,
Vincent
Collière
ab,
Jean-Pierre
Majoral
*ab,
Rosa María
Sebastián
*c and
Anne-Marie
Caminade
*ab
aCNRS, LCC (Laboratoire de Chimie de Coordination), 205, route de Narbonne, F-31077, Toulouse, France. E-mail: caminade@lcc-toulouse.fr; majoral@lcc-toulouse.fr; Fax: +33 56155 3003
bUniversité de Toulouse, UPS, INPT, LCC, F-31077, Toulouse, France
cDepartment of Chemistry, Universitat Autònoma de Barcelona, Cerdanyola, 08193, Barcelona, Spain. E-mail: rosamaria.sebastian@uab.es; Fax: +34 93581 1265
First published on 13th August 2009
Abstract
15-Membered tri-olefinic azamacrocycles as end-groups of phosphorus-containing dendrimers from generation 1 to 4 are used for obtaining Pt nanoparticles in mild conditions; remarkable and unprecedented branched supramolecular assemblies composed of dendrimers and coalesced Pt nanoparticles are obtained, which become larger for higher dendrimer generations, affording for the first time a very unique organization of organic dendritic structures interweaved with inorganic dendritic structures.
Introduction
Tuning the arrangement of nanometric building blocks attracts interest from many branches of science, and is believed to afford new perspectives for the elaboration of functional devices. Among nanometric building blocks, metallic nanoparticles have induced intense research activity for more than a decade.1 In a first approach, the focus was on the elaboration of the nanoparticles, generally from organometallic precursors, and their stabilization with suitable ligands. Colloidal templates were in particular shown to play a major role in controlling the size and shape of the inorganicnanoparticles.2 In addition, numerous experiments have used the tri-dimensional structure of dendrimers to elaborate nanoparticles,3 as we have recently exemplified for Pd nanoparticles.4 The second approach tries to organize the as-synthesized nanoparticles to elaborate size- and shape-controlled nanomaterials. One important method consists of using chemical interactions to direct the assembly of the nanoparticles in ordered arrays by the judicious choice of the coordinating ligand functionalities. Such an approach has in particular been used in self-assembled monolayers (SAMs) of nanoparticles,5 for connecting quantum dots,6 or for programmable nanoparticlecrystallization using DNA.7 We have previously proposed the unique example of dendrimers used for such a purpose,8 which induce the crystallization of gold nanoparticles.9 In order to simplify these processes, it appears highly desirable to find molecules/substrates able both to produce the nanoparticles from the organometallic precursor and then to organize the nanoparticles in defined networks, in an integrated bottom-up approach, which must be carried out in mild conditions to preserve both the substrate and the nanoparticles. In this communication we report the very first example of the use of dendrimers for both the synthesis and the organization of metallic nanoparticles in a one-pot process.Results and discussion
First of all, the type of dendrimer, the terminal groups, and the metal have to be chosen in order to maximize the chances of success of this unprecedented challenge. The elected dendritic backbone was previously synthesized by some of us over a decade ago,10 and we have already shown the compatibility of these phosphorus-containing dendrimers with numerous applications ranging from catalysis11 to biology,12 and in particular for the elaboration of nano-materials.13 The type of terminal group can be tuned at will, but we decided to choose 15-membered triolefinic triazamacrocycles.14 Indeed, these types of macrocycle were previously demonstrated by some of us to be able to afford nanoparticles in mild conditions, starting from organometallic precursors.15 Palladium nanoparticles were obtained from these types of macrocycle containing several long perfluorinated and long polyoxyethylene chains. They retain their ability to form discrete palladium nanoparticles when linked to the surface of phosphorus dendrimers, without the need for long chains.4 However, in none of these cases were such macrocycles (as monomer or linked to dendrimers) able to organize an exclusively ordered network of nanoparticles. Concerning the metal to be used, it appears that platinum is able to afford unique arrangements,16 such as hollow nanocages,17 nanospheres,18 circular nanosheets,19 and nanowires.20 Thus, we decided to use phosphorus dendrimers, possessing 15-membered triolefinic triazamacrocycles as terminal functions, to elaborate platinum nanoparticles, and if possible to organize them in networks.As indicated above, we have already reported the synthesis of dendrimers bearing two triolefinic triazamacrocycles linked to each terminal thiophosphoryl group.4 The compounds that we use in the present work possess the same type of macrocycle as terminal groups, but linked covalently to the dendrimer in a slightly different way than previously; in the present case, there is a single macrocycle per terminal thiophosphoryl group instead of two, and the orientation of the macrocycles relative to the surface of the dendrimer was linear in the former case, and perpendicular here, due to the formation of a 5-membered heterocycle21 (see Fig. 1). These dendrimers are obtained in basic conditions by reaction of the ethylenediamine derivative of the triolefinic triazamacrocycle M (macrocycle)4 with the P(S)Cl2 terminal groups of dendrimers of generations 1, 2, and 4, built from a cyclotriphosphazene core;22 their detailed synthesis will be reported elsewhere. We choose Pt2(dba)3 (dba = dibenzylideneacetone)23 as the organometallic precursor of platinum(0) nanoparticles. Indeed, in the case of palladium Pd2(dba)4 was previously found to be the best precursor of Pd(0) nanoparticles in mild conditions and without any reducing agent,4 affording either discrete complexes when an almost stoichiometric amount was used (1 to 1.5 Pd per macrocycle) or nanoparticles when a larger ratio of Pd was used. Thus we expected the same behavior in the case of the platinum derivatives.
