Metal imaging in non-denaturating 2D electrophoresis gels by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) for the detection of metalloproteins
J.
Susanne Becker†
*a,
Ryszard
Lobinski
a and
J.
Sabine Becker
b
aLaboratoire de Chimie Analytique Bio-inorganique et Environnement, UMR 5254, CNRS, Hélioparc, 64053 Pau, France. E-mail: susanne.becker@sandoz.com
bCentral Division of Analytical Chemistry, Research Centre Jülich, D-52425 Jülich, Germany
First published on 12th June 2009
Abstract
Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) was developed as a powerful analytical technique for metal imaging of 2D gels for the detection of metalloproteins in rat kidney after electrophoretic separation. Protein complexes, extracted with water, were separated in their native state in the first and second dimension by blue native gel electrophoresis (BN-PAGE). Essential and toxic metals, such as zinc, copper, iron, manganese and lead, were monitored by LA-ICP-MS after gel ablation by a focused laser beam in a way that the total surface of a selected fragment of the gel was totally ablated. The metal distribution of this part of the gel was then constructed by plotting the metal (isotope) signal intensity as a function of the x,y (isoelectric point, molecular mass) coordinates of the gel . The proteins at locations rich in metals were cut out, digested with trypsin and analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS).
1. Introduction
Two-dimensional gel electrophoresis (2D-GE) is a powerful separation technique, which allows the separation of thousands of proteins in one run.1Proteins are separated according to their isoelectric point in the first dimension (isoelectric focusing, IEF) and then, orthogonally, according to their molecular weight by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins in 2D gels are visualized by, e.g. Coomassie Blue or silver staining, excised, digested by trypsin and identified by MALDI-TOF-MS or 2D HPLC-ESI-MS/MS resulting in the principal workflow of the proteomics analysis.2Metals play a crucial role in all living processes – metals are critical as catalysts, gene expression regulators, and cofactors for enzymes (Cu/Zn superoxide dismutase – antioxidant defence) that protect the living organism from reactive oxygen species (ROS). Metalloproteins, which are believed to account for one-third of all the proteins in living organisms,3 are raising increasing interest in metallomics studies.4,5 However, they often escape the proteomics analysis because of the denaturating character of the protocol, possible metal losses during staining and the lack of metalloprotein detection specificity. Therefore, the use of non-denaturating protocols, such as e.g. Native Blue GE is advised.6 Detection of metals in protein spots in the gel was recognized to be indispensable in the metallomics workflow.7
Metal detection in metalloproteins in the gels has long been carried out by autoradiography, with its inherent use of radioactive isotopes, or by synchrotron radiation XRF (X-ray fluorescence analysis ) and PIXE (proton induced X-ray emission) with the need for hardly available facilities. Laser-ablation (LA)-ICP-MS, pioneered by Neilsen et al.,8 offers a competitive alternative for the in situ probing of the protein bands and spots for the presence of metals and metalloids. The laser ablated material is transported into the ICP by a continuous stream of argon as carrier gas, and the ions formed in the ICP source are analyzed using a quadrupole analyzer or sector field mass spectrometer. As a result, an electropherogram is obtained in which the quantity of a given element is a function of the position of the protein by which it is carried in the gel . Detection of metals by LA-ICP-MS is a potentially fast and fairly robust technology, because no further reaction or derivatization step is involved, and the signal is, theoretically, directly proportional to the quantity of the analyte element in the gel .9LA-ICP-MS allows fast screening of one- and two-dimensional gels in order to detect metals, metalloids and non-metals in protein bands and spots.9,10 Jakubowski’s group has developed labelling methods based on DOTA–lanthanide chelates commercially available with an SCN-linker molecule. Blot membranes with labelled proteins were scanned and analysed by LA-ICP-MS for lanthanides.11–15
In our recent work the formation of copper bindingproteins using an isotopic enriched copper tracer was studied by LA-ICP-MS. A fast zinc exchange by copper in bovine serum albumin proteins separated by one dimensional BN-PAGE was found via tracer experiments.11 The stability of Cu-, Zn- and Fe-containing human brain proteins separated by two dimensional gel (2D) electrophoresis of an Alzheimer diseased brain sample was studied using isotopic enriched tracers by LA-ICP-MS and MALDI-FTICR-MS.16 In the case of 2D gels, the LA-ICP-MS measurement was performed as a microlocal analysis of visible protein spots 17–20 or by ablating in the line scan modus passing through the most intense spots in the gel .8,21,22 These approaches are limited to metalloproteins, which are sufficiently abundant to be detected by conventional protein staining methods. Also, the staining procedure bears the risk of removing the metal which should be carefully studied.
