Ionic liquids with dual biological function: sweet and anti-microbial, hydrophobic quaternary ammonium-based salts†
Whitney L.
Hough-Troutman
a,
Marcin
Smiglak
a,
Scott
Griffin
a,
W.
Matthew Reichert
b,
Ilona
Mirska
c,
Jadwiga
Jodynis-Liebert
d,
Teresa
Adamska
d,
Jan
Nawrot
e,
Monika
Stasiewicz
f,
Robin D.
Rogers
*ag and
Juliusz
Pernak
*f
aThe University of Alabama, Department of Chemistry and Center for Green Manufacturing, Tuscaloosa, AL 35487, USA
bUS Naval Academy, Department of Chemistry, Annapolis, MD 21402, USA
cPoznań University of Medical Sciences, Department of Pharmaceutical Bacteriology, Święcickiego, 4 60-781, Poznań, Poland
dPoznań University of Medical Sciences, Department of Toxicology, Dojazd 30, 60-631, Poznań, Poland
eInstitute of Plant Protection, ul. Węgorka 20, 60-318, Poznań, Poland
fPoznań University of Technology, Faculty of Chemical Technology, pl. Skłodowskiej-Curie 2, 60-965, Poznań, Poland. E-mail: juliusz.pernak@put.poznan.pl
gThe Queen’s University of Belfast, QUILL, School of Chemistry and Chemical Engineering, Belfast, Northern Ireland BT9 5AG. E-mail: r.rogers@qub.ac.uk
First published on 22nd October 2008
Abstract
The dual nature of ionic liquids has been exploited to synthesize materials that contain two independent biological functions by combining anti-bacterial quaternary ammonium compounds with artificial sweetener anions. The synthesis and physical properties of eight new ionic liquids, didecyldimethylammonium saccharinate ([DDA][Sac]), didecyldimethylammonium acesulfamate ([DDA][Ace]), benzalkonium saccharinate ([BA][Sac]), benzalkonium acesulfamate ([BA][Ace]), hexadecylpyridinium saccharinate ([HEX][Sac]), hexadecylpyridinium acesulfamate ([HEX][Ace]), 3-hydroxy-1-octyloxymethylpyridinium saccharinate ([1-(OctOMe)-3-OH-Py][Sac]), and 3-hydroxy-1-octyloxymethylpyridinium acesulfamate ([1-(OctOMe)-3-OH-Py][Ace]), are reported, as well as the single crystal structures for [HEX][Ace] and [1-(OctOMe)-3-OH-Py][Sac]. Determination of anti-microbial activities is described for six of the ILs. While some exhibited decreased anti-microbial activity others showed a dramatic increase. For two of the ionic liquids, [DDA][Sac] and [DDA][ACE], oral toxicity, skin irritation, and deterrent activity was also established. Unfortunately, both ILs received a Category 4 (harmful) rating for oral toxicity and skin irritation. However, deterrent activity experiments point to use as an insect deterrent, as both ILs scored either “very good” or “good” against several types of insects.
Introduction
Ionic liquids (ILs) are currently defined as salts that are composed solely of cations and anions which melt below 100 °C. These salts have been studied for a variety of applications such as in electrochemistry,1–3 separation science,4–7 chemical synthesis,8–13 and catalysis,14–16 however, until recently, very few, if any, ILs had been used as liquid materials themselves.17,18 In addition, those material applications which have appeared, typically concentrated on a single desirable property brought by either the cation or the anion. But ILs, by definition, have at least two discrete types of ions, both of which can provide a unique property or function. Thus, our goal has been to explore how to exploit the dual nature of ILs by preparing materials that possess two functions, particularly two biological functions. Here, we present the combination of anti-bacterial quaternary ammonium compounds (QACs) with artificial sweeteners.The anti-bacterial properties of QACs were first discovered during the late 19th century, amongst carbonium dye compounds, such as auramin, methyl violet, and malachite green.19 Initially, QACs were found to be most effective against gram-positive organisms, until Jacobs and Heidelberger20–23 further exploited their anti-bacterial properties against other types of organisms. It was not until 1935 that the full potential of QACs was recognized by the chemical community, when the synthesis of benzalkonium chloride, a long-chain QAC, by Domagk24 and further characterization of its anti-bacterial activities, proved that QACs were effective against a wider variety of bacterial strains.
