Magnetically moveable bimetallic (nickel/silver) nanoparticle/carbon nanotube composites for methanol oxidation†
Guan-Ping
Jin
*ab,
Ronan
Baron
a,
Neil V.
Rees
a,
Lei
Xiao
a and
Richard G.
Compton
*a
aDepartment of Chemistry, Physical and Theoretical Chemistry Laboratory, Oxford University, South Parks Road, Oxford, UK OX1 3QZ. E-mail: richard.compton@chem.ox.ac.uk; Fax: 0044-1-865 275410; Tel: 0044-1-1865 275413
bAnhui Key Laboratory of Controllable Chemistry Reaction & Material Chemical Engineering, School of Chemical Engineering, Hefei University of Technology, Hefei, 230009, P. R. China. E-mail: jgp@hfut.edu.cn; Fax: 0086-0551-2902450; Tel: 0086-551-2901450
First published on 31st October 2008
Abstract
Multi-walled carbon nanotubes (CNTs) functionalized both by nickel and silver nanoparticles were obtained using a single step chemical deposition method in an ultrasonic bath. The new composite material was characterized by means of scanning electron microscopy (SEM), X-ray diffraction (XRD) and cyclic voltammetry (CV). The electroactivity of the bi-functionalized CNTs multi-walled carbon nanotubes was assessed in respect to the electrooxidation of methanol. It was found that the carbon nanotube supported silver nanoparticles have significantly higher catalytic properties than the bulk metal of the same surface area. Furthermore, it was shown that the presence of only a very small proportion of magnetic nickel nanoparticles (1.5% of the total number of metallic nanoparticles) allows the bi-functionalized carbon nanotubes to be moved magnetically in solution, making them easily recoverable after use whilst keeping an optimal electrocatalytic surface area.
1. Introduction
The synthesis of magnetic nanomaterials has attracted considerable attention in the last few years. One of the reasons for that interest is that magnetic material can be moved using a magnet and which makes the considered material recoverable both for economical and environmental issues.1–4 Nanomaterials that can be magnetically driven are also very promising for medical applications, such as drug delivery5 or complex biomanipulations.6The functionalization of carbon materials with magnetic particles is particularly attractive due to the properties and wide use of carbon materials. In particular, magnetic nanoparticles have been synthesized on the surface of carbon nanotubes using various different methodologies.6–10
As far as the use of magnetic nanomaterials in electrochemistry is concerned, there have been only a limited number of reports on the matter. Most notably Willner et al. studied the use of hydrophobic magnetic nanoparticles capable of blocking an electrode surface11–13 and Wang et al. addressed the use of magnetic nickel nanoparticles both for on-demand control of electrocatalytic processes and to reduce electrode surface fouling.14–16
A lot of interest is devoted to methanol electrocatalytic oxidation as it can be used in fuel cells and both bulk silver and silver nanoparticles are known to be efficient electrocatalysts for methanol oxidation in alkaline solutions17,18 with less poisoning observed than at platinum materials.19 However, even though silver is much less expensive than platinum its cost remains an issue.
In order to get the benefits of both the magnetic properties of magnetic nanoparticles and the catalytic properties of AgNPs we designed a hybrid material, which contains both. NiNPs and AgNPs were synthesized on CNTs using a one-pot chemical deposition in an ultrasonic bath using a methodology recently developed in our laboratory for the synthesis of NiNPs on glassy carbon microspheres.20 To date, this publication is the first report of the bi-functionalization of CNT with NiNPs and AgNPs. In addition, the electrocatalytic oxidation of methanol at the new hybrid material was studied.
2. Experimental
2.1 Reagents and equipment
Bamboo-like multi-walled carbon nanotubes (CNTs, diameter 30 ± 10 nm, 5–20 μm length, <95% purity) were purchased from NanoLab (Brighton, MA, USA). Nickel(II) chloride (NiCl2, 99.9%) was obtained from Alfa Aesar (Heysham, UK). L-Ascorbic acid (99.7%) and silver nitrate were supplied by BDH (Poole, UK). Nafion was purchased from Aldrich (Poole, UK). Acetonitrile (ACN) was supplied by Sigma-Aldrich (Gillingham, UK). All the reagents were used without further purification. All solutions were prepared using purified water from Vivendi UHQ grade water system with a resistivity of not less than 18.2 MΩ cm.Electrochemical measurements were recorded using an Autolab PGSTAT 30 computer-controlled potentiostat with a standard three-electrode setup. Either a home-made 4 mm diameter disc paraffin-impregnated graphite electrode21 or a 0.08 mm diameter disk bulk silver served as working electrodes. Paraffin-impregnated graphite electrodes have similar behaviour than other common graphite electrodes and have been chosen here because they are easy to fabricate and it is easy to renew their surface by a polishing step. A platinum wire was used as a counter electrode, and a silver wire used as the reference electrode completed the cell assembly. The paraffin-impregnated graphite electrode surface was renewed by successive mechanical polishing steps on alumina powders (Micropolish II, Buehler) of 1 to 0.3 μm in diameter. The electrode was sonicated for 5 min in deionized water after each polishing step. All experiments were carried at a temperature of 20 ± 1 °C. All the solutions were degassed with nitrogen prior to the electrochemical recordings.
