Independent metal-binding features of recombinant metallothioneins convergently draw a step gradation between Zn- and Cu-thioneins†
Roger
Bofill
a,
Mercè
Capdevila
*a and
Sílvia
Atrian
b
aDept. de Química, Fac. de Ciències, Universitat Autònoma de Barcelona, E-08193 Bellaterra, Barcelona, Spain. E-mail: merce.capdevila@uab.cat; Fax: +34 93 5813101; Tel: +34 93 5813323
bDept. de Genètica, Fac. de Biologia, Av. Diagonal 645, Universitat de Barcelona, E-08028, Barcelona, Spain. E-mail: satrian@ub.edu; Fax: +34 93 4034420; Tel: +34 93 4021501
First published on 24th March 2009
Abstract
Data on the metal-binding behaviour of circa 20 recombinant metallothioneins (MTs) from evolutionary divergent organisms, gathered after years of systematic research, are here comprehensively analyzed. The consideration of four independent in vivo and in vitro metal-binding features reveals a gradation of the metal-binding character of the MTs considered that significantly coincides in a robust new classification: a stepwise gradation between Zn- and Cu-thioneins. The intermediate positions in this list are occupied by a group of polyvalent MTs, exhibiting a merging Zn-/Cu-thionein character that would suit general metal handling purposes. In contrast, the extreme positions are respectively occupied by those MTs that would have evolved to fulfil specialized Zn- or Cu-related physiological roles. Overall, the analyzed trends allow the proposal of a chemically- and biologically-sound new reflection on MT classification criteria.
Introduction
Metallothioneins (MTs) constitute a large and heterogeneous family of ubiquitous, low molecular weight, Cys-rich proteins with outstanding heavy metal binding capacity.1 Since their discovery,2 nearly 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 articles have been devoted to many aspects of their study and today it is accepted that, besides their detoxifying properties, MTs are involved in Zn and Cu homeostasis, and may participate in a myriad of processes specific to the metabolism of each different group of organisms considered. So, although there is no question that MTs have unique function potentialities, it is perplexing that even after thousands of studies, there is still a lack of a definite classification evidencing MT basic biological function/s and associated patterns of differentiation through evolution.3 Significantly, the recent evidence of MT presence in eubacteria has raised even deeper questions about the original function of these polypeptides in primitive organisms.4
000 articles have been devoted to many aspects of their study and today it is accepted that, besides their detoxifying properties, MTs are involved in Zn and Cu homeostasis, and may participate in a myriad of processes specific to the metabolism of each different group of organisms considered. So, although there is no question that MTs have unique function potentialities, it is perplexing that even after thousands of studies, there is still a lack of a definite classification evidencing MT basic biological function/s and associated patterns of differentiation through evolution.3 Significantly, the recent evidence of MT presence in eubacteria has raised even deeper questions about the original function of these polypeptides in primitive organisms.4
Initially, MTs were classified in three classes on the basis of the similarity of their sequences to mammalian MTs.5 Afterwards, when the increase in reports of different taxon MTs made the so-called class II (those not homologous to mammalian isoforms) inadequate, a classification based on sequence similarities, derived from phylogenetic relationships, was proposed.6 Unfortunately, these distributions shed no light on the possible function or evolution relationships among MTs. In this scenario, some years ago we developed a new criterion7 based on the stoichiometric, spectroscopic and spectrometric features of the recombinant complexes yielded by the distinct MT polypeptides when binding a physiological metal ion – Zn(II) or Cu(I). This classification differentiated between two categories, considering as Zn-thioneins (Zn-ths) those that kept Zn(II) bound when folding in vivo even in the presence of high copper concentrations and hence generating Zn,Cu-mixed metal species; while defining as Cu-thioneins (Cu-ths) those capable of yielding homometallic Cu(I)–MT species under the same conditions. Significantly, protein distance trees fully reproduced the Zn-/Cu-thionein clustering resulting from the application of our criteria.7
Over the last 15 years, the number and phylogenetic distribution of the MTs of which we have studied the respective recombinant metal–MT complexes has been significantly increased (Table 1). Hence, we are now in a position that allows a comprehensive consideration, not only of the features of a great number of metal–MT complexes folded in vivo, but also of those formed in vitro by means of Zn/Cd or Zn/Cu replacement on the corresponding Zn–MT forms. The result of this analysis has provided us with a deeper insight and a fine-tuning of our previous classification. In brief, we propose a step gradation between genuine Zn- and Cu-thioneins, which converge in a central group of MTs exhibiting intermediate features. Significantly, this step gradation has been drawn on the basis of four independent grounds, which are detailed below and that render fully coincident results. Additionally, it is invariably coincident with the Zn- or Cu-thionein character previously proposed for a few MTs following other criteria, often related to the metal response pattern of the corresponding gene. In conclusion, the new MT overview here presented may be highly informative when attempting to describe the physiological role and/or evolutionary history of any MT polypeptide.