Later, in the 20th century, researchers became more interested in the synthesis of water-soluble QACs for potential applications as surfactants,25,26 anti-electrostatic agents,27 anti-corrosive agents,28disinfectants,29 and phase-transfer catalysts.30 These newly developed water-soluble QACs showed anti-bacterial action against not only gram-positive and gram-negative bacteria, but also pathogen species of fungi and protozoa.31 These discoveries led to applications for QACs in wood preservation32–34 and as preservatives in common household products,35 especially for general environmental sanitation in hospitals and food production facilities. Furthermore, QACs have been used as penetration enhancers for transnasal and transbuccal drug delivery, such as nasal vaccinations.36 The ability of QACs to penetrate and open cell membranes has been widely used in drug delivery such as liposomes, which consists of long alkyl chain QACs, and non-viral gene delivery.37
We have had specific interest in employing the IL concept to pair the biological activity of a class of compounds such as QACs, with a second biological activity inherent in the counterion.38 One such class of ions, which has also seen independent use in preparing ‘edible’ ILs, includes non-nutritive sweeteners such as saccharinate and acesulfame.39,40 Salts of these anions are currently used in food products and are approved as food additives by most national and global health agencies. Yet, only a handful of quaternary ammonium saccharinates and acesulfamates have been reported in the literature.41 Here we demonstrate the concept of preparing ILs by pairing the biological activity inherent in the cation with a separate biological function possessed by the anion with the synthesis, physical properties, anti-microbial activities, toxicity, and deterrent activity of new QAC-based ILs.
Results and discussion
Synthesis and characterization
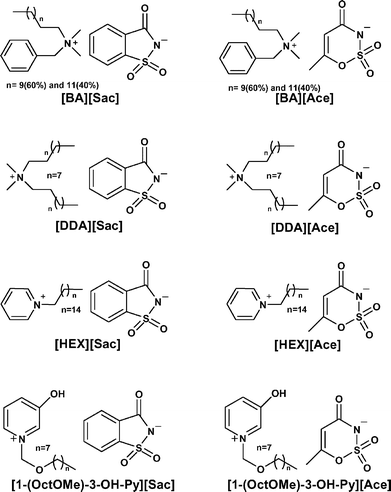 | ||
| Fig. 1 Structures of the synthesized ILs. | ||
All of the newly prepared ILs were found to be low melting solids at room temperature with the exception of [DDA]-containing salts, the only cation without an aromatic ring, which were found to be liquid at room temperature. The salts studied are only sparingly soluble in cold and hot water, but freely soluble and stable in many organic solvents (e.g., chloroform, methanol, ethanol, ethyl acetate, N,N-dimethylformamide (DMF), and dimethyl sulfoxide (DMSO)).
The packing diagram for [HEX][Ace] (Fig. 2) reveals that the cation tails interdigitate to create charge-rich and hydrophobic regions. Closer examination indicates that the two unique cations are not equivalent with slight differences in the orientation of the hexadecyl tail groups. This modest difference leads to completely different packing environments. One cation π-stacks in a polymeric fashion (Fig. 3) and has only three close contacts with the anions. The second cation forms a π-stacked dimer with anions capping each open face. These cations have five close contacts with the anions.
![Packing diagram along the a crystallographic axis for [HEX][Ace] (top) and overlay of the two cations in the asymmetric unit including the anions with close contacts to each (bottom).](/image/article/2009/NJ/b813213p/b813213p-f2.gif) | ||
| Fig. 2 Packing diagram along the a crystallographic axis for [HEX][Ace] (top) and overlay of the two cations in the asymmetric unit including the anions with close contacts to each (bottom). | ||
![One cation in [HEX][Ace] π-stacks in a polymeric fashion (interplanar spacing 3.5 and 3.6 Å) (top), while the second cation forms π-stacked dimers (interplanar spacing 3.4 Å) with acesulfamate anions capping both sides (bottom).](/image/article/2009/NJ/b813213p/b813213p-f3.gif) | ||
| Fig. 3 One cation in [HEX][Ace] π-stacks in a polymeric fashion (interplanar spacing 3.5 and 3.6 Å) (top), while the second cation forms π-stacked dimers (interplanar spacing 3.4 Å) with acesulfamate anions capping both sides (bottom). | ||
Fig. 4 illustrates the packing in the structure of [1-(OctOMe)-3-OH-Py][Sac]. Here the strong hydrogen bonding between the cation and anion dominates and a single cation/anion pair is found in the asymmetric unit. These hydrogen bonded ion pairs stack in alternate directions.