The objective of this research was to show, for the first time, the possibility of acquiring elemental images of 2D electrophoretic gels, which give a better possibility of not overlooking metal-complexing proteins and allow the monitoring of the metal abundance regardless of the protein abundance. The modus operandi chosen consists of ablating successive lines of the gels in a way that the total surface of a selected fragment is eventually ablated followed by the construction of an elemental image of this part of the gel by plotting the element (isotope) signal intensity as a function of the x,y (isoelectric point, molecular mass) coordinates of the gel . This method has successfully been used for elemental imaging of thin sections of biological tissue (10–20 μm thickness).23–26
2. Experimental section
2.1 LA-ICP-MS instrumentation
A double-focusing sector field ICP-MS (Element 1, Thermo Fisher Scientific, Bremen, Germany) and a quadrupole ICP-MS (Agilent 7500ce, Agilent Technologies, Tokyo, Japan) coupled to a laser ablation system UP-266 (New Wave Research, Fremont, CA, USA) were used for the analysis of metals in proteins separated by 2D BN-PAGE. LA-ICP-MS was performed with a frequency-quadrupled Nd-YAG laser (wavelength: 266 nm, repetition frequency: 20 Hz, lateral resolution was about 160 μm; laser power density: 1 × 109 W cm−2). The laser ablated material was transported by argon as a carrier gas into the inductively coupled plasma (ICP). The ions formed in the ICP were extracted in the double-focusing sector field mass spectrometer, analyzed and separated according to their mass-to-charge ratio. All LA-ICP-MS measurements using both ICP-MS were performed at the low mass resolution (m/Δm ∼ 300). A defined sample area (in general, several cm2) of a gel was scanned by LA-ICP-MS using the sector field ICP-MS at low mass resolution in Fig. 2 and 3. In order to detect more metals in protein spots the quadrupole ICP-MS Agilent 7500 was employed for imaging LA-ICP-MS illustrated in Fig. 4. Intensities of the analyte ions 64Zn+, 63Cu+, 57Fe+, 208Pb+ and 55Mn+ were measured in the selected gel area in the line scan modus. Sulfur is chosen as the internal standard element; 32S+ was measured in sector field ICP-MS at medium mass resolution but 34S+ was analyzed in quadrupole ICP-MS. Careful attention was paid to possible isobaric interferences of atomic ions of the analyte and polyatomic ions which can be observed in mass spectra. The experimental parameters were optimized to minimize interference problems during the LA-ICP-MS measurements. In general, the validation of the analytical data by isotope ratio measurements of at least two isotopes of the analyte (except the monoisotopic Mn) was performed. The background intensities of metal ions were determined directly in a gel blank by LA-ICP-MS. No signal suppression during the laser ablation in the investigated gel region was observed.2.2 Metal imaging of two-dimensional electrophoresis gels
LA-ICP-MS imaging was performed by moving the gel mounted on the target holder in the laser ablation chamber in the focused laser beam line by line in a way that the selected gel fragment was totally ablated. In order to obtain images with a high spatial resolution the spot size and laser scan speed were optimized in the range between 50 and 160 μm, and 20 μm s−1 and 60 μm s−1, respectively. The measurement time for imaging LA-ICP-MS (up to several hours) depended on the size of the gel section analyzed, the number of lines and the scan speed applied. As the gel matrix was responsible for a decrease in sensitivity in comparison with the tissue imaging, the laser beam diameter was set to 160 μm instead of 50 μm used in the tissue imaging. The distance between the lines was 10 μm to assure the maximum coverage of the gel surface. An instrumental drift was corrected by gas blank intensity of 13C+ measured between ablation of the analyzed sample.A defined sample area (in general, several cm2) of a gel was scanned. Intensities of the analyte ions 64Zn+, 63Cu+, 57Fe+, 208Pb+, 55Mn+ were measured by sector field ICP-MS. The images were plotted using programming script in MATLAB® 6.5 computing software. The developed script utilized a 2D plotting function of the graphical environment of MATLAB to combine the row data intensity from the measured by LA-ICP-MS lines and combine them into a 2D plot so, that the x- and y-axes correspond to the size of the sample; the z-axis represents the measured intensity signal as a function of color. Further details of the evaluation procedure are described elsewhere.23–26 Optimized experimental parameters of the LA-ICP-MS imaging procedure are summarized in Table 1.
