Microfluidic device using chemiluminescence and a DNA-arrayed thin film transistor photosensor for single nucleotide polymorphism genotyping of PCR amplicons from whole blood†
Keiichi
Hatakeyama
a,
Tsuyoshi
Tanaka
a,
Masahiro
Sawaguchi
b,
Akihito
Iwadate
b,
Yasushi
Mizutani
b,
Kazuhiro
Sasaki
b,
Naofumi
Tateishi
b and
Tadashi
Matsunaga
*a
aDepartment of Biotechnology, Tokyo University of Agriculture and Technology, 2-24-16, Naka-cho, Koganei, 184-8588, Tokyo, Japan. E-mail: tmatsuna@cc.tuat.ac.jp; Fax: +81 42-385-7713; Tel: +81 42-388-7020
bCasio Computer Co. Ltd., 6-2, Honmachi 1-chome, Shibuya-ku, Tokyo 151-8543, Japan
First published on 21st January 2009
Abstract
This work describes a novel microfluidic device using a thin film transistor (TFT) photosensor integrating a microfluidic channel, a DNA chip platform, and a photodetector for the discrimination of single nucleotide polymorphisms (SNPs). A DNA-arrayed TFT photosensor was used as a DNA chip platform and photo detecting device. Chemiluminescence was used for DNA sensing because chemiluminescence provides higher sensitivity and requires simpler instrumentation than fluorescence methods. The SNP of biotinylated target DNA was detected based on chemiluminescence by using horse radish peroxidase-conjugated streptavidin. The lower detection limit for a model biotinylated oligonucleotide (63-mer) was 0.5 nM, much lower than expected DNA concentrations in a practical application of this device. Furthermore, SNP detection in the aldehyde dehydrogenase 2 gene was successfully achieved using DNA-arrayed TFT photosensor without DNA extraction and DNA purification using PCR products. The assay was completed in less than one hour. Our technology will be a promising approach to developing a miniaturized, disposable DNA chip with high sensitivity.
Introduction
The use of integrated microfluidic devices for biosensing has been a topic of great interest over the last decade.1–3 Recently, miniaturized DNA chip devices that use electrochemical and optical detection have been intensively investigated as components of next-generation DNA sensors. Miniaturized chip devices have the potential to be portable, compact, and inexpensive DNA sensors.4–7Electrochemical detection has been preferred for disposable DNA chip technologies since it is easily miniaturized. More recently, miniaturized photodetectors, such as the phototransistor,8 micro-avalanche photodiode (µAPD)9 and PIN photodiode10,11 have been used in disposable photodetecting devices because their quantitative assay performance is superior to that of electrochemical detection. However, it is difficult to miniaturize the size of optical detection systems, compared with electrochemical detection, because hybridization on a DNA microarray is typically detected by fluorescence, which requires an external light source.Alternatively, chemiluminescence detection systems have gained much attention as miniaturized biosensing platforms,12,13 because chemiluminescence provides higher sensitivity and requires less instrumentation than fluorescence methods. The background signal in chemiluminescence detection is extremely low, but the generated signal is also very low, compared with fluorescence signals. Similarly, highly sensitive photodetectors, such as cooled CCD and photo multiplier tube, have been employed for signal imaging. Recently, a DNA-arrayed complementary metal oxide semiconductor (CMOS) has been used for the 2D measurement of chemiluminescence spots in DNA microarrays.14,15DNA probes have been directly immobilized onto the surface of a CMOS device to improve photon collection efficiency. Photon collection efficiency has been greatly improved by decreasing the separation distance between the detection element and luminescent source.16 Therefore, use of chemiluminescence in DNA-arrayed sensors appears to be a promising approach for the development of a miniaturized, disposable DNA chip device with high sensitivity.