Scanning electron microscopy (FEG-SEM, tungsten filament as electron source, acceleration voltage 20 keV) images and energy dispersion X-ray spectra analysis were performed using a JEOL 6300 F instrument. X-Ray diffraction patterns (XRD) were collected on a PANalytical X'Pert instrument with 40 kV and 40 mA settings.
Sonication was obtained using a D-78224 Singen/Htw sonicator (50/60 Hz, 80 W, UK).
2.2 Ultrasonic synthesis of silver and nickel nanoparticles on CNTs
The nickel and silver nanoparticles were synthesized onto the surface of CNTs using the following protocol: The CNTs were sonicated in conc. HClO4 + HNO3 (3:7, v:v) for 7 h in order to oxidize their surface, they were then filtered and extensively washed with deionized water to pH 7, and dried in air. Then, 2.9 mg NiCl2, 1.7 mg AgNO3 and 2.0 mg oxidized CNTs were added to 60 mL of acetonitrile in an airtight glass flask. The mixture was sonicated for one hour. 4.0 mg of L-ascorbic was then added in the flask and the pH was adjusted to 5.2 using 1 M NaOH. The reaction was allowed to proceed for 5 min at 65 °C under sonication. Finally, the products were separated by centrifugation, washed with acetonitrile and deionized water to remove any unreacted species. The multi-walled carbon nanotubes decorated with silver and nickel nanoparticles (AgNPs,NiNPs/CNTs) were allowed to air-dry for 24 h prior to use. Multi-walled carbon nanotubes decorated only with silver (AgNPs/CNTs) or nickel nanoparticles (NiNPs/CNTs) were obtained using the same method.2.3 Modification of the paraffin-impregnated graphite electrodes with CNTs
Films of CNTs on the surface of paraffin-impregnated graphite electrodes were obtained as follows: 2 mg of CNTs decorated with nanoparticles was suspended in 2 mL of Nafion (0.05%) and acetonitrile solution to form a “casting” suspension. The casting suspension was then briefly sonicated for 2 min in order to disperse the CNTs decorated with nanoparticles. Some of the suspension was then pipetted onto the surface of a freshly polished paraffin-impregnated graphite electrodes and let to dry in air.2.4 Movement of the AgNPs,NiNPs/CNTs composite material
The AgNPs,NiNPs/CNTs (AgNPs/CNTs, NiNPs/CNTs) composite materials were pipetted onto a 1.0 mm thick glass surface with some water. A magnet (NdFeB alloy rod magnet, purchased from e-magnets UK Ltd, Sheffield, UK) was positioned directly below the glass surface and moved by an electronic motor. The directed movement of the nanocomposites was observed and recorded using an optical microscope with a Bressler Visiomar video camera (160× magnification using a 320 × 240 pixel frame).3. Results and discussion
3.1 Synthesis and microscopic characterization of the nanoparticle-modified CNTs
The synthesis of the silver and nickel nanoparticles on the surface of the CNTs was obtained following the steps noted in Scheme 1. First the CNTs are treated with concentrated nitric and perchloric acids to generate carboxylic groups on their edges and defects. The negatively charged sites chelate silver and nickel cations added to the solution. It is then expected that the addition of ascorbic acid as a mild reducing agent in the presence of ultrasound results in the production of small nickel and silver nanostructures on the surface of the CNTs. Similar experimental routes were followed for the synthesis of nickel or silver nanoparticles separately on the CNTs. | ||
| Scheme 1 Preparation of multi-walled carbon nanotubes (CNTs) functionalized both by nickel and silver nanoparticles using a single step chemical deposition method in an ultrasonic bath and subsequent immobilization of the new composite material on an electrode surface. | ||