| Organism | Isoform | Swiss-Prot accession number | noCys | Sequence |
|---|---|---|---|---|
| C. elegans | CeMT1 | P17511 | 19 | MACKCDCKNKQCKCGDKCECSGDRCCEKYCCEEASEKKCCPAGCKGDCKCANCHCAEQKQCGDKTHQHQGTAAAH |
| M. edulis | MeMT | P80249 | 21 | MPAPCNCIETNVCICDTGCSGEGCRCGDACKCSGADCKCSGCKVVCKCSGSCACEGGCTGPSTCKCAPGCSCK |
| M. musculus | MT1 | P02802 | 20 | MDPNCSCSTGGSCTCTSSCACKNCKCTSCKKSCCSCCPVGCSKCAQGCVCKGAADKCTCCA |
| H. pomatia | HpCdMT | P33187 | 18 | MGKGKGEKCTSACRSEPCQCGSKCQCGEGCTCAACKTCNCTSDGCKCGKECTGPDSCKCGSSCSCK |
| T. pyriformis | TpyMT1 | O97388 | 31 | MDKVNNNCCCGENAKPCCTDPNSGCCCVSETNNCCKSDKKECCTGTGEGCKCTGCKCCQPAKSGCCCGDKAKACCTDPNSGCCCSSKTNKCCDSTNKTECKTCECCK |
| S. purpuratus | SpMTA | P04734 | 20 | MPDVKCVCCTEGKECACFGQDCCVTGECCKDGTCCGICTNAACKCANGCKCGSGCSCTEGNCAC |
| G. gallus | CkMT | P68497 | 20 | MDPQDCTCAAGDSCSCAGSCKCKNCRCRSCRKSCCSCCPAGCNNCAKGCVCKEPASSKCSCCH |
| H. americanus | MTH | P29499 | 18 | MPGPCCKDKCECAEGGCKTGCKCTSCRCAPCEKCTSGCKCPSKDECAKTCSKPCKCCP |
| C. elegans | CeMT2 | P17512 | 18 | MVCKCDCKNQNCSCNTGTKDCDCSDAKCCEQYCCPTASEKKCCKSGCAGGCKCANCECAQAAH |
| S. cerevisiae | Crs5 | P41902 | 19 | MTVKICDCEGECCKDSCHCGSTCLPSCSGGEKCKCDHSTGSPQCKSCGEKCKCETTCTCEKSKCNCEKC |
| M. musculus | MT4 | P47945 | 20 | MDPGECTCMSGGICICGDNCKCTTCSCKTCRKSCCPCCPPGCAKCARGCICKGGSDKCSCCP |
| Q. suber | QsMT | Q93X22 | 14 | MSCCGGNCGCGTGCKCGSGCGGCKMFPDISSEKTTTETLIVGVAPQKTHFEGSEMGVGAENGCKCGSNCTCDPCNCK |
| H. pomatia | HpCuMT | P55947 | 18 | MGRGKNCGGACNSNPCSCGNDCKCGAGCNCDRCSSCHCSNDDCKCGSQCTGSGSCKCGSACGCK |
| D. melanogaster | MtnA | P04357 | 12 | MPCPCGSGCKCASQATKGSCNCGSDCKCGGDKKSACGCSECCA |
| D. melanogaster | MtnB | P11956 | 12 | MVCKGCGTNCQCSAQKCGDNCACNKDCQCVCKNGPKDQCCSNK |
| S. cerevisiae | Cup1 | P07215 | 12 | QNEGHECQCQCGSCKNNEQCQKSCSCPTGCNSDDKCPCGNKSEETKKSCCSGK |
Experimental
The metal-binding abilities of all the MTs considered have been determined always following the same experimental rationale. In short, our strategy consists of (1) the recombinant synthesis of each MT peptide in Zn-, Cd- or Cu-supplemented cultures of the producing bacteria; (2) the characterization of the purified Zn–, Cd– and Cu–MT complexes, the so-called in vivo-folded complexes; (3) the parallel preparation and characterization of the complexes yielded in vitro by Zn(II)/Cd(II) or Zn(II)/Cu(I) replacement reactions, the so-called in vitro-complexes; and finally (4) the comparison of both kinds of metal–MT species.The synthesis of the MT peptides is achieved by recombinant expression of