We have developed a novel DNA chip analyzer based on fluorescent detection using a thin film transistor (TFT) photosensor consisting of a 200 × 240 pixel array.17 The surface was chemically modified with single-stranded DNA. This enables the detection of fluorescently-labeled target DNA based on DNA–DNA hybridization. The TFT photosensor provides disposable photodetection, and is thermostabile under the high temperatures required for the DNA hybridization and DNA denaturation. Furthermore, the TFT photosensor can detect light over a broad wavelength range with peak sensitivity at 450 nm. Therefore the TFT photosensor can be used for chemiluminescence-based DNA detection.
In the present study, we describe a chemiluminescence-based DNA chip platform using a DNA-arrayed TFT photosensor. Biotinylated target DNA was detected using horse radish peroxidase-conjugated streptavidin (HRP-SA). An integrated microchannel was fabricated on a DNA-arrayed TFT photosensor to enhance the hybridization efficiency of target DNA. The practical performance of the integrated system for single nucleotide polymorphism (SNP) detection in the aldehyde dehydrogenase 2 (ALDH2) gene was demonstrated. Assay time was reduced by shortening the PCR and hybridization times. The system performed direct PCR from whole blood using Ampdirect Plus, greatly simplifying the sample preparation. Moreover, unpurified PCR products were directly applied to the DNA chip, minimizing the sample preparation process. This system allows hybridization discrimination of SNPs in non-purified PCR amplicons from whole blood.
Experimental
Materials
Gamma-aminopropyltriethoxysilane (APTES) was purchased from Sigma-Aldrich (St Louis, MO). Sulfo-NHS acetate, HRP-conjugated streptavidin (HRP-SA) and SuperSignal ELISA Femto Maximum Sensitivity Substrate were obtained from Pierce Biotechnology (Rockford, IL). N-(γ-maleimidobutyloxy) succinimide ester (GMBS) was obtained from Dojindo Labratories (Kumamoto, Japan). All oligonucleotides were synthesized by Operon Biotechnologies (Huntsville, AL). Polydimethylsiloxane (PDMS) was obtained from Dow-corning (Midland, MI). The hybridization chamber (10 mm × 10 mm × 250 µm) was obtained from Takara Bio (Shiga, Japan). The PCR kit, Ampdirect Plus was purchased from Simadzu (Kyoto, Japan). Other chemicals and organic solvents were purchased from Wako Pure Chemical Industries (Osaka, Japan).Detection of HRP-SA on the TFT photosensor
An internal amplification photosensor using a double-gate structure TFT was used in this study. The TFT photosensor (Fig. 1A) consisted of a 200 × 240 pixel array (sensor area: 10 mm × 12 mm) with a 50 µm pitch17 (Fig. 1B). Its top-gate acts as a photosensing gate, while the bottom-gate acts as a pixel select gate. The photosensing mechanism is the photoinduced gate modulation. Noise cancelling in a-Si was carried out before each sensing process. The TFT photosensor detects light over a broad wavelength range, with peak sensitivity at 450 nm. To estimate the detection limit for HRP-SA as a luminescent marker, known concentrations of HRP-SA solutions (0 to 4.8 nM; spotting volume: 20 nl) were spotted onto the TFT photosensor using an inkjet dispenser (MicroSys 4100; Cartesian technologies, CA). HRP-SA was suspended in phosphate buffered saline (PBS, pH7.4) containing 20% PEG300 prior to spotting. After spotting and drying, a luminescence substrate (SuperSignal ELISA Femto Maximum Sensitivity Substrate) (25 µl) was gently dropped on the HRP-SA-spotted TFT photosensor surface. The luminescence signals were detected by the TFT photosensor. | ||
| Fig. 1 Photograph (A) and microscopic image (B) of TFT photosensor (scale bar = 20 μm). (C) Microfluidic channel on the TFT photosensor. ALDH2*1 and ALDH2*2 spots were alternately immobilized on the surface of the TFT photosensor. TAMRA-labeled oligonucleotide (ALDH2*1) was arrayed as a check spot for confirmation of immobilization. All spots were designed within microchannels. (D) Illustration showing fabrication of microfluidic TFT photosensor. (E) Photograph of microfluidic TFT photosensor on the peltier device. | ||
To evaluate the photon collection efficiency of the TFT photosensor, luminescent intensity was measured by placing a photon source (HRP-SA) various distances away from the sensor surface (0, 250, 500 and 750 µm from the surface). A hybridization chamber with a height of 250 µm to 750 µm was attached to the TFT photosensor. The hybridization chamber was covered with an HRP-SA-spotted glass slide, turning the spotted surface downwards. Then, the luminescent substrate for HRP was introduced into the hybridization chamber. To measure the luminescent intensity for the distance between the photon source and sensor surface equaling zero, HRP-SA solution was directly spotted onto the TFT photosensor.