A scanning electronic microscopy analysis of the samples reveals that silver and nickel nanoparticles of 100 nm in diameter in average are obtained on the CNTs (Fig. 1). The EDX (Fig. 2) and XRD spectra of the samples (Fig. 3) confirm the presence of both silver and nickel nanoparticles. It can be noticed that the peaks for Ni (111) and Ag (111) are larger than the other peaks, which reveals that the most common nanoparticles on the CNTs are face-centered cubic (fcc) nickel and silver. The average crystallite size calculated using Scherrer’s equation from the width at half peak maximum for the NiNPs is 30 ± 25, and 25 ± 15 nm, respectively for the NiNPs/CNTs and the AgNPs,NiNPs/CNTs and 20 ± 10 nm for the AgNPs of the AgNPs/CNTs and the AgNPs,NiNPs/CNTs. It can be further stated on the nature of the silver and nickel nanoparticles that those particles are distinct and do not crystallise in the form of alloys, as it is well known that Ni and Ag are immiscible in both the solid and liquid phases.22
 | ||
| Fig. 1 SEM images of the multi-walled carbon nanotubes functionalized both by nickel and silver nanoparticles (AgNPs,NiNPs/CNTs). The square on Fig. 1(A) indicates where the EDX spectrum in Fig. 2 was obtained. | ||
 | ||
| Fig. 2 EDX spectra of (A) AgNPs,NiNPs/CNTs, (B) NiNPs/CNTs and (C) AgNPs/CNTs. | ||
 | ||
| Fig. 3 XRD spectra of (a) Ni/CNTs, (b) AgNPs/CNTs and (c) AgNPsNi/CNTs. | ||
3.2 Electrode modification and characterization
The electrode surfaces were modified by the decorated CNTs in a Nafion film by following the protocol described in the Experimental section. The electrode surfaces can be characterized electrochemically by oxidizing the metallic nanoparticles. Some metal oxides have a well-defined and specific reduction potential and the corresponding reduction peak can be used to estimate the surface area of specific metals. Fig. 4 shows the cyclic voltammograms that were obtained in 0.1 M NaOH for the various modified electrodes. It can be observed that the voltammogram corresponding to the AgNPs,NiNPs/CNTs modified electrode corresponds to the superposition of the characteristic features of both the AgNPs/CNTs and the NiNPs/CNTs modified electrodes. Using the literature values of 790 and 270 μC cm−2 for the charge passed per unit area of surface area of nickel and silver,23–25 we can estimate the total surface area of each of the metals for the AgNPs,NiNPs/CNTs/Nafion material. The average loading of the nickel and silver nanostructures on the CNTs were estimated to be, respectively in the order of 3.4 × 10−2 and 2.2 cm2 mg−1. Which then shows that, for nanoparticles of about the same size, the NiNPs represent only 1.5% of the total number of nanoparticles.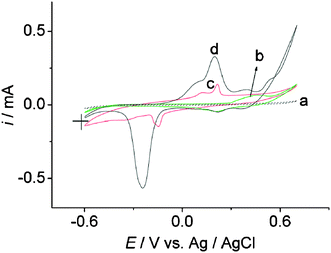 | ||
| Fig. 4 Cyclic voltammetry (30th cycle) in 0.1 M NaOH at a 4 mm diameter paraffin-impregnated graphite electrode modified with (a) 20 μL CNTs/Nafion, (b) 20 μL NiNPs/CNTs/Nafion, (c) 20 μL AgNPs/CNTs/Nafion and (d) 80 μL AgNPs,NiNPs/CNTs/Nafion casting solutions. Scan rate: 50 mV s−1. | ||
3.3 Electrocatalysis
The electroactivity of the mono- and bi-functionalized multi-walled carbon nanotubes was assessed and compared with the electroactivity of bulk silver macroelectrodes in respect to the electrooxidation of methanol. The cyclic voltammograms obtained for the second cycle are shown in Fig. 5. We choose to present the second cycle as a non-negligable decrease in intensity (ca. 25%) is observed from the first cycle to the second one. Measurements show a decrease of less than 10% is then observed between the second and the twentieth cycle. The electrochemical characterization of the modified electrode surfaces, conducted independently as described above, provides valuable data to compare the electrocatalytic efficiency of the different materials. Indeed, the catalytic currents obtained can be normalized with the total metal surface area to provide the current density per unit of electroactive surface (Table 1). The data reported in Table 1 show that the AgNPs/CNTs/Nafion- and AgNiNPs/CNTs/Nafion-modified electrodes have similar properties, with a current density about more than ten times higher than the current density obtained at the bulk silver macroelectrode. Such a higher electrocatalytic property can partially be explained by the higher substrate diffusion that we expect to observe at dispersed nanoparticles.26–31 Furthermore it has to be said from the results reported in Table 1 that the experimental results show that NiNPs and AgNPs have similar electrocatalytic properties towards the electrooxidation of methanol.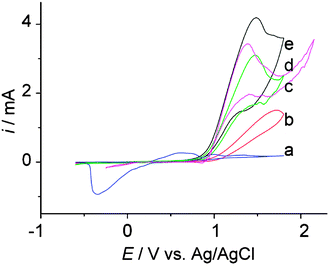 | ||
