Fabrication of fullerene-decorated carbon nanotubes and their application in flame-retarding polypropylene†
Pingan
Song
ab,
Yu
Shen
ab,
Baoxian
Du
ab,
Zhenghong
Guo
a and
Zhengping
Fang
*ab
aLaboratory of Polymer Materials and Engineering, Ningbo Institute of Technology, Zhejiang University, Ningbo 315100, P. R. China
bInstitute of Polymer Composites, Zhejiang University, Key Laboratory of Macromolecular Synthesis and Functionalization, Ministry of Education, Hangzhou 310027, China
First published on 28th August 2009
Abstract
Multi-walled carbon nanotubes were decorated with fullerene (C60) via a three-step chemical functionalization, with the goal of combining their unique physical and chemical characteristics and simultaneously improving the solubility of CNTs in organic solvents. C60 molecules, about 0.67% by molecule, were homogeneously bonded onto the surface of the CNTs. Electron microscopy clearly shows that C60 molecules are introduced onto the surface of the CNTs, and this is also evidenced by their UV–VIS absorption spectra. Cone calorimetry measurements showed that compared with pristine CNTs, fullerene-decorated CNTs further reduced the flammability of polypropylene, which may be due to the free-radical-trapping effect of C60 and the barrier effect of the CNT network.
Carbon nanotubes (CNTs) have been reported over the past few years to hold promising applications, ranging from nanodevices to nanocomposites, due to their remarkable electrical, mechanical, and thermal properties.1–3 However, the application of CNTs in devices has been hindered due to their poor dispersion and homogeneity within polymers, limiting their photovoltaic performance.4 Chemical or physical functionalization of CNTs may increase the interfacial binding between CNTs and polymer matrices.5 On the other hand, fullerene (C60), a strong electron acceptor, has been found to have high activity to free radicals.6 Considering the good solubility of C60 in many organic solvents, like toluene and dichlorobenzene, and its excellent free-radical-trapping properties, we tried to decorate the surface of CNTs with C60 molecules. This strategy may not only improve the solubility of CNTs, but also combine the unique performances of both C60 and CNTs and also extend their applications. Although the covalent and non-covalent attachment of C60 molecules to the surface of individual CNTs has been reported in the literature, the reaction conditions were severe and the procedure was inconvenient.7–9
The present work is the precursory part of a project to develop high-performance conjugated polymer/CNT composites. Herein, we presented a novel strategy for fabricating C60-decorated carbon nanotubes (C60-d-CNTs). This strategy consists of a three-step reaction: first hydroxylation using KOH/ethanol treatment, and then amino-functionalization using 3-aminopropyltriethyoxylsilane (APTES), and finally C60-decoration of CNTs through the addition reaction of C60 molecules with amino groups. Finally, we also investigated whether C60-d-CNTs could endow excellent flame retardancy to polypropylene.
The multi-walled carbon nanotubes used in this study, prepared by hydrogen direct-current arc discharge, were purchased from the Chendu Organic Chemistry Institute and purified according to the method reported previously.10 Other chemical agents were analytical grade and used as received.
The synthesis route for C60-decorated CNTs is schematically shown in Scheme 1. In a typical hydroxylation procedure, 100 mg pristine CNTs were added to a single-necked flask containing 100 mL ethanol and 5 g KOH. After ultrasonic dispersion for 30 min, the solution was refluxed for 8 h, then the mixture was filtered, washed thoroughly with ethanol and deionized water three times, and dried at 80 °C under vacuum for 12 h. The resultant product was designated as CNT-OH (thermogravimetric analysis (TGA) showed a 5 wt% weight loss occurred compared with pristine CNTs, see the ESI† ). Secondly, the amino-functionalization of CNTs was carried out using the following procedure. Typically, 50 mg CNT-OH was dispersed in tolueneviasonication for 30 min and then an excess of APTES solution was added dropwise and the mixture stirred at 80 °C for 8 h to enable the reaction to go to completion. The amino-functionalized CNTs were filtered, washed with toluene and ethanol, finally, dried at 80 °C under vacuum for 12 h and designated as CNT-NH2 (TGA showed a 6 wt% weight loss compared to CNT-OH, see the ESI† ). As for the preparation of C60-decorated CNTs, 20 mg CNT-NH2 was dispersed in 100 mL o-dichlorobenzene using sonication for 30 min. After sonication, an excess of C60 (10 mg) in o-dichlorobenzene was introduced and the mixture stirred for 8 h at 85 °C. The product was obtained by filtration and washing with o-dichlorobenzene, ethanol, and acetone sequentially. The C60-decorated CNTs were dried at 80 °C under vacuum for 12 h and designated as C60-d-CNTs. Around 20.9 mg was obtained.