Preparation of the DNA-arrayed TFT photosensor
Thiolated oligonucleotides (probe oligonucleotides) were immobilized on the photosensor surface using APTES and GMBS as cross-linking reagents. The sequences of the immobilized oligonucleotides are shown in Table 1. The oligonucleotides (ALDH2*1 and ALDH2*2) were alternately immobilized for SNP genotyping in the ALDH2gene. The surface density of oligonucleotide was controlled by spotting concentration of oligonucleotides using an inkjet dispenser (spotting volume: 5.2 nL). All spots were designed within microchannels. The TFT photosensor was exposed to oxygen plasma at an air flow rate of 40 ml/min for 40 sec. Then, the plasma-treated photosensor was immersed in 1% APTES in toluene for 30 min to introduce amino groups to the surface. After rinsing with toluene, the TFT photosensor was backed at 110 °C for 10 min.18 The aminated photosensor was immersed in 1 mM GMBS (in ethanol) for 60 min at room temperature to introduce maleimide groups. After rinsing with ethanol, thiolated oligonucleotide in 100% DMSO solution was spotted onto the photosensor surface using an inkjet dispenser. The TFT photosensor was incubated for 60 min in a humidified chamber (65% humidity) at room temperature to complete the cross-linking reaction between the thiol group of the oligonucleotide and the maleimide group on the photosensor surface.| Name | Sequences (5′ → 3′) | Length (mer) |
|---|---|---|
| a Bold: base mismatch; italic: complementary sequence to immobilized oligonucleotide. | ||
| Immobilized oligonucleotide | ||
| ALDH2*1 | SH-TTCACTTCAGTGTAT | 15 |
| ALDH2*2 | SH-TTTCACTTTAGTGTAT | 16 |
| Target oligonucleotide | ||
| ALDH2*1 | Biotin-GGAGTTGGGCGAGTACGGGCTGCAGGCATACACTGAAGTGAAAACTGTGAGTGTGGGACCTGC | 63 |
| ALDH2*2 | Biotin-GGAGTTGGGCGAGTACGGGCTGCAGGCATACACTAAAGTGAAAACTGTGAGTGTGGGACCTGC | 63 |
| PCR primers | ||
| F-primer | Biotin-GGAGTTGGGCGAGTACGG | 18 |
| R-primer | GCAGGTCCCACACTCACAG | 19 |
Following the incubation, the DNA-arrayed TFT photosensor was washed with 0.1% SDS and super pure water for 5 min. The DNA-arrayed TFT photosensor was then immersed in 1 mM Sulfo-NHS acetate in NaHCO3 (pH 8.5) for 60 min at room temperature, which inactivated unreacted amino groups on the surface. To estimate the number of oligonucleotides immobilized onto the photosensor, TAMRA-labeled oligonucleotides with or without a thiol group on the 5′-end were spotted onto the GMBS-modified substrate.17
Microfluidic device fabrication on the DNA-arrayed TFT photosensor
Polymethylmethacrylate (PMMA) substrate with a positive surface relief was used as a mold for the production of PDMS channel replicas. The PMMA master mold was created using the CAD/CAM system (Roland DG, Japan). PDMS was introduced onto the PMMA mold using a syringe. After degassing with a vacuum pump, the PDMS was heated at 85 °C for 30 min. The designed channel was 250 µm in depth and 600 µm in width (Fig. 1C). The PDMS channel replica was then fabricated onto the DNA-arrayed TFT photosensor. The microfluidic PDMS channel was clamped with a PMMA clamp (Fig. 1D) and connected to a syringe pump (KD Scientific, MA). The temperature of the PDMS channel and TFT photosensor was controlled with a peltier device (Cell systems, Japan) (Fig. 1E).SNP detection in the ALDH2gene
Biotin-labeled single stranded DNA (ssDNA) (63-mer) were used as model targets (Table 1), because the expected size of the PCR products was 63 bp.19 The target ssDNA was diluted in hybridization buffer (4 × SSC and 0.1% SDS) to a final concentration between 1 nM and 100 nM. The target ssDNA solution (25 µl) was introduced into the microfluidic PDMS channel and was shuttled using a syringe pump to enhance the hybridization efficiency. The volume of shuttling solution was set at 25 µl. The temperature of the microfluidic PDMS channel was kept at 30 °C. Following hybridization, the target ssDNA solution was pushed out from the channel. Then, the PDMS channel was washed with 50 µl of 2 × SSC