Metal ion binding of the first external loop of DCT1 in aqueous solution
Yuande
Song
,
Dan
Wang
,
Haiyan
Qi
,
Shuyan
Xiao
,
Rong
Xue
and
Fei
Li
*
State Key Laboratory of Supramolecular Structure and Materials, Jilin University, Changchun 130012, People’s Republic of China. E-mail: feili@jlu.edu.cn; Fax: +86-431-85193421; Tel: +86-431-85168548
First published on 21st July 2009
Abstract
The binding of the external loop1 of DCT1 with divalent metal cations was first verified by the NMR measurements of isolated peptides and the binding sites were determined.
DCT1 (divalent cation transporter 1) consisting of 12 transmembrane domains belongs to the Slc11 (solute carrier 11) or Nramp (natural resistance-associated macrophage protein) family and is mainly found at the apical membrane of epithelial cells (isoform I)1 and in recycling endosomes (isoform II).2 DCT1 has an unusually broad substrate range including Fe2+, Zn2+, Mn2+, Co2+, Cd2+, Cu2+, Ni2+ and Pb2+, and plays an important role in metal ion homeostasis.3 Structure–function studies of Nramp homologues using mutagenesis approaches have revealed that the segment extending from the highly conserved C-terminal end of TM1 to the N-terminal portion of TM2, named as external loop1, may be involved in metal ion binding and could contribute to the formation of the Nramp cation translocation pathway.4 However, direct proof of metal ion binding to the external loop1 has not been reported and the transport mechanism is still obscure. In order to verify the importance of the external loop1 for the metal ion binding of DCT1, we measured the interactions of some isolated peptides, including a segment from external loop1 of DCT1 and its site-directed mutants (Fig. 1), with metal cations by NMR. We first directly observed the binding of the external loop1 of DCT1 with metal ions then determined the binding sites. Our findings may be helpful in understanding the transport mechanism of DCT1.
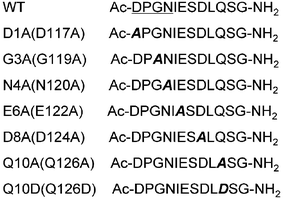 | ||
| Fig. 1 The sequences of the wildtype peptide (WT) and its mutants in this study. The numbers in parentheses are the sequence numbers of the residues in DCT1. | ||
The 2D NMR TOCSY spectra of the peptides in 10% D2O + 90% H2O at pH 5.5 were recorded before and after addition of the divalent metal ionsFe2+ (0.2 mM + 10 mM L-(+)-ascorbic acid sodium salt to prevent oxidation of Fe2+), Co2+ (0.4 mM), Ni2+ (0.2 mM), Mn2+ (0.05 mM), Zn2+ (10 mM), Cd2+ (10 mM) and different amounts of Pb2+ ions (from 0.05 to 0.5 mM with increments of 0.05 mM). The concentrations of the peptides were 0.5 mM for Pb2+titration experiments and 2 mM for other metal ion experiments. The intensities and chemical shifts of the spectra in the presence and absence of the cations were compared for the paramagnetic ions Fe2+, Co2+, Ni2+ and Mn2+, and only the chemical shifts were compared for the diamagnetic ions Zn2+, Cd2+ and Pb2+ ions. With the addition of small amounts of paramagnetic ions, the signals of the protons that are close to the binding sites would be much more broadened than the protons that are farther from the binding sites. Therefore, the residues that display a severe decrease in the intensity of the proton signals are considered to be involved in the coordination of metal ions. Similarly, the presence of paramagnetic ions would also lead to a larger change in the chemical shift of the residues that are in direct contact with the ions. On the other hand, the binding of the diamagnetic metal ions to peptides would alter the chemical shifts of some residues that have larger changes in their structures if the binding is strong enough.
Our results showed that for the WT peptide the signals mainly associated with the β-protons of the residues Asp1, Asn4, Asp8 and both β- and γ-protons of Glu6 are totally collapsed in the presence of Mn2+ and Co2+ ions or severely broadened in the presence of Ni2+ ions, while other residues of the peptide displayed less broadening of their signals (Fig. 2).