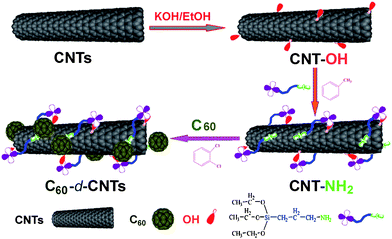 | ||
| Scheme 1 Schematic synthesis route for C60-d-CNTs. Note: the self-condensation of APTES actually occurred, as evidenced by IR spectrometry, but this is not described in the above scheme. | ||
Infrared spectrometry (IR, Vector-22 FT-IR), X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250) and UV–VIS absorption spectroscopy were exploited to characterize the changes in each step of the CNT chemical functionalization process. The morphology of the functionalized CNTs was observed using transmission electron microscopy (TEM, JEM-1200EX). The flame retardancy of the sample (100 × 100 × 3 mm3) was evaluated using a cone calorimeter and was performed in an FTT UK device according to ISO 5660 with an incident flux of 35 kW m−2. Typical results from cone calorimetry are reproducible to within around ±10% and the data reported here are the means of triplicate experiments.
Fig. 1A shows the IR spectrum for pristine CNTs. The appearance of a rather weak and broad absorption band at ca. 3440 cm−1 was due to the presence of –OH groups on the surface of the pristine CNTs, and was believed to result from either ambient atmospheric moisture bound to the CNTs or oxidation during purification of the raw CNTs.11 Another peak at ca. 1578 cm−1 was attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of quinine groups on the surface of the CNTs.11 In the IR spectrum of CNT-OH (Fig. 1B) a strong and broad band at 3400–3520 cm−1 was assigned to the –OH groups which arose from hydroxylation of the CNTs, the appearance of peaks at 1334 cm−1 and 1080 cm−1 was attributed to the bending vibration of O–H and the stretching of C–O, respectively. These changes confirmed the occurrence of hydroxylation of the CNTs. For the IR spectrum of CNT-NH2 (Fig. 1C), aside from the 3400–3520 cm−1 band becoming weaker and broader, the appearance of two new absorption peaks at 2853 cm−1 and 2922 cm−1 were assignable to stretching of the methylene (–CH2) groups from APTES, and the peak at 1334 cm−1 also disappeared. In addition to those, the broad band at 1000–1130 cm−1 was ascribed to the stretching of Si–O–Si groups, which were not described in schematic synthesis route of C60-d-CNTs (Scheme 1). At the same time, the stretching of Si–C bonds was also observed at ca. 695 cm−1. The above changes of absorption peaks and bands proved the occurrence of amino-functionalization of the CNTs. In the case of the spectrum of C60-d-CNTs (Fig. 1D), the appearance of two new peaks at 2975 cm−1 and 527 cm−1 were ascribed to the stretching of C60–H and the characteristic peaks of the C60 molecule, respectively.12 Another new, slightly stronger and broader peak at ca. 1651 cm−1 should be assigned to the bending mode of H–NR–C60 (R = CNTs), in comparison with the weak and narrow peak at 1630 cm−1 belonging to the bending vibration of N–H groups in CNT-NH2. The above changes verify the presence of C60 moieties on the surface of the CNTs.
O stretching vibration of quinine groups on the surface of the CNTs.11 In the IR spectrum of CNT-OH (Fig. 1B) a strong and broad band at 3400–3520 cm−1 was assigned to the –OH groups which arose from hydroxylation of the CNTs, the appearance of peaks at 1334 cm−1 and 1080 cm−1 was attributed to the bending vibration of O–H and the stretching of C–O, respectively. These changes confirmed the occurrence of hydroxylation of the CNTs. For the IR spectrum of CNT-NH2 (Fig. 1C), aside from the 3400–3520 cm−1 band becoming weaker and broader, the appearance of two new absorption peaks at 2853 cm−1 and 2922 cm−1 were assignable to stretching of the methylene (–CH2) groups from APTES, and the peak at 1334 cm−1 also disappeared. In addition to those, the broad band at 1000–1130 cm−1 was ascribed to the stretching of Si–O–Si groups, which were not described in schematic synthesis route of C60-d-CNTs (Scheme 1). At the same time, the stretching of Si–C bonds was also observed at ca. 695 cm−1. The above changes of absorption peaks and bands proved the occurrence of amino-functionalization of the CNTs. In the case of the spectrum of C60-d-CNTs (Fig. 1D), the appearance of two new peaks at 2975 cm−1 and 527 cm−1 were ascribed to the stretching of C60–H and the characteristic peaks of the C60 molecule, respectively.12 Another new, slightly stronger and broader peak at ca. 1651 cm−1 should be assigned to the bending mode of H–NR–C60 (R = CNTs), in comparison with the weak and narrow peak at 1630 cm−1 belonging to the bending vibration of N–H groups in CNT-NH2. The above changes verify the presence of C60 moieties on the surface of the CNTs.