the corresponding cDNAs cloned in the E. coli pGEX vector, which render GST-MT fusion proteins from which the MT moiety is further recovered after thrombin cleavage and FPLC purification. The expression construct is transformed into E. coli BL21 cells that, for preparative purposes, are grown in conventional media (LB) supplemented with 500 μM CuSO4, 300 μM ZnCl2 or 300 μM CdCl2, which allow direct (in vivo) synthesis of the MTs as Cu(I)-, Zn(II)- or Cd(II)-complexes. Full details of the synthesis and purification procedures have been extensively described.8,9 In the case of Cu(II)-enriched cultures, low aeration conditions are achieved by filling up the Erlenmeyer flasks to no less than 3/4 of their volume and a maximum agitation of 200 rpm during growth.10
The S, Zn, Cd and Cu content of the in vivo-MT preparations is analyzed by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES), and samples are routinely incubated in 1 M HCl at 65 °C for 5 min (acid ICP) prior to measurements in order to eliminate possible traces of labile sulfide ions.11Protein concentrations are calculated from the ICP-AES sulfur measure, assuming that all S is contributed by the Cys and Met residues of the MT peptides . The S2− content of the MT preparations is measured by Gas Chromatography with Flame Photometric Detection (GC-FPD) as previously described.11Circular dichroism (CD) and UV-visible spectrophotometry are performed at 25 °C, and all spectra are recorded with 1 cm capped quartz cuvettes, corrected for the dilution effects and processed using the GRAMS 32 programme.8 The metal–MT preparations are also analyzed by Time-of-Flight Electrospray Mass Spectrometry (QTOF-ESI-MS), following the procedures described elsewhere.12 The molecular masses, which are determined at least twice in order to ensure reproducibility, are calculated in accordance with the literature.10,13
The in vitro-folded metal–MT complexes are prepared through titration of the recombinant Zn(II)–MT preparations with Cd(II) or Cu(I) ions, at pH 7.0 and under Ar atmosphere, following the procedures described elsewhere.8,14 These titrations are monitored by CD and UV-visible spectroscopies, as well as by ESI-MS analysis. The spectroscopic and spectrometric data resulting from the titrations not only yield information about metal replacement pathways and the features of the corresponding final species (in vitro-folded metal–MT complexes), but also allow their comparison with their cognate in vivo complexes.