 | ||
| Fig. 2 The intensities of the TOCSY cross peaks for 2 mM WT peptide in the presence of 0.05 mM Mn2+, 0.4 mM Co2+ and 0.2 mM Ni2+ and for 2 mM Q10D mutant in the presence of 0.4 mM Co2+ relative to those in the absence of the metal ions. | ||
The effects of the paramagnetic metal ions on the chemical shifts of the WT peptide were also observed. The resonances that are associated with the β-protons of the residues Asn4 and Glu6 and the α-proton of Asp8 were down-field shifted remarkably in the presence of Co2+ ions (the signals of the γ-protons for Glu6 and the β-protons for Asp8 disappeared due to broadening). Similarly, much larger down-field shifting of the resonances from the sidechain protons of Asn4, Glu6 and Asp8 and larger down-field shifting from the sidechain protons of Asp1 were also observed in the presence of Fe2+ and Ni2+ ions. No obvious shifting of resonances was observed with addition of Mn2+ ions because the concentration of Mn2+ ions (0.05 mM) was too low to affect the chemical shifts of the WT peptide (data not shown). These results suggest that there is an interaction between the WT peptide and the paramagnetic ions and the interaction sites are correlated with the sidechains of Asp1, Asn4, Glu6 and Asp8.
Fig. 2 also displays the changes in the intensity of the TOCSY cross peaks for the Q10D mutant in the presence of Co2+ ions. One can find that the cross peaks associated with the amino protons of Asp8 and Leu9, and the sidechain protons of Asp10 and Ser11 are also severely broadened besides the cross peaks associated with the sidechain protons of Asp1, Asn4 and Glu6. Obviously, the substitution Q10D alters the binding mode of the peptide with metal ions.
The addition of Pb2+ ion into the WT peptide aqueous solution resulted in a larger shifting of some resonances associated with the residues Asp1, Ile5, Glu6, Ser7 and Asp8 (Fig. 3). When the residue Asp1 was replaced with Ala (D1A), the resonance shifting induced by Pb2+ ions nearly disappeared for Ala1 and remarkably reduced for Ile5, while the chemical shifts of other residues were less affected by Pb2+ ions. Similarly, the replacement of Ala for Gly in position 3 (G3A) resulted in a reduced shifting of the residues Asp1, Ile5 and Asp8. Although the evident change in the chemical shifts of Asn4 was unexpectedly not observed in the presence of Pb2+ ions for the WT peptide, the role of the residue in the binding was still explored by the substitution of N4A. Upon the substitution of Ala for Asn, the effect of Pb2+ ions on the chemical shifts of the residues Asp1 and Ile5 was nearly eliminated and the effect on the residue Asp8 was decreased. The dramatic effect of substitution on the interaction between Pb2+ ion and peptide was observed for E6A and D8A mutants, where the resonance shifting of the residues induced by Pb2+ ions was totally abolished when the Glu6 was replaced by Ala or remarkably reduced when Asp8 was replaced by Ala. In contrast, the chemical shifts of Q10A were less affected by Pb2+ ions, whereas the effect of Pb2+ ions on the chemical shifts of Q10D was larger and rather different from the WT peptide. For the Q10D mutant, the resonance shifting of the residues Asp1, Ile5, Glu6 and Ser7 was decreased, while the C-terminal residues Leu9, Asp10, Ser11 and Gly12 displayed a dramatic change in their chemical shifts. All these data indicate that the WT peptide coordinates with Pb2+ ions. The residues Asp1, Asn4, Glu6 and Asp8 may be involved in the coordination that leads to a larger change in the structure of the segment from Ile5 to Asp8. The dissociation constant of Pb2+ with the WT peptide was estimated to be ca. 10−4 M by the ion titration experiment (data not shown). The dramatic effect of Pb2+ on the resonances of the C-terminal residues of the Q10D mutant further suggests that the substitution of Asp for Gln10 alters the binding mode of the peptide with metal ions.
 | ||
| Fig. 3 The proton chemical shifts of the WT peptide and its mutants at concentrations of 0.5 mM in the presence of 0.5 mM Pb2+ ions relative to those in the absence of Pb2+ ions. | ||
The addition of Zn2+ and Cd2+ ions had no observed effect on the chemical shifts of the WT peptide, even when the concentration of the ions was increased up to 10 mM, indicating that the interactions of Zn2+ and Cd2+ ions with the WT peptide are rather weak.