| LA-ICP-SFMS | LA-ICP-QMS | |
|---|---|---|
| Inductively coupled plasma mass spectrometer | Element 1 (Thermo Fisher Scientific) | Agilent 7500ce (Agilent Technologies) |
| RF power/W | 1200 | 1500 |
| Cooling gas flow rate/L min−1 | 18 | 18 |
| Auxiliary gas flow rate/L min−1 | 0.65 | 0.83 |
| Carrier gas flow rate/L min−1 | 1.1 | 1.2 |
| Mass resolution, m/Δm | 300 (for S: 4400) | 300 |
| Laser ablation system | NewWave UP-266 | |
|---|---|---|
| Wavelength/nm | 266 | |
| Ablation mode | Scanning – line per line | |
| Ablation scan speed/μm s−1 | 30 | |
| Repetition rate/Hz | 20 | |
| Laser beam diameter/μm | 120 | |
2.3 Sample preparation
Deionized water (18 MΩ cm) obtained from a Millipore Milli-Q-Plus system was used throughout. The rat kidney tissue samples were freeze-dried and homogenized. The water-soluble proteins were extracted by using an ultrasonic probe and separated from the insoluble pellet by ultracentrifugation. For the separation of the proteins using native 2D gel electrophoresis the samples were diluted with sample buffer (2% IPG buffer Servalyt™ 4–7, Coger, Paris, France) and 0.01% Bromophenol Blue (Sigma-Aldrich, Lyon, France) in water.2.4 2D Blue-Native-PAGE
The proteins from the rat kidney tissue sample were separated using native 2D gel electrophoresis. The first dimension, isoelectric focusing (IEF), was carried out using IPG strips of 3–10NL (non linear) and 4–7 (Bio-Rad, Marnes-la-Coquette, France). Denaturating detergents, such as DTT (dithiothreitol) and SDS (sodium dodecyl sulfate), were avoided. The IPG strips were rehydrated overnight by passive rehydration using an Immobiline DryStrip reswelling tray, for 7–18 cm IPG strips (GE Healthcare, Orsay Cedex, France). An IEF chamber Horizont Plus was used together with the power supply EV 215 (Biostep GmbH, Jahnsdorf, Germany). It was cooled to a constant 10 °C during the run. After IEF, equilibration for reduction and alkylation of the proteins was omitted. Instead the IPG strips were colored with 0.01% (w/v) bromphenol blue in water. The second dimension was performed in the cooled Maxi-Protein chamber TV400YK (Biostep GmbH). For this chamber, 10% acrylamide : bis-acrylamide (30% : 0.8% w/v) gels with a size of 20 cm × 20 cm were used. The separation in the second dimension of the native 2D gel electrophoresis was similar to the 1D BN-PAGE described in a previous publication.27 The protein separation by native 2D gel electrophoresis was performed in duplicate (one gel was used for LA-ICP-MS measurements and the other for MALDI-MS studies). Several 2D gels of the samples were run before the LA-ICP-MS and MALDI-MS measurement to assure the reproducibility of this method. Besides some minor spots the major spots were shown in all the gels made. Different staining techniques were tried to get the highest reproducibility possible also for the weak spots. Therefore, and because of the better background for the LA-ICP-MS measurement, the gels were finally stained with silver staining, which was described elsewhere.18For all the LA-ICP-MS measurements, the gels were dried on filter paper (Whatman, VWR, Pessac, France) using a gel dryer (Model 583 Gel Dryer, Bio-Rad).