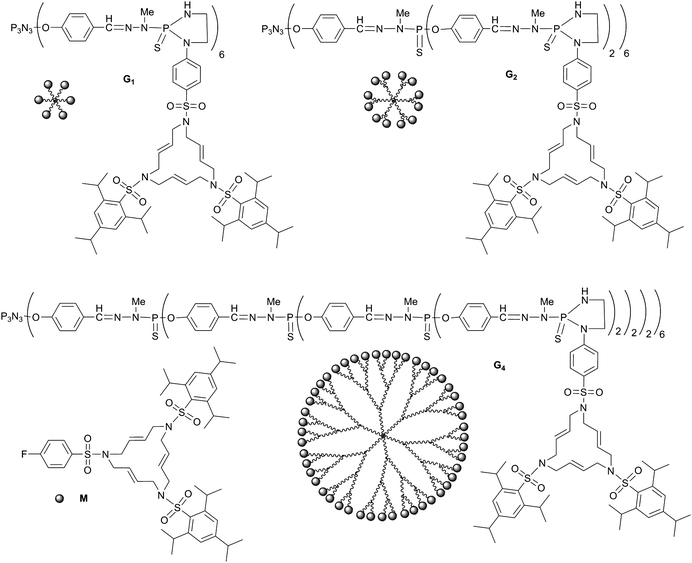 | ||
| Fig. 1 Chemical structure and schematization of the monomeric macrocycleM and of generations 1, 2, and 4 of the dendrimers issued from the grafting of the macrocycles onto the surface of phosphorus-containing dendrimersG1, G2, and G4, respectively (the fluorine of M is replaced by ethylenediamine for the grafting to the dendrimers). | ||
The reaction of Pt2(dba)3 with dendrimersG1, G2, G4 and with the monomeric macrocycleM are carried out overnight in refluxing THF and under air. In all cases, a stoichiometry of 1.2 Pt atoms per macrocycle is used. Such a stoichiometry was chosen first, since it was expected to afford discrete complexes, as we observed previously in the case of Pd, and we intended to increase the amount of Pt later to generate the nanoparticles. However, a deep black solution is formed in all cases, which cannot be analyzed by NMR. Only very broad signals are observed, which do not correspond to the expected discrete complexes, in contrast to what we have previously obtained in the case of palladium.4 We may anticipate that the size of the van der Waals radius of Pd (1.63 Å) fits better inside the macrocycle than in the case of Pt (1.75 Å). In order to understand what happened, a drop of the crude solution is diluted in THF, then a drop of the diluted solution is deposited onto a support for microscopy (carbon).†
Analysis by transmission electron microscopy (TEM) of the results of the reactions of Pt2(dba)3 with the monomer M and with the dendrimersG1, G2, G4 reveals totally unexpected results. Indeed, despite the very low amount of Pt used and the very mild conditions applied (no reducing agent and no protection against air or moisture under atmospheric pressure), oriented dendritic superstructures of Pt are observed in all cases, as shown by low resolution TEM (Fig. 2, with scale bar 200 nm). As indicated above, platinum has previously afforded shape controlled networks,18–21 but to the best of our knowledge, the type of arrangement that we observe here has never been previously reported. The shape of the network depends on the generation of the dendrimer. Large aggregates of Pt from which short but highly branched Pt networks emanate are obtained with the monomer M. With dendrimers, the size of Pt aggregates diminishes, as well as the number of ramifications of each Pt branch emanating from the aggregates, but the mean maximum length of branches increases and is directly proportional to the number of macrocycles per dendrimer (see insert in Fig. 2), affording an original example of “dendritic effect”. Furthermore, with increasing generations, the superstructure becomes more oriented, as particularly evidenced with the fourth generation, which affords “fan-shaped” (or “dendron-like”) Pt superstructures. Although branched superstructures were previously reported with platinum, and also with silver,24 they have in all cases a “dendrimer-like” structure, with branches emanating radially from a central point. The phenomenon that we observe here was predicted theoretically by Meakin 25 years ago in the case of a growth mechanism of clusters regulated by particle drift on diffusion-limited aggregation,25 and the structures he proposed are exactly analogous to the ones we observe in the case of the reaction with G4.