![Packing diagram along the a crystallographic axis for [1-(OctOMe)-3-OH-Py][Sac] (top) and close up of the hydrogen bonding and alternate stacking of the ion pairs (bottom).](/image/article/2009/NJ/b813213p/b813213p-f4.gif) | ||
| Fig. 4 Packing diagram along the a crystallographic axis for [1-(OctOMe)-3-OH-Py][Sac] (top) and close up of the hydrogen bonding and alternate stacking of the ion pairs (bottom). | ||
| T g | T c | T s−s | T m | T onset5% | T onset | |
|---|---|---|---|---|---|---|
| a Phase transition points (°C) were measured from transition onset temperatures determined by DSC from the second heating cycle at 5 °C min−1, after initially heating and then cooling of the samples to −100 °C unless otherwise indicated: Tg = glass transition temperature; Tc = crystallization temperature; Ts–s = solid–solid transition temperature on heating; Tm = melting point on heating. Decomposition temperatures were determined by TGA, heating at 5 °C min−1 under air atmosphere and are reported as (Tonset 5%) onset to 5 wt% mass loss and (Tonset) onset to total mass loss. b Transition measured on heating cycle. c Transition measured on cooling cycle. d Transition only during first heating e Visual melting point range via hot-plate apparatus. f Multiple transitions due to presence of water in starting material. g Multiple decomposition steps. | ||||||
| Ionic liquids | ||||||
| [BA][Sac] | — | 16b | — | 74 | 164 | 204 |
| [DDA][Sac] | −33 | 15c | — | 16 | 187 | 214 |
| [HEX][Sac] | — | 30c | — | 66 | 207 | 253/412g |
| [1-(OctOMe)-3-OH-Py][Sac] | — | — | — | 95–98e | 206 | 301 |
| [BA][Ace] | — | 30c | −36 | 90 | 184 | 187/249/394g |
| [DDA][Ace] | −53 | — | — | — | 189 | 232/426g |
| [HEX][Ace] | −11 | 5b | — | 57 | 212 | 267/494g |
| 18c | ||||||
| [1-(OctOMe)-3-OH-Py][Ace] | — | — | — | 79–81e | 203 | 267 |
| Starting materials | ||||||
| Na[Sac] | — | 98c | — | 120 | 431 | 459/541g |
| K[Ace] | — | — | — | 68 | 190 | 192/260g |
| [BA][Cl] | — | 16bd | — | — | 143 | 169 |
| [DDA][Br] f | — | — | — | — | 166 | 196 |
| [HEX][Cl] | — | 45c | — | 73 | 184 | 213 |
| [1-(OctOMe)-3-OH-Py][Cl] | — | — | — | 68–70e | 178 | 247 |
As seen in Table 1, all the ILs were found to be thermally stable to temperatures ranging between 160 and 210 °C. One-step decomposition was found for [BA][Sac], [DDA][Sac], [1-(OctOMe)-3-OH-Py][Sac] and [1-(OctOMe)-3-OH-Py][Ace]. The anions [Sac]− and [Ace]− normally display a two-step decomposition, suggesting that the cations, [BA]+, [DDA]+ and [1-(OctOMe)-3-OH-Py]+, play a role in the decomposition of these ILs resulting in the single decomposition step observed.
Two-step decomposition was observed for samples [HEX][Sac], [DDA][Ace] and [HEX][Ace]. Increase in the thermal stability (first decomposition step) in these salts, over the thermal stabilities of the starting materials may indicate an anion stabilizing effect on the parent cations; [HEX]+ and [DDA]+. Similarly, the stabilizing effect of the anion can be observed for the sample of [BA][Ace], which is the only sample that exhibits a three-step decomposition pathway.
Biological properties
| Ionic liquid | Starting materials | |||||
|---|---|---|---|---|---|---|
| Strain | [BA][Sac] | [DDA][Sac] | [BA][Ace] | [DDA][Ace] | [BA][Cl] | [DDA][Cl] |
| a In ppm. | ||||||
| S. aureus | 4 | 4 | 4 | 8 | 2 | 2 |
| S. aureus ( MRSA ) | 4 | 4 | 4 | 4 | 2 | 2 |
| E. faecium | 8 | 8 | 8 | 8 | 4 | 4 |
| E. coli | 16 | 16 | 31 | 16 | 8 | 8 |
| M. luteus | 8 | 4 | 8 | 8 | 4 | 2 |
| S. epidermidis | 4 | 4 | 4 | 4 | 2 | 2 |
| K. pneumoniae | 4 | 4 | 8 | 4 | 4 | 4 |
| C. albicans | 16 | 16 | 16 | 16 | 8 | 8 |
| R. rubra | 16 | 16 | 16 | 16 | 8 | 4 |
| S. mutans | 0.1 | 31 | 1 | 16 | 2 | 2 |
| Mean value | 8.0 | 10.7 | 10.0 | 10.0 | 4.4 | 3.8 |
| Ionic liquids | Starting materials | |||||
|---|---|---|---|---|---|---|
| Strain | [BA][Sac] | [DDA][Sac] | [BA][Ace] | [DDA][Ace] | [BA][Cl] | [DDA][Cl] |
| a In ppm. | ||||||
| S. aureus | 31.2 | 62.5 | 31.2 | 16 | 62.5 | 31.2 |