Results and discussion
The four independent criteria that led to the proposed step gradation between genuine Zn- and Cu-thioneins are fully commented on in separate sections below. Fig. 1 and Table 2 respectively display all the in vivo and in vitro features these foundations are based on.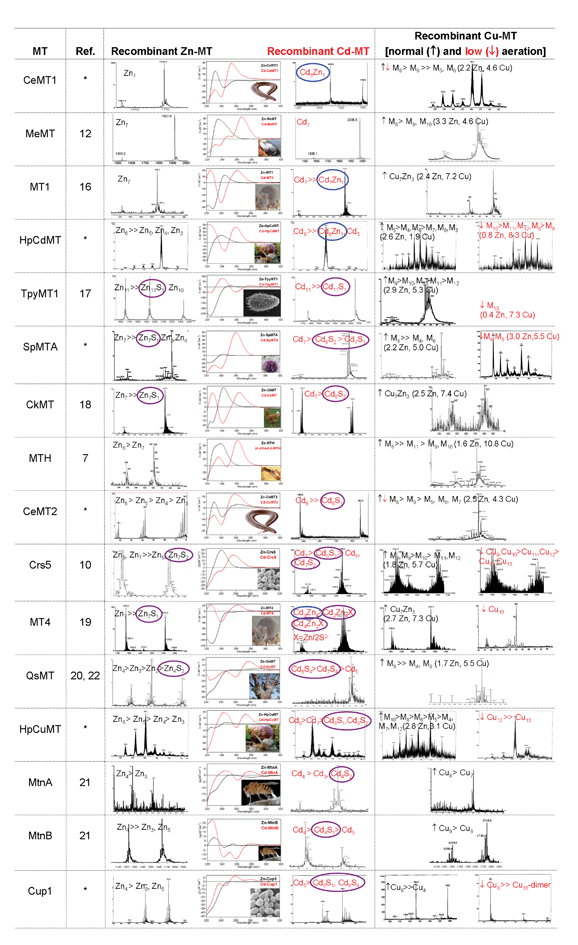 | ||
| Fig. 1 Electrospray Ionization Mass Spectrometry (ESI-MS) and Circular Dichroism (CD) data for the recombinant preparation of all the MTs considered in this work (first column) biosynthesized in Zn(II)- (black, third column), Cd(II)- (red, third column) and Cu(II)- (last column) enriched media, the later under regular (↑, black) and low (↓, red) aeration conditions. The proteins appear listed from genuine Zn-ths to genuine Cu-ths. Heterometallic Cd,Zn–MT and sulfide-containing complexes are respectively highlighted in blue and purple. In the speciation of the Cu–MT complexes, M stands for Zn and/or Cu in the heterometallic preparations. For these cases, ICP-AES quantification of their Zn and Cu content is included in parenthesis. *Unpublished data, personal communication. For enlarged 2-page version see ESI.† | ||
| Isoform | Ref. | Zn–MT + excess Cd(II)a | Cu(I) equiv. | Cys/Cu(I)b |
|---|---|---|---|---|
| a ESI-TOF-MS data. Bold: major species, italics: minor species. b Inverse ratio of the number of Cu(I) equiv. per-Cys residues needed to reproduce the in vivo preparations. c Unpublished data, personal communication. | ||||
| CeMT1 | c | Cd6Zn1, Cd7Zn1, Cd8Zn1 | 4 | 4.8 |
| MeMT | 12 | Cd6Zn1, Cd7, Cd5Zn2 | 6 | 3.5 |
| MT1 | 16 | Cd7, Cd6Zn2, Cd5Zn2, Cd6Zn1, Cd7Zn1, Cd8 | 7 | 2.9 |
| HpCdMT | c | Cd6, Cd6Zn1 | 6 | 3.0 |
| TpyMT1 | 17 | Cd11, Cd11S1 | 10 | 3.1 |
| SpMTA | c | Cd8, Cd9, Cd10 | 7 | 2.9 |
| CkMT | 18 | Cd7, Cd7S1 | 7 | 2.9 |
| MTH | 7 | Cd6, Cd4Zn2, Cd6Zn1 | 8 | 2.3 |
| CeMT2 | c | Cd6, Cd7, Cd8 | 8 | 2.3 |
| Crs5 | 10 | Cd7, Cd7S4 | 9 | 2.1 |
| MT4 | 19 | Cd7, Cd6Zn1 | 10 | 2.0 |
| QsMT | 20,22 | Cd4, Cd5, Cd3, Cd6 | 7 | 2.0 |
| HpCuMT | c | Cd7, Cd6, Cd8, Cd6Zn1, Cd7Zn1, Cd5Zn1, Cd5 | 10 | 1.8 |
| MtnA | 21 | Cd4, Cd3 | 7 | 1.7 |
| MtnB | 21 | Cd4, Cd5 | 9 | 1.3 |
| Cup1 | c | Cd5, Cd4, Cd6 | 10 | 1.2 |
Presence/absence of Zn(II) in the MT complexes biosynthesized in copper-enriched media
The first foundation of our proposal comes from the presence/absence of Zn(II) in the recombinant metal–MT complexes biosynthesized in cultures exclusively supplemented with Cu(I), a hint that was a key piece of evidence to differentiate between the two, firstly proposed MT classes, i.e. Zn-ths vs. Cu-ths.7 Recently, we further ascertained that the degree of aeration of the E. coli cultures could influence the presence of Zn(II) or the Zn(II)-to-Cu(I) ratio in the recovered complexes,10 in accordance with the well known decrease of copper levels in E. coli when grown aerobically.15 Therefore, both types of cultures (regular and limited aeration) have been routinely performed. A glance at the presence/absence of Zn(II) in complexes of several MTs synthesized in Cu-enriched media readily shows that the first 9 MTs listed in Fig. 1 generate heterometallic Zn,Cu-species whatever the aeration of the culture is, which indicates the intrinsic Zn-th character of these peptides – i.e. that Zn(II) is essential for their in vivo-folding in the presence of Cu(I) – and suggests that limited copper availability could be a drawback for non-strict Cu-ths to produce homometallic Cu-complexes. On the contrary, the last 3 MTs in Fig. 1 (MtnA, MtnB and Cup1) render homometallic Cu-complexes even under the most restrictive copper availability conditions, in accordance with a high Cu-th character. Interestingly, most MTs between these two sets exhibit intermediate properties, rendering homometallic Cu-species only when synthesized in low O2 conditions, but never under regular aeration. Therefore, a gradient in the Cu-th character can be inferred assuming that genuine Cu-ths are those that render homometallic Cu–MT species in vivo no matter what the aeration condition of the culture is, whereas MTs with a weaker Cu-preferring character only yield homometallic Cu-species under low O2 conditions. Concordantly, genuine Zn-th will always render heterometallic Zn,Cu–MT species independently of the aeration conditions.Number of Cu(I) equivalents required to reproduce the species obtained in vivo by in vitro Zn/Cu displacement