containing 0.1% SDS, 0.1 × SSC and 0.1% PBS containing 0.1% Tween 20 (PBST). The microfluidic PDMS channel was then incubated with 25 µl of HRP-SA (6 nM) for 10 min, followed by washing with 0.1% PBST. After washing, the luminescence substrate for HRP was introduced into the microfluidic PDMS channel. The luminescence signal was detected by the TFT photosensor. SNP genotyping in the ALDH2gene using PCR products (63 bp) was examined using the same procedures described above.Direct PCR from whole blood, without DNA purification, was performed using a PCR kit (Ampdirect Plus). The fragment in the ALDH2gene was amplified using previously reported primers (Table 1)19 with the following protocol: preheating at 95 °C for 5 min, 35 cycles at 95 °C for 0 sec, 58 °C for 3 sec, and 72 °C for 3 sec, with a final extension at 72 °C for 10 sec (processing time; 25 min). The reaction mixture contained 0.5 µl whole blood, PCR kit solution (containing MgCl2 and dNTPs), 1 µM primers and 0.5 U Nova Taq Hot DNA polymerase, in a final volume of 20 µl. The PCR products (5 µl) were mixed with 20 µl hybridization buffer (4 × SSC and 0.1%SDS), and heated to 95 °C for 5 min. The mixture was then immediately incubated on ice for 5 min. The mixture was applied to a microfluidic PDMS channel on the DNA-arrayed TFT photosensor. ALDH2 genotypes of sample DNA were confirmed by PCR-restriction fragment length polymorphism (RFLP) analysis.20
Signal processing
The luminescence signal was digitized and visualized for 2D imaging (8 bit, BMP format). The image was captured at an exposure time of 1.2 to 30 sec. One spot corresponded to approximately 100 units on the TFT elemental device. Photons generated by the enzymatic reaction were detected with a proximal TFT elemental device (equivalent to one pixel) and converted into electrons. The data was visualized to grey scale or false-color images. Background images were also measured just before the introduction of the luminescence substrate onto the DNA-arrayed TFT photosensor. Background images were subtracted from raw images to remove background signals at each pixel.Results and discussion
Characteristics of the TFT photosensor for luminescence detection
To estimate the detection limits of HRP-SA, the luminescence intensity of HRP-SA solutions were measured using a TFT photosensor. The luminescence intensity increased as the amount of HRP-SA and exposure time of the photosensor increased, reaching saturation at more than 150 molecules/µm2 of HRP-SA and 4.8 sec of exposure time (Fig. 2A). Because a higher linearity was obtained in the range of 0 to 59.0 molecules/µm2 of HRP-SA at 4.8 sec of exposure time, the limit of detection (LOD) was estimated at these conditions (Fig. 2B). The LOD of the photosensor was 2.5 molecules/µm2 (10.2 zmol per a detection element). The LOD was defined as a value three times higher than the standard deviation of the blank (3σb). In our previous work, the lowest detection limits of the TFT photosensor for the fluorescent signal of quantum dots (Qdot 565; Invitrogen) were 3.0 × 102 molecules/µm2.17 Thus, the LOD of chemiluminescent methods was 100 times lower than that of fluorescent methods. Furthermore, the performance of the TFT photosensor was similar to commercially available CMOS sensors; the LOD of CMOS for HRP was estimated as less than 1 molecule/µm2.14 | ||
| Fig. 2 (A) Luminescence intensity of spotted HRP-SA on the TFT photosensor at various exposure times. (B) Calibration curve for HRP-SA at 4.8 sec of exposure time. Standard deviations were calculated from the luminescence intensity of triplicates. σb was defined as standard deviation of the blank. | ||