| S. aureus ( MRSA ) | 31.2 | 31.2 | 31.2 | 31.2 | 31.2 | 31.2 |
| E. faecium | 16 | 16 | 31.2 | 31.2 | 31.2 | 31.2 |
| E. coli | 62.5 | 16 | 125 | 62.5 | 62.5 | 31.2 |
| M. luteus | 62.5 | 31.2 | 62.5 | 62.5 | 31.2 | 31.2 |
| S. epidermidis | 31.2 | 16 | 62.5 | 31.2 | 16 | 31.2 |
| K. pneumoniae | 62.5 | 16 | 31.2 | 31.2 | 31.2 | 16 |
| C. albicans | 31.2 | 16 | 31.2 | 31.2 | 16 | 16 |
| R. rubra | 62.5 | 31.2 | 62.5 | 62.5 | 31.2 | 31.2 |
| S. mutans | 0.5 | 62.5 | 16 | 125 | 16 | 16 |
| Mean value | 39.1 | 29.9 | 48.5 | 48.5 | 32.9 | 26.6 |
It is thought that 1-alkoxymethylpyridinium chlorides are strongly active against microbes, yet in previous research,43 it was concluded that the antimicrobial activities depended on the substituent at the 3-position of the pyridine ring. Unfortunately, [1-(OctOMe)-3-OH-Py][Cl] and the ILs, [1-(OctOMe)-3-OH-Py][Sac] and [1-(OctOMe)-3-OH-Py][Ace] exhibited no antimicrobial activity.
The total coefficient value T is compared to standard values for deterrent activity in Table 4, where a value of 0 equals neutral activity and a value of +150 to +200 corresponds to very high deterrent activity. The results of deterrent activity for [DDA][Ace] and [DDA][Sac] are compared to a natural deterrent, azadirachtin, in Table 5. The ILs received either ‘very good’ or ‘good’ deterrent activity for all tested insects. In particular, [DDA][Sac] exhibited the same deterrent activity toward Tribolium confusum (larvae and beetles) as azadirachtin and thus, could be classified as a potential synthetic insect deterrent.
| Total coefficient | Deterrent activity |
|---|---|
| 200–151 | Very good |
| 150–101 | Good |
| 100–51 | Medium |
| 50–0 | Weak |
| Ionic liquid | Relative coefficient | Absolute coefficient | Total coefficient | Deterrent activity |
|---|---|---|---|---|
| a Natural deterrent. b The least significant differences at the 5% level of significance. | ||||
| Sitophilus granarius (beetles) | ||||
| [DDA][Ace] | 97.5 | 57.9 | 155.5 | Very good |
| [DDA][Sac] | 57.8 | 56.6 | 114.5 | Good |
| Azadirachtin a | 100.0 | 74.3 | 174.3 | Very good |
| LSD0.05b | 57.8 | 28.8 | 60.1 | |
| Trogoderma granarium (larvae) | ||||
| [DDA][Ace] | 94.0 | 85.0 | 179.0 | Very good |
| [DDA][Sac] | 94.2 | 86.1 | 180.3 | Very good |
| Azadirachtin a | 100.0 | 94.2 | 194.2 | Very good |
| LSD0.05b | 0.3 | 7.6 | 7.8 | |
| Tribolium confusum (beetles) | ||||
| [DDA][Ace] | 96.2 | 19.1 | 115.3 | Good |
| [DDA][Sac] | 95.0 | 90.7 | 186.6 | Very good |
| Azadirachtin a | 100.0 | 85.0 | 185.0 | Very good |
| LSD0.05b | 0.6 | 9.2 | 9.0 | |
| Tribolium confusum (larvae) | ||||
| [DDA][Ace] | 95.0 | 64.1 | 159.1 | Very good |
| [DDA][Sac] | 95.3 | 88.8 | 184.1 | Very good |
| Azadirachtin a | 100.0 | 88.4 | 188.4 | Very good |
| LSD0.05b | 2.1 | 29.4 | 29.1 | |
Conclusions
We have prepared ILs of two biologically active ions by combining anti-microbial QACs cations with sweetener anions. Some of these ILs demonstrate properties such as limited water solubility, high thermal stability, and good deterrent activity against insects; which suggest potential application as an insecticide. Although oral toxicity and skin irritation values were higher than hoped, there is still potential use, not only for these ILs, but also for new, related ILs which can be prepared by tuning the composition in such a manner to reduce toxicity.In general, research in the IL field has begun to shift from random combinations of ions to a design scheme in which both the cation and anion are chosen based on the desired physical, chemical, and biological properties. All procedures performed on these animals were in accordance with established guidelines and were reviewed and approved by the University of Alabama’s Institutional Animal Care and Use committee. As our fundamental understanding of IL behavior increases, more control over the resultant properties of the salts will be possible, and the number of potential applications, such as those presented here, will continue to grow.