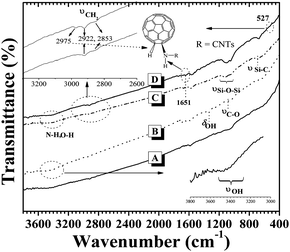 | ||
| Fig. 1 IR spectra of (A) pristine CNTs, (B) CNT-OH, (C) CNT-NH2, and (D) C60-d-CNTs. | ||
XPS spectra for pristine CNTs, CNT-OH, CNT-NH2, and C60-d-CNTs, as well as N 1s spectra for CNT-NH2 and C60-d-CNT are shown in Fig. 2, with the chemical compositions listed in Fig. 2A. For the pristine CNTs, aside from the main C–C peak at 284.5 eV, another weak photoemission present at a higher binding energy (BE) of ca. 286.1 eV (O 1s, atm%: 1.78) was attributed to atmospheric moisture or oxidation during the purification process,13 which is consistent with the IR results mentioned above. As expected, CNT-OH displayed a much stronger photoemission at a BE of 286.1 eV belonging to O 1s. The concentration of oxygen increased up to 8.86 atm% and the C 1s spectrum showed a BE of 531.5 eV assigned to C–OH, which implied the CNTs were successfully hydroxylated. In comparison with CNT-OH, two new photoemission peaks appeared in the XPS spectrum of CNT-NH2, namely at BEs of 102.8 eV (Si 2p, atm%: 1.99) and ca. 399.3 eV (N 1s, atm%: 2.02), and the higher surface oxygen content (as high as 13.14 atm%) was attributed to the hydrolysis of APTES, which provided the proof for amino-functionalization of the CNTs. As for C60-d-CNTs, a very important point is the increase in surface content of carbon, increasing from 82.85 atm% for CNT-NH2 to 86.49 atm% for C60-d-CNTs, which was due to the introduction of C60 molecules onto the surface of CNT-NH2, and also suggested about 0.67% C60 molecules were attached onto the surface of CNTs (ca. 2.71 wt% by mass obtained from TGA). Another direct proof was the splitting of the N 1s peak (see Fig. 2B), namely from one photoemission peak at a BE of ca. 399.6 eV for CNT-NH2, to three peaks at BEs of 399.5 eV (C–N), 400.3 eV and 401.4 eV for C60-d-CNTs respectively, indicating that the primary amino group (–NH2) was chemically bonded to another atom. In our system, it was obvious that the amino groups were only likely to react with C60 molecules via an amine-addition reaction reported by plenty of organic chemists. The BEs at 400.3 and 401.4 eV for C60-d-CNTs were 0.7 eV and 2.0 eV higher than those for CNT-NH2, this higher binding energy may be due to the strong electron-withdrawing effect of the C60 molecules. Because C60 has an electron-withdrawing effect, the BE at 400.3 eV may be due to the photoemission peak of N 1s in the bi-addition product, CNT–N(C60)2, while 401.4 eV belongs to the peak of N 1s in the mono-addition product, CNT–NH–C60.
 | ||
| Fig. 2 XPS spectra of pristine CNTs, CNT-OH, CNT-NH2, and C60-d-CNTs, as well as N 1s spectra of CNT-NH2, and C60-d-CNTs. | ||
On the basis of IR and XPS results, we have made sure, on the whole, that C60 molecules were successfully introduced onto the surface of CNTs. Furthermore, we also employed TEM, SEM and UV–VIS absorption spectroscopy to characterize our products, as discussed in the following sections.