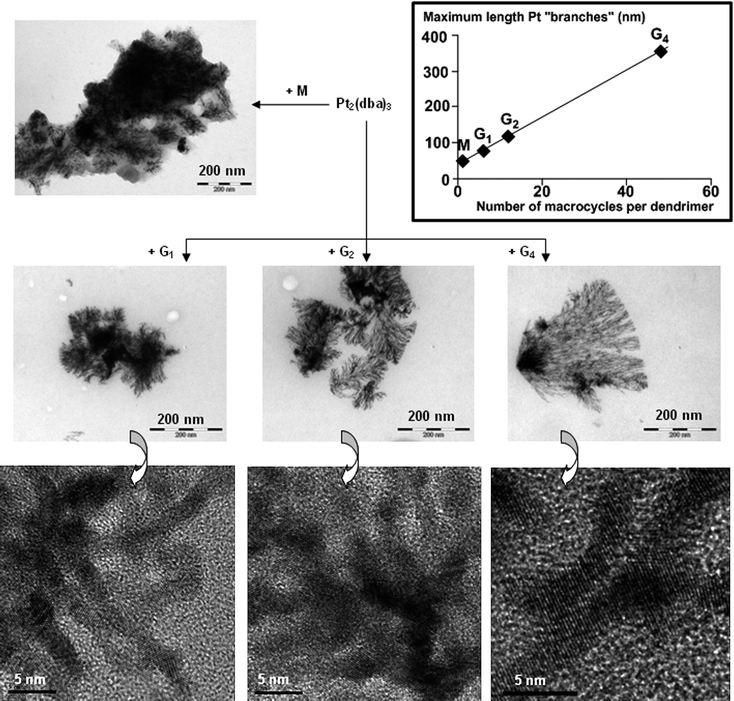 | ||
| Fig. 2 Results of the reactions of monomer M and dendrimersG1, G2, and G4 with Pt2(dba)3. TEM images (upper part, 200 nm: low resolution TEM; lower part, 5 nm: high resolution TEM). Insert: variation of the maximum length of nanoparticle “branches” versus the number of macrocycles per dendrimer. | ||
The next step for characterizing and understanding the phenomenon observed consists of verifying the presence of Pt nanoparticles and of dendrimers in the network. For this purpose, high resolution (HR) TEM images were obtained (Fig. 2, with scale bar 5 nm). HRTEM permits the observation of details of nanoparticles. The arrangement of Pt(0) atoms in the nanoparticles corresponds to classical Pt(fcc) (measured from the HRTEM image resulting from the reaction with G4). These images reveal the presence of coalesced nanoparticles, creating ribbons of 2–3 nm, composed of oriented layers of Pt atoms. Individual ribbons contain nanoparticles organized parallel, at least for a few nm, as can be seen in the HRTEM image obtained with G4. The branching is generally created by a partial superimposition with another ribbon.