| Fig. 5 Second cycle cyclic voltammetric curves obtained in 0.56 M methanol and 0.1 M NaOH at a 0.08 mm diameter silver electrode (curve a) and at a 4 mm diameter paraffin-impregnated graphite electrode modified with casting solutions made of 80 μL CNTs/Nafion (curve b), 80 μL NiNPs/CNTs/Nafion (curve c), 80 μL AgNPs/CNTs/Nafion (curve d) and 120 μL AgNPs,NiNPs/CNTs/Nafion (curve e). Scan rate: 50 mV s−1. | ||
| Electrode | A/cm2 | I p/mA | J p /mA cm−2 |
|---|---|---|---|
| Ag bulk | 0.16 ± 0.05 | 9.28 × 10−2 | 0.58 ± 0.2 |
| NiNPs/CNTs/Nafion | 0.29 ± 0.1 | 3.00 | 10.3 ± 2 |
| AgNPs/CNTs/Nafion | 0.30 ± 0.1 | 3.3 | 11.0 ± 2 |
| AgNPs,NiNPs/CNTs/Nafion | 0.56 ± 0.2 | 4.14 | 7.4 ± 2 |
3.4 Characterization of magnetically driven movement of the AgNPs,NiNPs/CNTs composites
The possibility of magnetically recovering the electrocatalytic nanomaterial was explored by assessing the possibility to move it with a magnet using the setup described in the Experimental section. As it can be seen in Fig. 6 and Video S1 (ESI†), with the shift of a magnet positioned directly below the glass surface, the AgNPs,NiNPs/CNTs composites move from right bottom to middle, then, to left up in the video screen, suggesting an obvious movement for AgNPs,NiNPs/CNTs composites on the surface of glass. The same test was undertaken for the NiNPs/CNTs and AgNPs/CNTs nanocomposites. A similar response can be seen for NiNPs/CNTs composites (Video S2, ESI†). However, no move was observed for AgNPs/CNTs nanocomposites (Video S3, ESI†). It is then possible to conclude that the bifunctionalisation of the CNTs provides the possibility to magnetically drive them to a specific location in a solution. This added property to the new electroactive nanomaterial allows, for example, the recovery of the catalyst once the reaction has taken place. | ||
| Fig. 6 Optical microscopy images of the AgNPs,NiNPs/CNTs material, taken at 10 s time-intervals and following the movement of a magnet going forward (images (A) to (C)) and then going backward (images (D) to (F)). These pictures were extracted from Video S1 (ESI†). | ||
4. Conclusions
Multi-walled carbon nanotubes functionalized either by nickel or silver nanoparticles or by both were obtained using a single step chemical deposition method in an ultrasonic bath. The electroactivity of the bi-functionalized CNT multi-walled carbon nanotubes was assessed in respect to the electrooxidation of methanol. It was found that they have significantly higher catalytic properties than the bulk silver of the same surface area. Furthermore, it was shown that the addition of a minute fraction (1.5%) of NiNPs in respect to the total number of nanoparticles adds to their electrocatalytic properties the possibility to easily move them in solution using a magnet. The bi-functionalized carbon nanotubes are then easily recoverable after use.Acknowledgements
G.-P. J. gratefully acknowledges financial support from Natural Science Foundation of Anhui Province of China (No. 070415210), Science and Technology Program Foundation of Hefei City (No. 20071032), and Doctor Foundation of Hefei University of Technology (2005). R. B. and N. V. R. are grateful to EPSRC for funding.References
- M. J. Jacinto, P. K. Masunaga, H. Sueli, R. F. Jardim and L. M. Rossi, Appl. Catal., A, 2008, 338, 52 CrossRef CAS.
- B. Baruwati, D. Guin and S. W. Manorama, Org. Lett., 2007, 9, 5377 CrossRef CAS.
- G. Chouhan, D. Wang and H. Alper, Chem. Commun., 2007, 4809 RSC.