Photon collection efficiency is greatly affected by the separation distance between the detection element and the photon source.16 The TFT photosensor does not involve optical lenses, unlike CCD or CMOS devices. Therefore, investigation of relationship between separation distance and luminescent intensity is important to estimate the effective area to collect photons. Luminescent intensity was measured by placing a photon source (HRP-SA) various distances away from the sensor surface (Fig. 3). The luminescent intensity decreased exponentially as the separation distance increased. Furthermore, the luminescent area broadened out with increasing distance. These results indicate that the most effective distance to collect luminescent signals and minimize cross-talk signals was at least less than 250 µm. Based on the above results, the depth of the micro channel on the TFT photosensor was set at 250 µm. If the luminescence yield in a 250 µm high micro-channel filled with luminescent solution is 100%, the detectable luminescent yield by TFT photosensor is expected to be approximately 71% (Fig. 3). Assuming that contact imaging has 50% collection efficiency,21 the net collection efficiency of TFT photosensor in this study was estimated to be 36% of the total photons emitted.
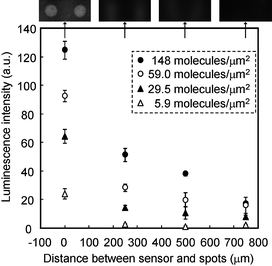 | ||
| Fig. 3 Relationship between luminescent intensity and the separation distance between the HRP-SA spot and the sensor surface. Images at 148 molecules/µm2 of HRP-SA were visualized using the photosensor at each distance. Standard deviations were calculated from the luminescence intensity of triplicates. Exposure time was set at 4.8 sec. | ||
SNP detection in the ALDH2gene using model oligonucleotides by DNA-arrayed TFT photosensor
Prior to the estimation of LOD for target DNA (63-mer), the optimum flow rate for hybridization was investigated. The hybridization time was set at 60 min. The hybridization efficiency at 120 µl/min of shuttled flow was 1.6 times higher than at the static condition (Fig. S1, ESI†). Therefore, the flow rate was fixed at 120 µl/min for subsequent experiments. Target oligonucleotides at various concentrations were detected by the DNA-arrayed TFT photosensor to estimate the LOD for biotinylated target DNA (Fig. 4). The ALDH2*1 target oligonucleotide was used as a model target. The luminescence signal markedly increased until the amount of target DNA on the ALDH2*1 detection spot reached 100 nM, while lower signals were obtained on the ALDH2*2 detection spot (Fig. 4A). The LOD of the microfluidic TFT photosensor was estimated to be 0.5 nM (Fig. 4B). The amount of hybridized target DNA (i.e. HRP-SA amount) at 0.5 nM was calculated from the luminescent intensity using the calibration curve in Fig. 2. The amount of target DNA was estimated to be 6.3 molecules/µm2, which was similar to the LOD value (2.5 molecules/µm2) of HRP-SA estimated in Fig. 2. | ||
| Fig. 4 (A) Hybridization signal of 63-mer ALDH2*1/*1 target at various target concentrations in the microfluidic TFT photosensor. (B) Evaluation of the detection limit of the ALDH2*1/*1 target. Standard deviations were calculated from the luminescence intensity of triplicates. | ||
Using fluorescence-based detection, 1 µM of biotinylated DNA (21- or 22-mer target of ALDH2gene) was detectable on the DNA-arrayed TFT photosensor.17 Therefore, the chemiluminescence-based method shows more than 1000 times higher sensitivity than the fluorescent-based method. Because the LOD of the chemiluminescent maker (2.5 molecules/µm2 of HRP-SA) was 100 times lower than of the LOD of the fluorescent marker (3.0 × 102 molecules/µm2 of quantum dot) as mentioned above, the chemiluminescence-based method showed 10 times higher sensitivity than expected. This unexpected result could be due to a higher background in fluorescent method caused by non-specific light scattering of the excitation light source. Moreover, the obtainable concentration of PCR products is expected to be submicromolar level, since primer concentration is typically set between 400 and 1000 nM. Therefore, the LOD (0.5 nM) was expected to be much lower than any DNA concentration in a practical application of this device. These results indicate that the microfluidic TFT photosensor is applicable to SNP genotyping using PCR amplicon.