In order to detect the presence of dendrimers in these networks (which cannot be detected by TEM), EDX analyses are carried out, in particular in the case of the network obtained from the G2dendrimer. Three different areas are analyzed (see Fig. 3): one inside an aggregate of Pt (numbered 1), another outside any network or aggregate (numbered 2), and a third one in the branches of the inorganic network (numbered 3). In the first case, all the signals corresponding to Pt are observed, in particular the most intense at 2.051 keV (Pt Ma1) and 9.441 keV (Pt La1). The signal of phosphorus cannot be unambiguously attributed, since it is overlapped with the Pt Ma1 signal; however, the signal corresponding to sulfur is detectable, demonstrating the presence of small quantities of dendrimers, even in this area with a high density of Pt. The second experiment is carried out outside any network, but the presence of low amounts of both Pt and sulfur (due to the dendrimers) is detected. It might correspond to discrete Pt complexes (one Pt(0) atom inside macrocycles linked to the dendrimer). The most important experiment is the third one, because it demonstrates that the branches of the network are not only composed of Pt but also of dendrimers, in an important proportion (even if EDX is not quantitative). The HRTEM images display a continuous network of Pt nanoparticles, thus we deduce from the EDX experiments that the dendrimers wrap the ribbons of nanoparticles to ensure their cohesion, and can also probably connect the ribbons to induce an almost linear continuation of the network. It is understandable that large dendrimers having numerous macrocycles as terminal groups should wrap Pt more efficiently and produce longer ribbons. Furthermore a single large dendrimer may also connect two parallel ribbons and may explain why experiments with the G4dendrimer produce longer but less branched networks than smaller compounds.
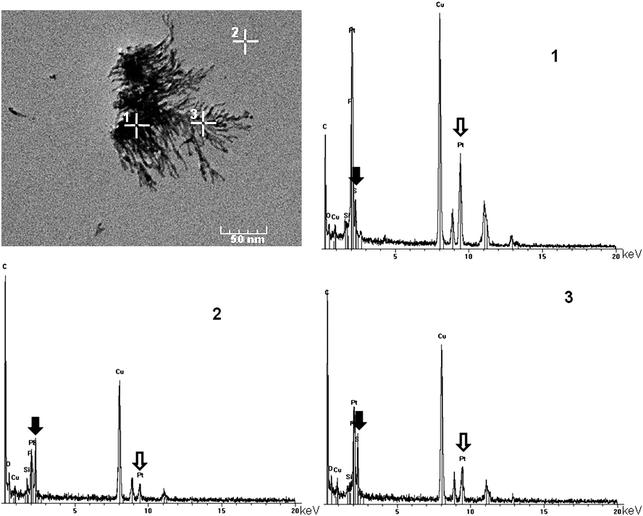 | ||
| Fig. 3 EDX analyses of the reaction of G2 with Pt2(dba)3 in three different areas (numbered 1, 2, and 3). Black arrows: sulfur (from the dendrimer); hollow arrows: platinum (Cu is from the microscope grid). | ||
Conclusion
In summary, we have demonstrated for the first time that specifically engineered dendrimers induce the elaboration of Pt nanoparticles in very mild conditions and then organize them in original hyperbranched networks of coalesced nanoparticles, for which both the size and the degree of branching vary with the generation of the dendrimer. To the best of our knowledge, this is the very first example of a self-integrated sequence of reactions using only 2 molecular components (the organometallic precursor and the dendrimer) to elaborate highly sophisticated networks. The methodology described in this report does not require any special equipment (no glove box, on the contrary the experiments were carried out in air), no reducing agent, and needs only a low stoichiometry of metal precursor. Thus it should be usable with other types of metal to produce other original nanomaterials.References
-
Metal Nanoparticles, ed. D. L. Feldheim and C. A. Foss Jr, Marcel Dekker, New York, 2002 Search PubMed
; G. Schmidt, Nanoparticles, from theory to applications, Wiley-VCH, Weinheim, 2004 Search PubMed
; V. Rotello, Nanoparticles: building blocks for nanotechnology, Springer Science, New York, 2004 Search PubMed
; Nanoparticle assemblies and superstructures, ed. N. A. Kotov, CRC Press, Taylor & Francis, Boca Raton, FL, 2006 Search PubMed
.
- M. P. Pileni, Nat. Mater., 2003, 2, 145 CrossRef CAS
; A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310 CrossRef CAS
.