- C. O. Dalaigh, S. A. Corr, Y. Gun’ko and S. J. Connon, Angew. Chem., Int. Ed., 2007, 46, 4329 CrossRef CAS.
- F. Yang, D. L. Fu, J. Long and Q. X. Ni, Med. Hypotheses, 2008, 70, 765 CrossRef CAS.
- C. Gao, W. Li, H. Morimoto, Y. Nagaoka and T. Maekawa, J. Phys. Chem. B, 2006, 110, 7213 CrossRef CAS.
- S. Qu, J. Wang, J. Kong, P. Yang and G. Chen, Talanta, 2007, 71, 1096 CrossRef CAS.
- H. Cao, M. Zhu, Y. Li, J. Liu, Z. Ni and Z. Qin, J. Solid State Chem., 2007, 180, 3218 CrossRef CAS.
- A. Reyhani, S. Z. Mortazavi, O. Akhavan, A. Z. Moshfegh and S. Lahooti, Appl. Surf. Sci., 2007, 253, 8458 CrossRef CAS.
- B. Jia, L. Gao and J. Sun, Carbon, 2007, 45, 1476 CrossRef CAS.
- I. Willner and E. Katz, Langmuir, 2006, 22, 1409 CrossRef CAS.
- E. Katz, L. Sheeney-Haj-Ichia, B. Basnar, I. Felner and I. Willner, Langmuir, 2004, 20, 9714 CrossRef CAS.
- E. Katz, R. Baron and I. Willner, J. Am. Chem. Soc., 2005, 127, 4060 CrossRef CAS.
- J. Wang, M. Musameh and R. Laocharoensuk, Electrochem. Commun., 2005, 7, 652 CrossRef CAS.
- J. Wang, M. Musameh, R. Laocharoensuk, O. Gonzalez-Garcia, J. Oni and D. Gervasio, Electrochem. Commun., 2006, 8, 1106 CrossRef CAS.
- R. Laocharoensuk, A. Bulbarello, S. B. Hocevar, S. Mannino, B. Ogorevc and J. Wang, J. Am. Chem. Soc., 2007, 129, 7774 CrossRef CAS.
- D. J. Guo and H. L. Li, Carbon, 2005, 43, 1259 CrossRef CAS.
- M. Avramov-Ivic, S. Strbac and V. Mitrovic, Electrochim. Acta, 2001, 46, 3175 CrossRef CAS.
- R. Baron, F. W. Campbell, I. Streeter, L. Xiao and R. G. Compton, Int. J. Electrochem. Sci., 2008, 3, 556 Search PubMed.
- G.-P. Jin, R. Baron, L. Xiao and R. G. Compton, J. Nanosci. Nanotechnol. DOI:10.1166/jnn.2008.462.
- G.-P. Jin and X. Q. Lin, Electrochim. Acta, 2005, 50, 3556 CrossRef CAS.
- J. Xu, U. Herr, T. Klassen and R. S. Averback, J. Appl. Phys., 2006, 79, 3935.
- L. M. Abrantes, M. C. Oliveira, J. P. Bellver and J. Lecoeur, Electrochim. Acta, 1994, 39, 1915 CrossRef CAS.
- K. A. Soliman 1 and L. A. Kibler, Electrochim. Acta, 2007, 52, 5654 CrossRef.
- M. J. Esplandiu, M. A. Schneeweiss and D. M. Kolb, Phys. Chem. Chem. Phys., 1999, 1, 4847 RSC.
- R. G. Compton, G. G. Wildgoose, N. V. Rees, I. Streeter and R. Baron, Chem. Phys. Lett., 2008, 459, 1 CrossRef CAS.
- I. Streeter, R. Baron and R. G. Compton, J. Phys. Chem. C, 2007, 111, 17008 CrossRef CAS.
- R. Baron, B. Sljukic, C. Salter, A. Crossley and R. G. Compton, Electroanalysis, 2007, 19, 1062 CrossRef CAS.
- R. Baron, G. G. Wildgoose and R. G. Compton, J. Nanosci. Nanotechnol. DOI:10.1166/jnn.2008.470.
- B. Šljukić, R. Baron, C. Salter, A. Crossley and R. G. Compton, Anal. Chim. Acta, 2007, 590, 67 CrossRef.
- R. Baron, B. Šljukić, C. Salter, A. Crossley and R. G. Compton, Russ. J. Phys. Chem., 2007, 81, 1443 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Videos S1–3. See DOI: 10.1039/b814630f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2009 |