As shown in Fig. 3, apparently compared with pristine CNTs (A), the diameter of CNT-OH (B) remained at 20–50 nm, but the surface of the latter was much more smooth, due to the fact that the hydroxylation reaction got rid of the amorphous carbon on the surface of the pristine CNTs. However, after CNT-OH was amino-functionalized using APTES, not only did the diameter of the CNTs increase to some degree, but also their surfaces were much more rough relative to CNT-OH, and a thin polymeric layer could be observed (see Fig. 3C, marked by arrows). Using the above IR and XPS analyses, we were able to draw the conclusion that the layer is the self-condensation product of APTES. Spherical C60 nanoparticles with a diameter of 80–130 nm can be readily observed in Fig. 3D. While for C60-d-CNTs (E) compared with CNT-NH2, besides the further increase in diameter, up to 90 nm, especially for the end of CNTs because of high activation at the ends of tubes, some salient parts could be obviously observed (marked by arrows). The size of the C60 molecules attached to the surface of the CNTs was much larger than those reported in the literature.7–9 Since the raw C60-d-CNT product was washed viaultrasonication using o-dichlorobenzene three times to remove the unreacted and physically adsorbed C60 molecules, and the C60 molecule has a diameter of only ca. 0.7 nm, a possible reason may be that the condensation polymerization of APTES created a large number of amino groups which produced more multi-adducts [CNT-N (C60)2] when reacting with excess C60. To further verify the above analysis, a TEM image of a physical mixture of CNTs and C60 is also presented for comparison (F). Obviously, the C60 molecules are in the form of crystalline self-aggregates and are separated from the CNTs, supporting the above observations.
 | ||
| Fig. 3 TEM images of (A) pristine CNTs, (B) CNT-OH, (C) CNT-NH2, (D) C60, (E) C60-d-CNTs, and (F) a physical mixture of C60 and CNTs. | ||
SEM images of CNTs, CNT-OH, CNT-NH2 and C60-d-CNTs are shown in Fig. 4. On the whole, the SEM observations were consistent with those from TEM, like the surface of CNT-OH being more smooth than pristine CNTs, while the surface of CNT-NH2 was more rough and some aggregates can be observed compared with CNT-OH. For C60-d-CNTs, some salient parts appeared, which can be clearly observed in the partially magnification image (marked by arrows in Fig. 4D), in good agreement with the TEM observations.
 | ||
| Fig. 4 SEM images of (A) pristine CNTs, (B) CNT-OH, (C) CNT-NH2, (D) C60-d-CNTs. | ||
Fig. 5 shows the UV–VIS spectra of pristine C60 and pristine CNTs, and their derivatives in tetrahydrofuran (THF). It should be noted that the solubility of C60 in THF was not good, thus, the measurements for C60/THF were performed shortly after sonication for 5 min. While for other samples, after sonication for 5 min, the solution was allowed to remain stable for 30 min before the tests were conducted. For the CNT derivatives (A–C), the UV–VIS absorption spectra showed basically no differences. While for C60-d-CNTs (D), a new weak absorption peak at a wavelength of 328 nm appeared, slightly shorter than the 330 nm peak belonging to the characteristic absorption of C60. The reduction of absorption wavelength was due to the reduction in π-electrons in C60 molecules caused by the amine-addition reaction.12 From the digital photos (see Fig. 5), we could observe that C60-d-CNTs in THF solution looked black and slightly yellow relative to black for CNT-NH2 and yellow for pristine C60. On the other hand, C60-d-CNTs exhibited better solubility in toluene than pristine CNTs, which may favor the dispersion of CNTs in polymer matrices. As for the detailed flame retardant mechanism, it will be reported in the near future.
 | ||
| Fig. 5 UV–VIS absorption spectra of (A) pristine CNTs, (B) CNT-OH, (C) CNT-NH2, (D) C60-d-CNTs, and (E) pristine C60 in THF at room temperature. Digital photos of the solubility in THF for the latter three samples are also given. | ||
Fig. 6 presents the heat-release rate curves of polypropylene (PP) and its composites with 1 wt% CNTs or C60-d-CNTs. As is clearly evident, the incorporation of CNTs considerably reduced the peak heat-release rate (PHRR) of PP (around 66% reduction). While at the same loading level, C60-d-CNTs not only decrease the PHRR further, but also slow down the combustion process, indicating that C60-d-CNTs confer better flame retardancy to PP relative to pristine CNTs. To clarify whether the grafting effect or the effect of a simple combination of C60 and CNTs was responsible for this observation, we conducted a control experiment by physically mixing C60 and CNTs in PP. The PHRR value for this composite was higher than for C60-d-CNTs at the same loading level, which indicated it was the grafting effect, not the effect of a simple combination of two that was responsible. According to the investigations reported by T. Kashiwagi14,15 and our group,16,17 the improvement in flame retardancy may be due to the free-radical-trapping effect of C60 and the barrier effect of the CNT network. Since the size of pristine C60 is too small to aggregate in a PP matrix, it was not easy to optimize its action. Whilst in C60-d-CNTs, the grafting will make C60 disperse better in PP and therefore endow it with better flame retardancy.