The second criterion of our proposal considers the number of Cu(I) equivalents required to reproduce the recombinant Cu–MT complexes by Cu(I) titration of the Zn–MT forms. It is noteworthy that for most of the studied MTs, the recombinant Cu–MT preparations are reproduced at some stage of the in vitro Zn/Cu replacement process. This Zn/Cu substitution is complete in strict Cu-ths, while only partial in MTs of a weaker Cu-th character, and therefore a lower amount of Cu(I) is required for the latter to reproduce the in vivo complexes by in vitrotitration . This simple but robust reasoning led us to estimate the number of Cu(I) equiv. required compared to the number of Cys of each MT sequence, in order to normalize the data in relation to the number of coordinating residues in each polypeptide (i.e. Cu(I) equiv.-to-Cys ratio). In Table 2, this relationship is shown in inverse terms (i.e., Cys/Cu(I) ratios) for the sake of clarity. It gradates from ca. 5 on the top to values close to 1 at the bottom. This tendency will be highly useful when seeking to place some controversial MTs in the Zn-th/Cu-th gradation (see below).Presence/absence of sulfide ligands and/or of Zn(II) in the MT complexes biosynthesized in cadmium-enriched media
The third basis for our gradation comes from the presence/absence of S2− ligands and/or of Zn(II) in the recombinant metal–MT complexes biosynthesized in cultures supplemented with Cd(II). This criterion originates from the fact that when we reported the presence of S2− (acid-labile) ligands in recombinant Zn–MT, and especially Cd–MT complexes,11 data already suggested that the S2−/MT ratio was greater for those MTs previously classified as Cu-ths, and thus not optimized for divalent metal binding. Since then, we have systematically analyzed the presence of S2− in all the metal–MT complexes studied, and effectively, only the 4 most extreme Zn-ths (cf. Fig. 1 list) do not show any trace of S2−-containing complexes in the production resulting from Cd-rich cultures. In all the other cases, the final Cd–MT preparations contain S2− ligands in some of the intermediate or minor species. Eventually, some of these MTs also give rise to minor sulfide-containing Zn–MT complexes when biosynthesized in Zn-rich media. The absence of sulfide ligands in the former peptides indicates that MTs with a strong Zn-th character are highly proficient at divalent metal binding and, therefore, do not require the contribution of non-proteic ligands. Concordantly, the presence of S2− in the biosynthesized Cd–MT complexes increases in direct relation to the Cu-th character of the peptide. Cd-QsMT is a special case since even the major species contains this inorganic component, but the particular primary structure of plant MTs may affect its metal binding performance.22Another feature to take into account regarding the in vivo folded Cd–MT species is the presence of Zn(II) in some of them, i.e.biosynthesis of heterometallic Zn,Cd–MT species, which could be another indicator of strong Zn-th character of the protein. Therefore, a gradation in the Zn-th character can also be envisaged by considering this aspect. Hence, CeMT1, MT1 and HpCdMT include Zn(II) in their Cd-complexes, and in the case of CeMT1, the mixed Zn,Cd-complex is the major species (Fig. 1). In summary, a structural role of Zn(II) does seem evident in the in vivo-folding of genuine Zn-th in the presence of Cd(II), under the same terms as those described in the presence of Cu(I).