- R. M. Crooks, M. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181 CrossRef CAS
; T. Vassilieff, A. Sutton and A. K. Kakkar, J. Mater. Chem., 2008, 18, 4031 RSC
; N. Satoh, T. Nakashima, K. Kamikura and K. Yamamoto, Nat. Nanotechnol., 2008, 3, 106 Search PubMed
.
- E. Badetti, A. M. Caminade, J. P. Majoral, M. Moreno-Mañas and R. M. Sebastián, Langmuir, 2008, 24, 2090 CrossRef CAS
.
- Z. M. Fresco and J. M. J. Fréchet, J. Am. Chem. Soc., 2005, 127, 8302 CrossRef CAS
; O. Crespo-Biel, B. Dordi, D. N. Reinhoudt and J. Huskens, J. Am. Chem. Soc., 2005, 127, 7594 CrossRef CAS
.
- M. Ouyang and D. A. Awschalom, Science, 2003, 301, 1074 CrossRef CAS
.
- S. Y. Park, A. K. R. Lytton-Jean, B. Lee, S. Weigand, G. C. Schatz and C. A. Minkin, Nature, 2008, 451, 553 CrossRef CAS
.
- For reviews about dendrimers, see in particular: Dendrimers and Dendrons, ed. G. R. Newkome, C. N. Moorefield and F. Vögtle, Wiley-VCH, Weinheim, 2001 Search PubMed
; Dendrimers and other dendritic polymers, ed. J. M. J. Fréchet and D. A. Tomalia, Wiley, Chinchester, 2001 Search PubMed
; J. P. Majoral and A. M. Caminade, Chem. Rev., 1999, 99, 845 Search PubMed
; G. R. Newkome and C. D. Shreiner, Polymer, 2008, 49, 1 Search PubMed
.
- G. Schmid, W. Meyer-Zaika, R. Pugin, T. Sawitowski, J. P. Majoral, A. M. Caminade and C.-O. Turrin, Chem.–Eur. J., 2000, 6, 1693 CrossRef CAS
; G. Schmid, E. Emmrich, J. P. Majoral and A. M. Caminade, Small, 2005, 1, 73 CrossRef CAS
.
- N. Launay, A. M. Caminade, R. Lahana and J. P. Majoral, Angew. Chem., Int. Ed. Engl., 1994, 33, 1589 CrossRef
; N. Launay, A. M. Caminade and J. P. Majoral, J. Am. Chem. Soc., 1995, 117, 3282 CrossRef CAS
.
- A. Ouali, R. Laurent, A. M. Caminade, J. P. Majoral and M. Taillefer, J. Am. Chem. Soc., 2006, 128, 15990 CrossRef CAS
; A. M. Caminade, P. Servin, R. Laurent and J. P. Majoral, Chem. Soc. Rev., 2008, 37, 56 RSC
.
- L. Griffe, M. Poupot, P. Marchand, A. Maraval, C.-O. Turrin, O. Rolland, P. Métivier, G. Bacquet, J. J. Fournié, A. M. Caminade, R. Poupot and J. P. Majoral, Angew. Chem., Int. Ed., 2007, 46, 2523 CrossRef CAS
; T. R. Krishna, M. Parent, M. H. V. Werts, L. Moreaux, S. Gmouh, S. Charpak, A. M. Caminade, J. P. Majoral and M. Blanchard-Desce, Angew. Chem., Int. Ed., 2006, 45, 4645 CrossRef CAS
; A. M. Caminade, C.-O. Turrin and J. P. Majoral, Chem.–Eur. J., 2008, 14, 7422 CrossRef CAS
.
- A. M. Caminade and J. P. Majoral, Acc. Chem. Res., 2004, 37, 341 CrossRef CAS
; J. Leclaire, Y. Coppel, A. M. Caminade and J. P. Majoral, J. Am. Chem. Soc., 2004, 126, 2304 CrossRef CAS
; D. H. Kim, P. Karan, P. Göring, J. Leclaire, A. M. Caminade, J. P. Majoral, U. Gösele, M. Steinhart and W. Knoll, Small, 2005, 1, 99 CrossRef CAS
; C. L. Feng, X. H. Zhong, M. Steinhart, A. M. Caminade, J. P. Majoral and W. Knoll, Small, 2008, 4, 566 CrossRef CAS
; E. Martínez-Ferrero, G. Franc, S. Mazères, C.-O. Turrin, C. Boissière, A. M. Caminade, J. P. Majoral and C. Sanchez, Chem.–Eur. J., 2008, 14, 7658 CrossRef CAS
.