Based on these results, SNP genotyping was performed by a DNA-arrayed TFT photosensor using an oligonucleotide at 5 nM target DNA. The genotypes of ALDH2gene were categorized as homozygous (ALDH2*1/*1), heterozygous (ALDH2*1/*2) or mutant (ALDH2*2/*2).20Fig. 5 shows the result of identification of three genotypes in the ALDH2 gene using model oligonucleotides by the DNA-arrayed TFT photosensor. Specific spot patterns of luminescence were observed based on the three genotypes, ALDH2*1/*1, ALDH2*1/*2 and ALDH2*2/*2, respectively (Fig. 5A). Spot positions of these TFT images corresponded to spots shown in Fig. 1C. The mean values of luminescence intensities from each photosensor are presented in Fig. 5B when immobilized DNA was 1.69 × 105 molecules/µm2. In the homozygote target DNA (ALDH2*1/*1 and ALDH2*2/*2), 1-base mismatched DNA provided less than 90% decrease in the luminescence intensity compared to complete matched DNA. In the heterozygote target DNA (ALDH2*1/*2), luminescent signals were observed on both ALDH2*1 and ALDH2*2 detection oligonucleotide spots. Therefore, discrimination of all genotyping in ALDH2 was successfully achieved using our microfluidic TFT photosensor based on chemiluminescence detection. Lower luminescent intensity in ALDH2*2*2 target DNA versus ALDH2*1*1 target DNA could be explained by differences in Tm values in both immobilized oligonucleotides, because the Tm value of ALDH2*2 was lower than the Tm value of ALDH2*1.
 | ||
| Fig. 5 Genotyping analysis with microfluidic TFT photosensor. (A) Luminescence images of the microfluidic TFT photosensor hybridized with ALDH2allele-specific targets at 5 nM target concentration. Three possible diploid genotypes of ALDH2alleles are shown. The left, center and right bars are described for a homozygous wild type, heterozygote and a homozygous mutant, respectively. The array pattern was the same position as described in Fig. 1C. (B) Luminescence intensities at 1.69 × 105 molecules/μm2 of immobilized DNA in (A) are represented. | ||
Hybridization time was investigated using 100 nM target oligonucleotides. A remarkable increase in luminescence signal was observed until 60 min (Fig. S2, ESI†). The signal was nearly saturated at 60 min. Alternatively, the specific signal was confirmed after only 1 min of hybridization time. These results suggest that the hybridization time could be reduced even if PCR products were employed.