Reluctance to in vitro Zn/Cd substitution
Finally, the above mentioned presence of Zn(II) in some biosynthesized Cd–MT complexes prompted us to check whether these MTs would be reluctant to fully exchange Zn(II) by Cd(II) in an in vitro replacement process, confirming our fourth piece of evidence. Thus, consideration of the final species achieved after titration of the distinct Zn–MT forms performed up to a clear excess of Cd(II) (i.e. until total saturation of the protein, Table 2) revealed a significant inertness against Zn/Cd substitution for the first two MTs in Fig. 1, CeMT1 and MeMT. Also, but to a lesser extent, reluctance to Zn/Cd replacement is patent for the mouse MT1 and snail HpCdMT isoforms. Finally, three other MTs (MTH, MT4 and HpCuMT) also retain Zn(II) at the end of the Cd(II) titrations. In these cases their Cys/Cu(I) ratio has been extremely helpful in placing those MTs in the global gradation here proposed (Fig. 1). All other MTs easily render homometallic Cd–MT species in vitro. Here, it is worth considering that data on Zn/Cd replacement refers to in vitro-forced conditions, which do not necessarily exist in normal physiological environments. In fact, it seems unlikely that the emergence and evolutionary differentiation of MTs has been driven by their ability to bind, and thus detoxify, cadmium. Somehow, it seems more likely that cadmium detoxification is an adopted property of MTs rather than its original function.23 Therefore, assumption of the hypothesis that MTs were not initially designed for Cd(II) binding would sensibly explain why, in two thirds of the cases, recombinant Zn– and Cd–MT complexes show clearly distinct CD envelopes, which may be an indication of their lack of isostructurality (Fig. 1). Although both divalent cations coordinate to MTs under a tetrahedral coordination environment, the Cd(II) ionic radius is 1.31 times that of Zn(II). Hence, steric constraints may be the factor dictating the capacity of a Zn-th to become optimal or not for Cd(II) coordination, and thus for detoxification purposes.Conclusions
In conclusion, the comprehensive consideration of all the analyzed properties of a series of distinct MTs converges in a coincident classification for these metal-binding peptides : a step-wise gradation between extreme Zn-ths and extreme Cu-ths.The following overall guidelines can be drawn, taking into account that invariably, the less marked a specific character of an MT (Zn- or Cu-th) the more pronounced the contrary character (Scheme 1). On the one hand, genuine Zn-ths render (i) unique species when synthesized as Zn–MT complexes; (ii) mixed Zn,Cd–MT complexes when obtained in Cd-enriched media and (iii) show a clear in vitro-reluctance to total Zn/Cd exchange. In regards to Cu(I) coordination, Zn-ths biosynthesized in Cu-rich media yield several mixed Zn,Cu–MT complexes of different stoichiometries.