Experimental
Chemicals and microorganisms
Benzalkonium chloride [BA][Cl] (molecular formula C6H5CH2N(CH3)2RCl where R = C12H25 (60%) and C14H29 (40%)), didecyldimethylammonium bromide [DDA][Br] (tech., 75 wt% gel in water), hexadecylpyridinium chloride [HEX][Cl] (monohydrate, minimum 99%) and sodium saccharinate Na[Sac] (hydrate, minimum 98%) were purchased from Sigma Aldrich. Potassium acesulfamate K[Ace] (≥ 99%) was purchased from Fluka.The following microorganisms were used: bacteria Staphylococcus aureusATCC 6538, Staphylococcus aureus (MARSA) ATCC 43300, Enterococcus faeciumATCC 49474, Escherichia coli ATCC 2592,2 Micrococcus luteus ATCC 9341, Staphylococcus epidermidis ATCC 12228, Klebsiella pneumoniaeATCC 4352, and fungi Candida albicans ATCC 10231, Rhodotorula rubraPhB and Streptococcus mutans PCM (Polish Collection of Microorganisms) 2502. The Rhodotorula rubra was obtained from the Department of Pharmaceutical Bacteriology, Poznań University of Medical Sciences, Poland.
General synthesis47
Solid (0.001 mol) Na[Sac] or K[Ace] was dissolved in distilled water and added to hot aqueous solutions containing 0.001 mol of [BA][Cl], [DDA][Br] or [HEX][Cl]. The mixtures were stirred at 60 °C for 1 h and then cooled to room temperature. The hydrophobic product was extracted with chloroform and purified using distilled water washes, until chloride or bromide ions were no longer detected in the product phase using AgNO3. The chloroform was evaporated and the IL was dried under vacuum.The starting material 3-hydroxy-1-octyloxymethylpyridinium chloride was prepared according to previous literature.43 Solid (0.03 mol) K[Ace] or Na[Sac] was dissolved in distilled water and then added to an aqueous solution containing 0.03 mol [1-(OctOMe)-3-OH-Py][Cl]. The reaction was completed by gentle heating and stirring in a water bath for 2 h. The heat was removed and stirring was continued at room temperature for 24 h. The mixture was filtered, and the precipitate was washed with cold distilled water (3 × 20 mL) to give an oil or solid IL. The IL was dried under vacuum, and recrystallized from ethyl acetate and then dried again under vacuum. Karl–Fischer analysis indicated the water content of all dried ILs to be less than 500 ppm.
Thermal analysis
Melting points and other thermal transitions of the ILs were determined by DSC, with a TA Instruments model 2920 Modulated DSC (Newcastle, DE), cooled with a liquid nitrogen cryostat. The calorimeter was calibrated for temperature and cell constants using indium standard (mp 156.61 °C, ΔH = 28.71 J g−1). Data were collected at constant atmospheric pressure where the ILs were placed in aluminum pans with sample sizes from 5 to 15 mg. An empty sample pan was used as reference. All experiments were performed at a heating rate and a cooling rate of 5 °C min−1. The DSC was adjusted so zero heat flow was between 0 and −0.5 mW, and the baseline drift was less than 0.1 mW over the temperature range 0–180 °C.Thermal decomposition temperatures were measured in the dynamic heating regime using a TGA, 2950 TA Instrument, under air atmosphere. The amount of IL used was between 2 and 10 mg in each case, and the samples were heated from 40 to 800 °C at a constant heating rate of 5 °C min−1. Decomposition temperatures (T5%dec) were determined from onset to 5 wt% mass loss; this provides a more realistic representation of thermal stability at elevated temperatures.