2.5 MALDI-TOF-MS instrumentation and measurement
MALDI-TOF-MS measurements were performed with a Voyager-DETM STR instrument (Applied Biosystems, Toronto, Canada). A spot was excised and digested with trypsin as described elsewhere.21 The pulsed nitrogen laser was operated at 337 nm. A solution of α-cyano-4-hydroxycinnamic acid (LaserBio Labs, Sophia Antipolis Cedex, France) in acetonitrile: 0.1% TFA in water (2 : 1) was used as the matrix. 0.7 μL of the matrix solution and 0.7 μL of the sample solution were mixed on the stainless steel MALDI sample target and allowed to dry. Calibration was performed with a standard peptide mixture with the m/z range of approximately 3000 (BioRad, Marnes-la-Coquette, France). This matrix offered better results than 2,5-dihydroxybenzoic acid (DHB, Sigma-Aldrich Chimie, Lyon, France) used under the same conditions. Proteins were identified by peptide fingerprinting using publicly available databases: www.matrixscience.com and http://prowl.rockefeller.edu.3. Results and discussion
3.1 Elemental imaging of 2D BN-PAGE gel using LA-ICP-MS
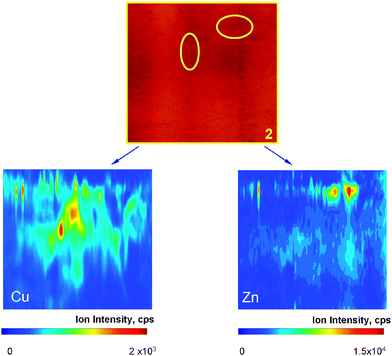
A 2D BN-PAGE gel obtained after separation of rat kidney proteins in a water extract was selected as an example sample for the purpose of this study. Experimental parameters for metals detection and their local distribution analysis in the selected regions of the gel were optimized with respect to maximum 63Cu+ ion intensity.Several pieces of the gel (shown in Fig. 1) were investigated. The metal distribution of the sections 1, 2 and 3 are shown in Fig. 2, 3 and 4, respectively. The concentrations of metals in the other sections analyzed (4–7) were too low to produce exploitable metal images. Nevertheless, when the most intense spots within sections 4 and 7 were analyzed by laser ablation ICP-MS, measurable signals were obtained for Zn, Cu and Pb. This suggests that the imaging mode is slightly (a factor of 2–3) less sensitive that the directed spot-to-spot hopping mode.
Fig. 2 shows two well defined spots strongly enriched in Cu and Zn. The correlation between the intensities of these signals is a measure of the metal–protein complex stoichiometry. Note that a number of other more intense spots are visible in this section but no metals are associated with the proteins observed. Fig. 3 shows a number of spots enriched in either Cu or Zn, but contrary to the section in Fig. 2 only one of these spots contains both elements in stoichiometric amounts. The Cu and Zn concentration images correspond to the protein image in Fig. 2. But in Fig. 3 metals could be detected in places where there are no spots visible in the silver stained gel .