SNP genotyping of unpurified PCR amplicons from whole blood
SNP detection was examined using PCR amplicons from whole blood. The genotype of homogeneous ALDH2*1/*1, which was confirmed by PCR-RFLP, was used as a blood sample. Direct PCR was used to minimize sample preparation. The PCR products were applied to the microfluidic device without being purified. Fig. 6 shows the TFT photosensor image of the DNA array after PCR amplicon was exposed to various amounts of immobilized oligonucleotides, after which HRP-SA was reacted with the biotin-labeled DNA on the surface of the TFT photosensor. The luminescent image was converted into a false color image for visualization. Luminescent spots were observed only on the ALDH2*1 oligonucleotide-arrayed photosensor. Additionally, part of the image was converted to a spectrum (Fig. 6C). The specific signal increased with increasing amounts of immobilized DNA. These results indicate that SNP detection by our DNA-arrayed TFT photosensor was successfully achieved using unpurified PCR products. | ||
| Fig. 6 SNP discrimination by microfluidic TFT photosensor hybridized with unpurified PCR amplicon from whole blood. (A) Pattern of false color and positions of ALDH2*1 and ALDH2*2 detection spot. (B) Luminescence image of TFT photosensor were reacted with the target at various hybridization time. (C) The luminescence intensities at the dotted line (B) are represented. | ||
To investigate the feasibility of a reduction in hybridization time, luminescent spots were visualized at various hybridization times. The luminescent signals were observed after more than 5 min of hybridization time (Fig. 6B). The SNP discrimination from whole blood was completed in less than an hour (see whole flow in Table S1, ESI†).
The amount of hybridized DNA calculated from luminescence intensities was 15.9 molecules/µm2, which corresponds to 2.3 nM of target DNA concentration. On the other hand, PCR amplicon concentration determined by densitometry after agarose-gel electrophoresis was approximately 100 nM. One possible reason for this lower DNA hybridization could be a partial inhibition of DNA hybridization by unpurified PCR products. To specify the effect of unpurified PCR products on hybridization, hybridization efficiency was investigated using purified single- or double-stranded DNA and unpurified double-stranded DNA (PCR product) (Fig. S3, ESI†). Purified single- or double-stranded DNA was dissolved in PCR reagents. No significant difference in hybridization efficiencies was observed between purified double-stranded DNA (dsDNA) and single-stranded DNA (dsDNA), although the nonspecific adsorption on the ALDH2*2 spot was slightly increased when dsDNA was used. This result indicates that DNA was efficiently hybridized on immobilized DNA regardless of whether an ssDNA preparation process was performed. However, the specific signal of unpurified dsDNA (PCR product) was remarkably lower than that of other samples. This could be due to the inhibition of hybridization by impurities in whole blood. These results suggest that the use of unpurified PCR products reduces assay time but sacrifices hybridization efficiency. Although our proposed system showed enough high sensitivity to detect unpurified PCR products, direct PCR conditions (including blood dilution or lysis) should be further investigated in future work.
Conclusions
We describe a disposable DNA chip device using a microfluidic DNA-arrayed TFT photosensor. DNA hybridization was detected by luminescent signals of HRP. The detection limit for target DNA was 0.5 nM, which is much lower than the expected DNA concentrations in a practical application of the device. SNP detection was achieved in less than 1 h using the TFT photosensor, without DNA extraction, purification, and/or asymmetric PCR. This photosensor would be a valuable component of microarray-type gene, protein, peptide, and cell array biochips in the future. The photosensor may also be useful in investigating real-time imaging of interactions between biomolecules on a solid surface.Acknowledgements
This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for the Japan Society for the Promotion of Science (JSPS) Fellows, 19.06807, 2007. We are grateful to Tetsushi Mori for assistance in preparing the English manuscript.References
- P. S. Dittrich, K. Tachikawa and A. Manz, Anal Chem, 2006, 78, 3887–3908 CrossRef CAS.
- J. Melin and S. R. Quake, Annu Rev Biophys Biomol Struct, 2007, 36, 213–231 CrossRef CAS.
- J. West, M. Becker, S. Tombrink and A. Manz, Anal Chem, 2008, 80, 4403–4419 CrossRef CAS.
- R. H. Liu, J. Yang, R. Lenigk, J. Bonanno and P. Grodzinski, Anal Chem, 2004, 76, 1824–1831 CrossRef CAS.
- B. Elsholz, R. Worl, L. Blohm, J. Albers, H. Feucht, T. Grunwald, B. Jurgen, T. Schweder and R. Hintsche, Anal Chem, 2006, 78, 4794–4802 CrossRef CAS.