The motif DPGN (corresponding to Asp1-Asn4 in this study) at the end of the first putative transmembrane segment is conserved in the Slc11 homologues. Other residues in the external loop1 of Slc11 homologues, except for a glycine residue at position 128, are not strictly conserved. However, some amino acid substitutions in this region resulted in a dramatic effect on the metal ion transport of Slc11 proteins.4 Studies of site-directed mutagenesis demonstrated that the conservative mutations of four sites Asp34, Pro35, Gly36 and Asn37 in MntH (corresponding to Asp1-Asn4 in this study), the Slc11 homologue of Escherichia coli, result in severe defectiveness in Mn2+ transport, even loss of transport activity (for the mutation D34E, N). In DCT1, the mutations of D117A (corresponding to D1A in this study), G119A (G3A), E122A (E6A), D124A (D8A) and Q126D (Q10D) resulted in a substantial loss of transport activity. Among these mutations, G119A and Q126D abolished the function of DCT1. In addition, the substitution of five residues ESDLQ in the external loop1 of DCT1 with the corresponding sequence STAVD in Smf1p, the Slc11 homologue of yeast, abolished the transport activity of the resulting DCT1 mutant. The significant impact of so many negatively charged residues on the metal ion transport of DCT1 may imply the involvement of the external loop1 in the metal ion binding of DCT1. Through NMR measurements of the peptides from the external loop1 of DCT1 and some of its substituents in the presence and absence of metal ions, we observed interactions of the external loop1 with metal ions, at least Mn2+, Co2+, Ni2+, Fe2+ and Pb2+. The negatively charged residues Asp117 (Asp1 in this study), Asn120 (Asn4), Glu122 (Glu6) and Asp124 (Asp8) may be involved in the metal ion binding of DCT1. Our results indicate that the residue Gln10 (Gln126 in DCT1) does not participate in metal ion binding. However, when the Gln residue is replaced with Asp (Q126D in DCT1 or Q10D in this study), the binding mode is changed. The Asp10 and its neighboring residues may be also involved in metal ion binding for the mutating peptide besides the residues Asp1, Asn4, Glu6 and Asp8. The different binding mode of the Q10D or Q126D mutant from the WT peptide or protein may lead to the difference in their functions. The G3A (G119A in DCT1) substitution only has a large effect on the N-terminal binding of the peptide. Whether the partial change in the binding is correlated to the dramatic abolishment of the metal ion transport observed in the electrophysiological study of DCT1/G119A is not clear yet.
In conclusion, we first directly observed the binding of the external loop1 of DCT1 with metal ionsMn2+, Co2+, Ni2+, Fe2+ and Pb2+ from the isolated peptides using an NMR method based on the changes in the resonance intensity and chemical shift induced by metal ions. The binding sites were found to be correlated to the negatively charged residues Asp117, Asn120, Glu122 and Asp124. The dramatic effect of Q126D substitution on the function of DCT1 may arise from the change in the binding structure of the mutant.
We thank the NSFC (20673043) for funding.
Notes and references
- F. Canonne-Hergaux, S. Gruenheid, P. Ponka and P. Gros, Blood, 1999, 93, 4406 CAS; F. Canonne-Hergaux, J. Calafat, E. Richer, M. Cellier, S. Grinstein, N. Borregaard and P. Gros, Blood, 2002, 100, 268 CrossRef CAS; D. Trinder, D. J. Macey and J. K. Olynyk, Int. J. Mol. Med., 2000, 6, 607 Search PubMed; S. Tandy, M. Willians, A. Leggett, M. Lopez-Jimenez, M. Dedes, B. Ramesh, S. K. Srai and P. Sharp, J. Biol. Chem., 2000, 275, 1023 CrossRef CAS.
- S. Gruenheid, F. Canonne-Hergaux, S. Gauthier, D. J. Hackam, S. Grinstein and P. Gros, J. Exp. Med., 1999, 189, 831 CrossRef CAS; N. Touret, W. Furuya, J. Forbes, P. Gros and S. Grinstein, J. Biol. Chem., 2003, 278, 25548 CrossRef CAS.
- H. Gunshin, B. Mackenzie, U. V. Berger, Y. Gunshin, M. F. Romero, W. F. Boron, S. Nussberger, J. L. Gollan and M. A. Hediger, Nature, 1997, 388, 482 CrossRef CAS; Y. Nevo and N. Nelson, J. Biol. Chem., 2004, 27951, 53056 CrossRef; A. Sacher, A. Cohen and N. Nelson, J. Exp. Biol., 2001, 2046, 1053 Search PubMed; P. Marciani, D. Trotti, M. A. Hediger and G. Monticelli, J. Membr. Biol., 2004, 197, 91 CrossRef CAS; M. Okubo, K. Yamada, M. Hosoyamada, T. Shibasaki and H. Endou, Toxicol. Appl. Pharmacol., 2003, 187, 162 CrossRef CAS.
- H. A. Haemig and R. J. Brooker, J. Membr. Biol., 2004, 201, 97 CrossRef CAS; A. Cohen, Y. Nevo and N. Nelson, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10694 CrossRef CAS; S. Lam-Yuk-Tseung, G. Govoni, J. Forbes and P. Gros, Blood, 2003, 101, 3699 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