X-Ray diffraction
Crystalline samples of [HEX][Ace] and [1-(OctOMe)-3-OH-Py][Sac] were mounted on a glass fiber on a goniometer head of a Siemens SMART CCD diffractometer equipped with a Mo-Kα source (λ = 0.71073 Å) and a graphite monochromator. Data collection was conducted at −100 °C which was achieved by streaming cold nitrogen over the crystal. Final unit cell parameters were determined by least-squares refinement of the hemispherical data set obtained from 20 s exposures. Data were corrected for Lorentz and polarization effects and absorption using SADABS.48 The initial structure solution was carried out using the direct methods option in SHELXTL version 5.49 The positions of all non-hydrogen atoms were refined anisotropically. The hydrogen atoms were added and allowed to refine unconstrained in order to obtain proper close contact interactions.![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; T = 173 K; a = 7.921(3), b = 13.374(5), c = 25.689(10) Å, α = 76.755(7), β = 82.225(7), γ = 89.260(7)°; Z = 4; V = 2624.2(17) Å3; Dc = 1.181 g cm−3; 7459 independent (Rint = 0.0249) and 5755 observed ([I > 2σ(I)]) reflections; GooF = 1.070; R1, wR2 [I > 2σ(I)] = 0.0462, 0.1208; R1, wR2 (all data) = 0.0641, 0.1411
; T = 173 K; a = 7.921(3), b = 13.374(5), c = 25.689(10) Å, α = 76.755(7), β = 82.225(7), γ = 89.260(7)°; Z = 4; V = 2624.2(17) Å3; Dc = 1.181 g cm−3; 7459 independent (Rint = 0.0249) and 5755 observed ([I > 2σ(I)]) reflections; GooF = 1.070; R1, wR2 [I > 2σ(I)] = 0.0462, 0.1208; R1, wR2 (all data) = 0.0641, 0.1411
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; T = 173 K; a = 8.1626(15), b = 8.7141(16), c = 16.756(3) Å, α = 81.872(3), β = 80.780(3), γ = 62.850(3)°; Z = 2; V = 1043.7(3) Å3; Dc = 1.338 g cm−3; 2969 independent (Rint = 0.0170) and 2515 observed ([I > 2σ(I)]) reflections; GooF = 1.033; R1, wR2 [I > 2σ(I)] = 0.0362, 0.0871; R1, wR2 (all data) = 0.0472, 0.0937
; T = 173 K; a = 8.1626(15), b = 8.7141(16), c = 16.756(3) Å, α = 81.872(3), β = 80.780(3), γ = 62.850(3)°; Z = 2; V = 1043.7(3) Å3; Dc = 1.338 g cm−3; 2969 independent (Rint = 0.0170) and 2515 observed ([I > 2σ(I)]) reflections; GooF = 1.033; R1, wR2 [I > 2σ(I)] = 0.0362, 0.0871; R1, wR2 (all data) = 0.0472, 0.0937
Antimicrobial characteristics
Anti-microbial activity was determined by the tube dilution method. Bacteria strains were cultured in Mueller–Hinton broth for 24 h and fungi were cultured on Sabouraud agar for 48 h. Suspensions of the above microorganisms, at a concentration of 106 cfu mL−1, were prepared from each culture. Two milliliters of serial twofold dilutions of IL were inoculated with the above-mentioned suspension to obtain a final concentration of (1–5) × 105 cfu mL−1.Growth of the microorganism (or its lack) was determined visually after incubation for 24 h at 35 °C (bacteria) or 48 h at 22 °C (fungi). The lowest concentration at which there was no visible growth (turbidity) was determined to be the minimal inhibitory concentration (MIC). Then, from each tube content, 10 mL (calibrated loop) was smeared on an agar medium with inactivates (0.3% lecithin, 3% polysorbate 80, and 0.1% L-cysteine) and incubated for 48 h at 35 °C (bacteria) or for 5 days at 22 °C (fungi). The lowest concentration of the IL that killed 99.9% or more of the microorganism was defined as the minimum biocidal concentration (MBC).
Acute oral toxicity test
The toxicity was tested according to the method of acute toxic class.44 Three male (250 ± 25 g) and three female (170 ± 17 g) Wistar rats were used for each IL tested. The ILs were first suspended in distilled water and then administered intragastrically at doses of 300 mg/kg b.w. and 2000 mg/kg b.w. After the dose was administered, the rats were observed for 14 days.Skin irritation tests
Each IL was tested on 3 male New Zealand albino rabbits, where the fur was previously removed from the back of the rabbit. Half a milliliter of the ILs (100%, pure) was distributed on two 6 cm3 sites of the same animal. The application site was then covered with a porous gauze dressing and secured in place with tape. After a 4 h exposure, the dressing was removed and the application site was gently washed with water. Observations were then conducted at 1, 24, 48, and 72 h, where the test sites were evaluated for erythema and edema using a prescribed scale.45Feeding deterrent activity tests
Three species of insects were selected for testing: Tribolium confusum Duv. (larvae and beetles), Sitophilus granarius L. (beetles), and Trogoderma granariumEv. (larvae). Insects were grown on a wheat grain or whole-wheat meal diet in laboratory colonies which was maintained at 26 ± 1 °C and 60 ± 5% relative humidity. The laboratory assay was conducted according to the method developed and standardized for storage insects feeding activity for both choice and no-choice test.46Wheat wafer discs (1 cm in diameter × 1 mm thick) were saturated by dipping in either ethanol (96%) only (control) or in a 1% ethanol solution of [DDA][Ace] or [DDA][Sac]. After evaporation of the solvent by air-drying (30 min), the wafers were weighed and offered as the only food source for the insects over a five day period. The feeding of the insects was recorded under three conditions: (a) control test (two control discs (CC)), (b) choice test (a choice between one treated disc (T) and one control disc (C)), and (c) no-choice test (two treated discs (TT)). Each of the three experiments was repeated five times with 3 beetles of Sitophilus granarius, 20 beetles and 10 larvae of Tribolium confusum, and 10 larvae of Trogoderma granarium. The number of individual insects depended on the intensity of their food consumption. The beetles utilized in the experiments were unsexed, 7–10 days old, and the larvae were 5–30 days old. After five days of feeding, the discs were reweighed. The data from the experiments have been statistically corrected by an analysis of variance.