- R. H. Liu, T. Nguyen, K. Schwarzkopf, H. S. Fuji, A. Petrova, T. Siuda, K. Peyvan, M. Bizak, D. Danley and A. McShea, Anal Chem, 2006, 78, 1980–1986 CrossRef CAS.
- S. W. Yeung, T. M. Lee, H. Cai and I. M. Hsing, Nucleic Acids Res, 2006, 34, e118 CrossRef.
- T. Vo-Dinh, J. P. Alarie, N. Isola, D. Landis, A. L. Wintenberg and M. N. Ericson, Anal Chem, 1999, 71, 358–363 CrossRef CAS.
- M. L. Chabinyc, D. T. Chiu, J. C. McDonald, A. D. Stroock, J. F. Christian, A. M. Karger and G. M. Whitesides, Anal Chem, 2001, 73, 4491–4498 CrossRef CAS.
- T. Kamei, B. M. Paegel, J. R. Scherer, A. M. Skelley, R. A. Street and R. A. Mathies, Anal Chem, 2003, 75, 5300–5305 CrossRef CAS.
- T. Kamei, N. M. Toriello, E. T. Lagally, R. G. Blazej, J. R. Scherer, R. A. Street and R. A. Mathies, Biomed Microdevices, 2005, 7, 147–152 CrossRef CAS.
- B. J. Cheek, A. B. Steel, M. P. Torres, Y. Y. Yu and H. Yang, Anal Chem, 2001, 73, 5777–5783 CrossRef CAS.
- K. A. Heyries, M. G. Loughran, D. Hoffmann, A. Homsy, L. J. Blum and C. A. Marquette, Biosens Bioelectron, 2008, 23, 1812–1818 CrossRef CAS.
- F. Mallard, G. Marchand, F. Ginot and R. Campagnolo, Biosens Bioelectron, 2005, 20, 1813–1820 CrossRef CAS.
- G. Marchand, P. Broyer, V. Lanet, C. Delattre, F. Foucault, L. Menou, B. Calvas, D. Roller, F. Ginot, R. Campagnolo and F. Mallard, Biomed Microdevices, 2008, 10, 35–45 CrossRef CAS.
- K. Salama, H. Eltoukhy, A. Hassibi and A. El-Gamal, Biosens Bioelectron, 2004, 19, 1377–1386 CrossRef CAS.
- T. Tanaka, K. Hatakeyama, M. Sawaguchi, A. Iwadate, Y. Mizutani, K. Sasaki, N. Tateishi, H. Takeyama and T. Matsunaga, Biotechnol Bioeng, 2006, 95, 22–28 CrossRef CAS.
- S. Taylor, S. Smith, B. Windle and A. Guiseppi-Elie, Nucleic Acids Res, 2003, 31, e87 CrossRef.
- T. Nakagawa, R. Hashimoto, K. Maruyama, T. Tanaka, H. Takeyama and T. Matsunaga, Biotechnol Bioeng, 2006, 94, 862–868 CrossRef CAS.
- W. J. Chen, E. W. Loh, Y. P. Hsu and A. T. Cheng, Biol Psychiatry, 1997, 41, 703–709 CrossRef CAS.
- M. Eggers, M. Hogan, R. K. Reich, J. Lamture, D. Ehrlich, M. Hollis, B. Kosicki, T. Powdrill, K. Beattie and S. Smith, Biotechniques, 1994, 17, 516–525 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Relationship between hybridization reaction and flow rate in the microchannel (Fig. S1); signal increment of ALDH2*1/*1 target (63-mer, 100 nM) hybridized to the ALDH2*1 and ALDH2*2 spots on the TFT photosensor (Fig. S2); comparison of hybridization efficiency based on various target conditions (Fig. S3); and total time required for SNP detection (Table S1). See DOI: 10.1039/b817427j |
| This journal is © The Royal Society of Chemistry 2009 |