 | ||
| Fig. 2 Cu- and Zn-containing protein spots in section 1 of 2D-BN gel of rat kidney water extract (cf. Fig. 1). | ||
 | ||
| Fig. 3 Cu- and Zn-containing protein spots in section 2 of 2D-BN gel of rat kidney water extract (cf. Fig. 1). | ||
The image of Zn and Cu shown in Fig. 4 does not totally follow the protein spot of serum albumin from rat kidney in the gel . But correlation with the protein spots in some parts of this gel cut can be observed. We found the presence of an intense Cu and Zn-containing spot which is not visible in the silver stained gel (see Fig. 2). This indicates a higher sensitivity of the metalloprotein detection via the complexed metal ions in comparison to the protein staining.
 | ||
| Fig. 4 Images of Zn, Cu, Fe, Pb, Mn and Ag in section 3 of 2D-BN gel of rat kidney water extract (cf.Fig. 1). | ||
3.2 Identification of proteins by MALDI-TOF-MS
Identification of some intense protein spots in gel sections investigated by gel imaging was attempted by MALDI-TOF-MS following spot excision and tryptic in-gel digestion. The results of mass spectrometric measurements with respect to identified proteins from 2D BN-PAGE gel of rat kidney are summarized in Table 2. The staining marker, bovine serum albumin (68.7 kDa), is clearly present in the most intense band, including spots e, f and g by peptide matching as shown in Fig. 5. No metal-containing peptides could be found due to low mass resolution of the mass spectrometer applied. It could also be possible that the attached metals were lost during the tryptic digest. The proteins in the spots shown in Fig. 2 containing Cu and Zn could not be identified. Either the proteins in the spots are not included in the database used for identification. Or the peptides contained post-translational modifications which makes identification more difficult. | ||
| Fig. 5 MALDI-TOF mass spectrum of serum albumin cut out from section 3 of the gel with the identified peptides . | ||
| Protein spot | Identified protein | MW/kDa |
|---|---|---|
| c | Superoxide dismutase [Cu–-Zn] | 15.9 |
| d | Aldose 1-epimerase | 37.9 |
| e–g | Serum albumin | 68.7 |
| n, o | Hydroxy acid oxidase 2 | 39.1 |
Future studies will focus on identification of metal-containing protein spots present in sections 1 and 2 by high resolution FTICR-MS (Fourier Transform Ion Cyclotron Resonance Mass Spectrometer) using MALDI or ESI ionization. Also fragmentation methods will be used to identify other post-translational modifications besides metals bound to the proteins (e.g.phosphorylationetc.). These methods will also be used to identify the metal binding sites in the metal-containing peptides .
4. Conclusions
This paper is the first to present laser ablationICP MS for metal imaging in two dimensional electrophoresis gels. The technique developed for bioimaging of metals in thin tissue sections of brain samples applied to 2D gels allows the detection of metalloproteins in gels, and thus optimization of non-denaturating separation of metalloproteins, which is the sine qua non condition for in vivo metalloproteomics. A critical step is the identification of the detected metalloproteins which would give information on the number of S atoms and thus allow stoichiometric studies. The identification is likely to be achieved by FTICR-MS,27 which together with elemental imaging is likely to become an indispensable analytical tool for heteroatom-tagged proteomics.Acknowledgements
The financial support of the French National Scientific Research Agency (ANR) and of the Aquitaine Region is acknowledged.References
- D. E. Garfin, TrAC, Trends Anal. Chem., 2003, 22, 263 CrossRef CAS.
- A. Shevchenko, M. Wilm, O. Vorm and M. Mann, Anal. Chem., 1996, 68, 850 CrossRef CAS.
- S. S. Hasnain, J. Synchrotron Radiat., 2004, 11, 7 CAS.
- J. Szpunar, Analyst, 2005, 130, 442 RSC.
- W. Shi and M. R. Chance, Cell. Mol. Life Sci., 2008 Search PubMed.
- I. Wittig, H.-P. Braun and H. Schägger, Nat. Protocols, 2006, 1, 418 CAS.
- J. Szpunar, Anal. Bioanal. Chem., 2004, 378, 54 CAS.