- For reviews see: M. Moreno-Mañas, R. Pleixats, A. Roglans, R. M. Sebastián and A. Vallribera, Arkivoc, 2004,(iv), 109 Search PubMed
, available from http://www.arkat-usa.org; M. Moreno-Mañas, R. Pleixats, R. M. Sebastián, A. Vallribera and A. Roglans, J. Organomet. Chem., 2004, 689, 3669 CAS
.
- A. Serra-Muns, R. Soler, E. Badetti, P. de Mendoza, M. Moreno-Mañas, R. Pleixats, R. M. Sebastián and A. Vallribera, New J. Chem., 2006, 30, 1584 RSC
.
- F. Wen, N. Waldöfner, W. Schmidt, K. Angermund, H. Bönnemann, S. Modrow, S. Zinoveva, H. Modrow, J. Hormes, L. Beuermann, S. Rudenkiy, W. Maus-Friedrichs, V. Kempter, T. Vad and H. G. Haubold, Eur. J. Inorg. Chem., 2005, 3625 CrossRef CAS
.
- Y. Song, R. M. Garcia, R. M. Dorin, H. Wang, Y. Qiu and J. A. Shelnutt, Angew. Chem., Int. Ed., 2006, 45, 8126 CrossRef CAS
.
- Y. Song, W. A. Steen, D. Peña, Y. B. Jiang, C. J. Medforth, Q. Huo, J. L. Pincus, Y. Qiu, D. Y. Sasaki, J. E. Miller and J. A. Shelnutt, Chem. Mater., 2006, 18, 2335 CrossRef CAS
.
- Y. Song, Y. Yang, C. J. Medforth, E. Pereira, A. K. Singh, H. Xu, Y. Jiang, C. J. Brinker, F. van Swol and J. A. Shelnutt, J. Am. Chem. Soc., 2004, 126, 635 CrossRef CAS
.
- Y. Song, R. M. Garcia, R. M. Dorin, H. Wang, Y. Qiu, E. N. Coker, W. A. Steen, J. E. Miller and J. A. Shelnutt, Nano Lett., 2007, 7, 3650 CrossRef CAS
; E. Ramirez, L. Eradès, K. Philippot, P. Lecante and B. Chaudret, Adv. Funct. Mater., 2007, 17, 2219 CrossRef CAS
.
- D. Prévôté, B. Donnadieu, M. Moreno-Mañas, A. M. Caminade and J. P. Majoral, Eur. J. Org. Chem., 1999, 1701 CAS
.
- N. Launay, A. M. Caminade and J. P. Majoral, J. Organomet. Chem., 1997, 529, 51 CrossRef CAS
.
- K. Moseley and P. M. Maitlis, J. Chem. Soc. D, 1971, 982 RSC
.
- X. Wang, K. Naka, H. Itoh, S. Park and Y. Chujo, Chem. Commun., 2002, 1300 RSC
.
- P. Meakin, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 28, 5221 CrossRef
.
Footnote |
| † Typical treatment and deposition: DendrimerGn (n = 1, 2, 4) and Pt2(dba)3 (1.2 eq per azamacrocycle) were mixed together in THF to form a rather concentrated solution. The mixture was left overnight in refluxing THF under strong stirring. Next morning the mixture, which had turned to deep black, was then allowed to reach room temperature. Subsequently, a few drops of the dark mixture were dissolved in THF in order to obtain a clear dilute solution. A small drop of the latter was then deposited on a grid to perform the TEM and EDX analyses. The grid was home-made from a commercial copper grid (diameter 3.05 mm) which was covered by a thin collodion membrane, on which carbon was evaporated (approximately 50 nm thickness). Low resolution TEM analyses were performed on a JEOL JEM 1011 (100 kV, resolution 4 Å). A wide angle Megaview III (SIS) camera was used for routine imaging. High resolution TEM analyses and EDX analyses were performed on the TEM-FEG (Field Emission Gun) JEOL JEM 2100F (200 kV, resolution 2.3 Å), Analyses X PGT (resolution 135 eV). Images were acquired with a CDD Gatan 2K × 2K camera. |
| This journal is © The Royal Society of Chemistry 2009 |