Acknowledgements
This work was supported by Poznań University of Technology, BW 32-222/2008.References
- F. Endres, ChemPhysChem, 2002, 3, 145.
- P. Wang, S. M. Zakeeruddin, I. Exnar and M. Graetzel, Chem. Commun., 2002, 2972 RSC.
- T. Sato, G. Masuda, M. Kotani and S. Iizuka, Chem. Abstr., 2004, 140, 245004.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, S. T. Griffin and R. D. Rogers, Ind. Eng. Chem. Res., 2000, 39, 3596 CrossRef CAS.
- A. E. Visser, R. P. Swatloski, W. M. Reichert, J. H. Davis, Jr, R. D. Rogers, R. Mayton, S. Sheff and A. Wierzbicki, Chem. Commun., 2001, 135 RSC.
- S. Dai, Y. H. Ju and C. E. Barnes, J. Chem. Soc., Dalton Trans., 1999, 1201 RSC.
- P. Wasserscheid, A. Boesmann, A. Jess, L. Datsevitch, C. Schmitz and A. Lauter, Chem. Abstr., 2003, 138, 370660.
- C. Chiappe and D. Pieraccini, ARKIVOC, 2002, 11, 249.
- L. Kiss, T. Kurtán, S. Antus and H. Brunner, ARKIVOC, 2003, 5, 69.
- T. Welton, Coord. Chem. Rev., 2004, 248, 2459 CrossRef CAS.
- J. Mo, L. Xu and J. Xiao, J. Am. Chem. Soc., 2005, 127, 751 CrossRef CAS.
- J. Ding, V. Desikan, X. Han, T. L. Xiao, R. Ding, W. S. Jenks and D. W. Armstrong, Org. Lett., 2005, 7, 335 CrossRef CAS.
- E. Janus, I. Goc-Maciejewska, M. Łożyński and J. Pernak, Tetrahedron Lett., 2006, 47, 4079 CrossRef CAS.
- M. T. Carter, C. L. Hussey, S. K. D. Strubinger and R. A. Osteryoung, Inorg. Chem., 1991, 30, 1149 CrossRef CAS.
- K. N. Marsh, J. A. Boxall and R. Lichtenthaler, Fluid Phase Equilib., 2004, 219, 93 CrossRef CAS.
- A. Hammerl, M. A. Hiskey, G. Holl, T. M. Klapotke, K. Polborn, J. Stierstorfer and J. J. Weigand, Chem. Mater., 2005, 17, 3784 CrossRef CAS.
- A. Pernak, K. Iwanik, P. Majewski, M. Grzymisławski and J. Pernak, Acta Histochem., 2005, 107, 149 CrossRef CAS.
- Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities—Transformations and Processes, eds. R. D. Rogers and K. R. Seddon, ACS Symposium Series 902, American Chemical Society, Washington DC, 2005 Search PubMed.
- R. S. Shelton, M. G. Van Campen, C. H. Tilford, H. C. Lang, L. Nisonger, F. J. Bandelin and H. L. Rubenkoenig, J. Am. Chem. Soc., 1949, 69, 753.
- W. A. Jacobs and M. Heidelberger, Proc. Natl. Acad. Sci. U. S. A., 1915, 1, 226 CrossRef CAS.
- W. A. Jacobs, J. Exp. Med., 1916, 23, 563 CrossRef CAS.
- W. A. Jacobs, M. Heidelberger and H. L. Amoss, J. Exp. Med., 1916, 23, 569 CrossRef CAS.
- W. A. Jacobs, M. Heidelberger and C. G. Bull, J. Exp. Med., 1916, 23, 577 CrossRef CAS.
- G. Domagk, Dtsch. Med. Wochenschr., 1935, 61, 829 CrossRef CAS.