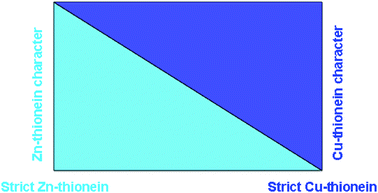 | ||
| Scheme 1 Schematic drawing representing the gradation between the Zn- and the Cu-thionein character for all considered MTs. The light and dark blue colours respectively illustrate the Zn-th and Cu-th character of a MT peptide. Thus, it is graphically displayed how, for a given peptide, the increase of one character concomitantly entails the decrease of the other, and vice versa. | ||
On the other hand, strict Cu-ths (iv) are able to render unique, homometallic Cu-species when biosynthesized in Cu-rich media whatever the culture aeration conditions are, while (v) they yield a mixture of species of different stoichiometry when synthesized as Zn- or Cd-complexes, with (vi) a significant presence of S2− ligands in their metal-complexes, and (vii) they do not show inertness against in vitro Zn/Cd substitution.
In fact, this rationale is made patently clear when observing the spectrometric results included in Fig. 1, as the MTs positioned on top – those with the most strict Zn-th character – render ESI-MS spectra exhibiting single peaks – unique species – when biosynthesized in Zn- or Cd- enriched media, but multiple peaks – a mixture of species – when produced in Cu-enriched media. These features are the opposite for the extreme Cu-ths, i.e. clean ESI-MS spectra when folded in the presence of Cu(I), and intricate spectra when affording divalent-metal complexes.
The gradation from strict Zn-ths to strict Cu-ths and vice versa converges in a group of MTs with intermediate properties, such as the yeast Crs5 and the mammalian MT4 isoforms, which have to be considered ambivalent metal bindingpeptides , and highly flexible in their metal-preference abilities. Significantly, these peptides have been considered as representatives of the most primitive MTs (in eukaryotes10 and mammals,24 respectively), which is concordant with peptides exhibiting a low degree of functional specificity. Furthermore, it is worth noting that the classification proposed here nicely matches, for those cases where it is known, the classification of MTs previously reported according to native characterization, or at gene response level, this is, according to the metal ion that induces its synthesis in overexposed organisms. This criterion has been commonly used to deduce putative physiological roles, and our current results show, once again, how functional specialization is a feature that may have been achieved in evolution combining gene regulation and protein function specificities. Hence, to mention some outstanding cases, mammalian MT1 has been long considered the paradigm of divalent metal ion binding MTs;2Tetrahymena MT1s are clear cadmium-inducible isoforms;25 yeast Cup1 honours its name by appearing as the most extreme Cu-th,26 and finally, both snail (H. pomatia) isoforms are classified according to their distinct native preferences.27
We cast no doubt on the fact that the position of each MT peptide in the proposed classification encloses information about its physiological role and evolutionary history. Therefore, it may be worth considering this gradation when attempting to unveil the putative physiological significance of isoform differentiation in this controversial protein superfamily.
Acknowledgements
S.A.’s and M.C.’s research is supported by the Spanish MICINN grants BIO2006-14420-C02/01 and /02, respectively. We also thank Dr Òscar Palacios and Mr Rubén Orihuela for personal communication of unpublished data.References
- Metal Ions in Life Sciences, ed. A. Sigel, H. Sigel and R. K. O. Sigel, Royal Society of Chemistry, Cambridge, UK, 2009, vol. 5, pp. 1–514 Search PubMed.
- M. Margoshes and B. L. Vallee, J. Am. Chem. Soc., 1957, 79, 4813–4814 CrossRef CAS.
- R. D. Palmiter, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 8428–8430 CrossRef CAS.
- N. J. Robinson, Nat. Chem. Biol., 2008, 4, 582–583 CrossRef CAS.
- B. A. Fowler, C. F. Hildebrand, Y. Kojima and M. Webb, in Metallothionein II, ed. J. H. R. Kägi and Y. Kojima, Birkhäuser Verlag, Basel, 1987, vol. 52, pp. 19–22 Search PubMed.
- http://www.bioc.unizh.ch/mtpage/classif.html (accessed Dec. 10, 2008).
- M. Valls, R. Bofill, R. Gonzàlez-Duarte, P. Gonzàlez-Duarte, M. Capdevila and S. Atrian, J. Biol. Chem., 2001, 276, 32835–32843 CrossRef CAS.