 | ||
| Fig. 6 Heat-release rate curves for pure PP, PP/1 wt% CNTs, PP/1 wt% C60-d-CNTs, and 1 wt% of a physical mixture C60 and CNTs at a heat flux of 35 kW m−2. Note: PHRR is the peak-heat-release rate. | ||
In conclusion, we have developed a new strategy to functionalize CNTs using fullerenevia a three-step reaction. C60 molecules (about 3.6% by atom or 0.67% by molecule), homogeneously reside on the surface of the CNTs by covalent chemical bonds. The C60-decorated CNTs are more soluble in organic solvents than pristine CNTs. Furthermore, compared with pristine CNTs, C60-d-CNTs confer better flame retardancy to PP, and the improvement in flame retardancy may be due to the free-radical-trapping effect of C60 and the barrier effect of the CNT network. As for more flame retardancy results and the corresponding mechanism, this work is in progress and will be reported in the near future.
Acknowledgements
The authors acknowledge financial support from the Natural Science Foundation of China (50873092).References
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- C. Gao, Macromol. Rapid Commun., 2006, 27, 841 CrossRef CAS.
- M. Moniruzzaman and K. I. Winey, Macromolecules, 2006, 39, 5194 CrossRef CAS.
- G. Kalita, S. Adhikari, H. R. Aryal, R. Afre, T. Soga, M. Sharon and M. Umeno, Curr. Appl. Phys., 2009, 9, 346 CrossRef.
- J. Chen, M. A. Hamon, H. Hu, Y. Chen, A. M. Rao, P. C. Eklund and R. C. Haddon, Science, 1998, 282, 95 CrossRef CAS.
- P. J. Krusic, E. Wasserman, P. N. Keizer, J. R. Morton and K. F. Preston, Science, 1991, 254, 1183 CrossRef CAS.
- W. Wu, H. R. Zhu, L. Z. Fan and S. H. Yang, Chem.–Eur. J., 2008, 14, 5981 CrossRef CAS.
- D. M. Guldi, E. Menna, M. Maggini, M. Marcaccio, D. Paolucci and F. Paolucci et al., Chem.–Eur. J., 2006, 12, 3975 CrossRef CAS.
- J. L. Delgado, P. D. L. Cruz, A. Urbina, J. T. L. Navarrete, J. Casado and F. Langa, Carbon, 2007, 45, 2250 CrossRef CAS.
- I. W. Chiang, B. E. Brinson, R. E. Smalley, J. L. Margrave and R. H. Hauge, J. Phys. Chem. B, 2001, 105, 1157 CrossRef CAS.
- P. C. Ma, J. K. Kim and B. Z. Tang, Carbon, 2006, 44, 3232 CrossRef CAS.
- D. E. Weeks and W. G. Harter, J. Chem. Phys., 1989, 90, 4744 CrossRef CAS.
- T. Ramanathan, F. T. Fisher, R. S. Ruoff and L. C. Brinson, Chem. Mater., 2005, 17, 1290 CrossRef CAS.
- T. Kashiwagi, F. M. Du, J. F. Douglas, K. I. Winey, R. H. Harris and J. R. Shields, Nat. Mater., 2005, 4, 928 CrossRef CAS.
- T. Kashiwagi, M. F. Mu, K. Winey, B. Cipriano, S. R. Raghavan, S. Pack, M. Rafailovich, E. Grulke, J. Shields, R. Harris and J. Douglas, Polymer, 2008, 49, 4358 CrossRef CAS.
- P. G. Song, Y. Zhu, L. F. Tong and Z. P. Fang, Nanotechnology, 2008, 19, 225707 CrossRef.
- P. G. Song, H. Liu, Y. Shen, B. X. Du and Z. P. Fang, J. Mater. Chem., 2009, 19, 1305–1313 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional TGA curves and SEM and TEM images. See DOI: 10.1039/b9nr00026g |
| This journal is © The Royal Society of Chemistry 2009 |