- Cationic Surfactants, ed. J. M. Richmond, Marcel Dekker, New York, 1990 Search PubMed.
- Cationic Surfactants: Physical Chemistry, eds. D. N. Rubingh and P. M. Holland, Marcel Dekker, New York, 1991 Search PubMed.
- S.-W. Kwak, E.-J. Lee, M.-S. Song, G.-I. Jung and J.-W. Ha, PCT Int. Appl., 2 006 057 506, 2006.
- Y. I. Kuznetsov, L. V. Frolova and E. V. Tomina, Prot. Met., 2006, 42, 215 Search PubMed.
- A. N. Petrocci, Disinfection, Sterilization and Preservation, ed. S. S. Block, Lea & Febiger, Philadelphia, 1983 Search PubMed.
- M. Makosza, Pure Appl. Chem., 2000, 72, 1399 CrossRef CAS.
- F. C. Kull, P. C. Eisma and H. D. Sylwestrowicz, Environ. Microbiol., 1961, 9, 538 CAS.
- J. Oertel, Holztechnologie, 1965, 6, 243 Search PubMed.
- J. A. Butcher, A. F. Preston and J. Drysdale, For. Prod. J., 1977, 27, 19 Search PubMed.
- J. A. Butcher and J. Drysdale, N. Z. J. For. Sci., 1978, 8, 403 Search PubMed.
- J. Cross, in Introduction, Cationic Surfactants, Analytical and Biological Evaluation Surfactant Science Series, eds. J. Cross and E. J. Singer, Marcel Dekker, New York, 1994, vol. 53 Search PubMed.
- C. Klinguer, A. Beck, P. De-Lys, M. C. Bussat, A. Blaecke, F. Derouet, J. Y. Bonnefoy, T. N. Nguyen, N. Corvaia and D. Velin, Vaccine, 2001, 19, 4236 CrossRef CAS.
- F. Liu and L. Huang, J. Controlled Release, 2002, 78, 259 CrossRef CAS.
- W. L. Hough, M. Smiglak, H. Rodríguez, R. P. Swatloski, S. K. Spear, D. T. Daly, J. Pernak, J. E. Grisel, R. D. Carliss, M. D. Soutullo, J. H. Davis, Jr and R. D. Rogers, New J. Chem., 2007, 31, 1429 RSC.
- E. B. Carter, S. L. Culver, P. A. Fox, R. D. Goode, I. Ntai, M. D. Tickell, R. K. Traylor, N. W. Hoffman and J. H. Davis, Jr, Chem. Commun., 2004, 630 RSC.
- J. Pernak, F. Stefaniak and J. Weglewski, Eur. J. Org. Chem., 2005, 650 CrossRef CAS; M. Stasiewicz, A. Fojutowski, A. Kropacz and J. Pernak, Holzforschung, 2008, 62, 309 CrossRef CAS.
- A. Burgard, Eur. Pat., 1 270 580, 2003; A. Burgard, US Pat., 2003023084 A1, 2003.
- Z.-B. Zhou, H. Matsumoto and K. Tatsumi, Chem.–Eur. J., 2005, 11, 752 CrossRef CAS; J. Pernak, K. Sobaszkiewicz and I. Mirska, Green Chem., 2003, 5, 52 RSC.
- J. Pernak and M. Branicka, J. Surfactants Deterg., 2003, 6, 119 Search PubMed.
- OECD Guideline for Testing of Chemicals No. 434: Acute Oral Toxicity—Acute Toxic Class Method, 2001, 1.
- OECD Guideline for Testing of Chemicals No. 404: Acute Dermal Irritation/Corrosion, 2002, 1.
- J. Nawrot, E. Bloszyk, J. Harmatha, L. Nowotny and B. Drożdż, Acta Entomol. Bohemosl., 1986, 83, 327 Search PubMed.
- Additional information regarding the ionic liquids synthesized is available in the ESI†.
- G. M. Sheldrick, Program for Semiempirical Absorption Correction of Area Detector Data, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, SHELXTL, version 5.05, Siemens Analytical X-ray Instruments Inc., 1996 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data. Fig. S1: ORTEP (50% probability thermal ellipsoids) of the asymmetric unit of [HEX][Ace]. Fig. S2: Close contacts around the cations in [HEX][Ace]. Fig. S3: π-Stacking modes of the polymeric cation in [HEX][Ace]. Fig. S4: π-Stacking mode of the dimeric cation in [HEX][Ace]. Fig. S5: ORTEP (50% probability thermal ellipsoids) of the asymmetric unit of [1-(OctOMe)-3-OH-Py][Sac]. CCDC reference numbers 687477 and 687478. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b813213p |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2009 |