- M. Capdevila, N. Cols, N. Romero-Isart, R. Gonzàlez-Duarte, S. Atrian and P. Gonzàlez-Duarte, Cell. Mol. Life Sci., 1997, 53, 681–688 CrossRef CAS.
- N. Cols, N. Romero-Isart, M. Capdevila, B. Oliva, P. Gonzàlez-Duarte, R. Gonzàlez-Duarte and S. Atrian S, J. Inorg. Biochem., 1997, 68, 157–166 CrossRef CAS.
- A. Pagani, L. Villarreal, M. Capdevila and S. Atrian, Mol. Microbiol., 2007, 63, 256–269 CrossRef CAS.
- M. Capdevila, J. Domènech, A. Pagani, L. Tío, L. Villarreal and S. Atrian, Angew. Chem., Int. Ed., 2005, 44, 4618–4622 CrossRef CAS.
- R. Orihuela, J. Domènech, R. Bofill, C. You, E. A. Mackay, J. H. R. Kägi, M. Capdevila and S. Atrian, J. Biol. Inorg. Chem., 2008, 13, 801–812 CrossRef CAS.
- D. Fabris, J. Zaia, Y. Hathout and C. Fesenlau, J. Am. Chem. Soc., 1996, 118, 12242–12243 CrossRef CAS.
- R. Bofill, O. Palacios, M. Capdevila, N. Cols, R. Gonzàlez-Duarte, S. Atrian and P. Gonzàlez-Duarte, J. Inorg. Biochem., 1999, 73, 57–64 CrossRef CAS.
- F. W. Outten, D. L. Huffman, J. H. Hale and T. V. O’Halloran, J. Biol. Chem., 2001, 276, 30670–30677 CrossRef CAS.
- R. Bofill, M. Capdevila, N. Cols, S. Atrian and P. Gonzàlez-Duarte, J. Biol. Inorg. Chem., 2001, 6, 405–417 CrossRef CAS.
- J. Domènech, R. Bofill, A. Tinti, A. Torreggiani, S. Atrian and M. Capdevila, Biochim. Biophys. Acta, 2008, 1784, 693–704 CAS.
- L. Villarreal, L. Tío, M. Capdevila and S. Atrian, FEBS J., 2006, 273, 523–535 CrossRef CAS.
- L. Tío, L. Villarreal, S. Atrian and M. Capdevila, J. Biol. Chem., 2004, 279, 24403–24413 CrossRef CAS.
- J. Domènech, G. Mir, G. Huguet, M. Capdevila, M. Molinas and S. Atrian, Biochimie, 2006, 88, 583–593 CrossRef CAS.
- D. Egli, J. Domènech, A. Selvaraj, K. Balamurugan, H. Hua, M. Capdevila, O. Georgiev, W. Schaffner and S. Atrian, Genes Cells, 2006, 11, 647–658 CrossRef CAS.
- J. Domènech, R. Orihuela, G. Mir, M. Molinas, S. Atrian and M. Capdevila, J. Biol. Inorg. Chem., 2007, 12, 867–882 CrossRef CAS.
- B. L. Vallee, Experientia Suppl., 1987, 52, 5–16 Search PubMed.
- L. Tío, L. Villarreal, S. Atrian and M. Capdevila, J. Biol. Chem., 2004, 279, 24403–24413 CrossRef CAS.
- S. Díaz, F. Amaro, D. Rico, V. Campos, L. Benítez, A. Martín-González, E. P. Hamilton, E. Orias and J. C. Gutiérrez, PLoS ONE, 2007, 2, e291–e304 CrossRef.
- D. R. Winge, K. B. Nielson, W. R. Gray and D. H. Hamer, J. Biol. Chem., 1985, 260, 14464–14470 CAS.
- R. Dallinger, B. Berger, P. E. Hunziker and J. H. R. Kägi, Nature, 1997, 388, 237–238 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Two-page version of Fig. 1. See DOI: 10.1039/b904953c |
| This journal is © The Royal Society of Chemistry 2009 |