- J. L. Neilsen, A. Abildtrup, J. Christensen, P. Watson, A. Cox and C. W. McLeod, Spectrochim. Acta, Part B, 1998, 53, 339 CrossRef.
- R. Ma, C. W. McLeod, K. Tomlinson and R. K. Poole, Electrophoresis, 2004, 25, 2469 CrossRef CAS.
- G. Ballihaut, C. Pecheyran, S. Mounicou, H. Preud’homme, R. Grimaud and R. Lobinski, TrAC, Trends Anal. Chem., 2007, 26, 183 CrossRef CAS.
- I. Feldmann, C. U. Koehler, P. H. Roos and N. Jakubowski, J. Anal. At. Spectrom., 2006, 21, 1006 RSC.
- A. Venkatachalam, C. U. Koehler, I. Feldmann, P. Lampen, A. Manz, P. H. Roos and N. Jakubowski, J. Anal. At. Spectrom., 2007, 22, 1023 RSC.
- A. Polatajko, M. Azzolini, I. Feldmann, T. Stuezel and N. Jakubowski, J. Anal. At. Spectrom., 2007, 22, 878 RSC.
- N. Jakubowski, L. Waentig, H. Hayen, A. Venkatachalam, A. von Bohlen, P. H. Roos and A. Manz, J. Anal. At. Spectrom., 2008, 23, 1497 RSC.
- P. H. Roos, A. Venkatachalam, A. Manz, L. Waentig, C. U. Koehler and N. Jakubowski, Anal. Bioanal. Chem., 2008, 392, 1135 CrossRef CAS.
- J. Su. Becker, D. Pozebon, V. L. Dressler, R. Lobinski and J. S. Becker, J. Anal. At. Spectrom., 2008, 23, 1076 RSC.
- J. Su. Becker, M. Zoriy, C. Pickhardt, M. Przybylski and J. S. Becker, Int. J. Mass Spectrom., 2005, 242, 135 Search PubMed.
- J. S. Becker, M. Zoriy, U. Krause-Buchholz, J. Su. Becker, C. Pickhardt, M. Przybylski, W. Pompe and G. Rödel, J. Anal. At. Spectrom., 2004, 19, 1236 RSC.
- J. S. Becker, M. Zoriy, J. Su. Becker, C. Pickhardt and M. Przybylski, J. Anal. At. Spectrom., 2004, 19, 149 RSC.
- C. C. Chery, D. Gunther, R. Cornelis, F. Vanhaecke and L. Moens, Electrophoresis, 2003, 24, 3305 CrossRef.
- L. Tastet, D. Schaumlöffel and R. Lobinski, J. Anal. At. Spectrom., 2008, 23, 309 RSC.
- J. S. Becker, M. V. Zoriy, C. Pickhardt, N. Palomero-Gallagher and K. Zilles, Anal. Chem., 2005, 77, 3208 CrossRef CAS.
- J. S. Becker, M. Zoriy, J. Su. Becker, J. Dobrowolska and A. Matusch, J. Anal. At. Spectrom., 2007, 22, 736 RSC.
- J. S. Becker, A. Matusch, C. Depboylu, J. Dobrowolska and M. V. Zoriy, Anal. Chem., 2007, 79, 6074 CrossRef CAS.
- J. Dobrowolska, M. Dehnhardt, A. Matusch, M. Zoriy, N. Palomero-Gallagher, P. Koscielniak, K. Zilles and J. S. Becker, Talanta, 2008, 74, 717 CrossRef CAS.
- J. Su. Becker, S. Mounicou, M. V. Zoriy and R. Lobinski, Talanta, 2008, 76, 1183 CrossRef CAS.
- J. S. Becker, M. Zoriy, C. Pickhardt, E. Damoc, G. Juhacz, M. Palkovits and M. Przybylski, Anal. Chem., 2005, 77, 5851 CrossRef CAS.
Footnote |
| † Current address: Aeropharm GmbH, D-07407 Rudolstadt, Germany. |
| This journal is © The Royal Society of Chemistry 2009 |

