Microfluidic emulsification and sorting assisted preparation of monodisperse chitosan microparticles†
Chih-Hui
Yang
*a,
Yung-Sheng
Lin
b,
Keng-Shiang
Huang
*ac,
Yu-Che
Huang
b,
Eng-Chi
Wang
*d,
Jia-Ying
Jhong
ad and
Chun-Yen
Kuo
a
aDepartment of Biological Science & Technology, I-Shou University, Taiwan. E-mail: chyang@isu.edu.tw; Fax: +886-7-615-1959; Tel: +886-7-615-1100 ext. 7312
bInstrument Technology Research Center, National Applied Research Laboratories, Taiwan
cDepartment of Chemistry, National Cheng Kung University, Taiwan. E-mail: huangks@mail.ncku.edu.tw
dFaculty of Medicinal and Applied Chemistry, Kaohsiung Medical University, Taiwan. E-mail: enchwa@kmu.edu.tw
First published on 21st October 2008
Abstract
A microfluidic device for generating monodisperse chitosan microparticles and separating the desired particle from smaller particles created as a byproduct of this process was described. The purpose of this study is to separate the satellite droplets from their parent droplets to enhance the size uniformity of the desired microparticles. A double T-junction design was first employed to control the emulsification and the separation, respectively. The results show that the size and gap of the parent droplets are tunable by adjusting the water and oil flow rates. A separation ratio of the satellite droplets of more than 99% was observed. The proposed microfluidic chip is capable of generating relatively uniform micro-droplets with well controllable diameter, and it has the added advantages of being a simple, low cost, and high throughput process. In the future this apparatus can be used to fabricate size-controlled monodisperse microparticles to act as drug carriers for biotechnology and biomedicine applications.
1 Introduction
Chitosan is a linear polysaccharide composed of arbitrarily distributed N-acetyl-D-glucosamine (acetylated unit) and β-(1–4)-linked D-glucosamine (deacetylated unit). Due to its high biocompatibility and biodegradability, chitosan is currently gaining a great deal of attention for medical and pharmaceutical applications.1 When preparing chitosan particles, conventional methods such as phase separation, solvent evaporation, spray drying, and others2 have the common drawback of a large particle-size distribution, and being unable to easily control the resulting sizes. It is important to control the particle size and size distribution in preparing drug-loaded microspheres for controlled-release drug delivery, because they influence the clearance rate by the body and ultimately determine the drug dosage.3 Basically, an ideal particle size provides the optimal release rate and route of administration.The concept of making monodisperse4 microparticles has gained attention in the past few years. The methods to prepare uniform microparticles can be liquid/liquid injection, membrane emulsification, electrostatic extrusion, or acoustic excitation.3,5 The microfluidic chip is a novel and emerging tool for the manufacture of microparticles with precisely controlled and monodisperse size distributions.6 The mechanism of droplet formation in a liquid/liquid emulsification system has been studied extensively from both theoretical and experimental perspectives7 because of the wide variety of applications.8,9
Satellite droplets are byproducts in the droplet formation process for the preparation of uniform microparticles. In order to have uniform microparticles, droplet sorting is used to allow for isolation and purification on the microfluidic chip. Methods for sorting droplets can be active or passive.6e,8a Active sorting needs an additional accessory such as an electrical, optical, mechanical, or magnetic control10 and is an additional expenditure, as well as detrimental to the miniaturization of the system required for droplet formation. Passive sorting can be achieved by either gravity or channel geometry.11 To have a better gravity-driven sorting, much effort is required for the theoretical hydrodynamic analysis or experimental try and error under tuning flow conditions. The typical channel geometry for sorting is the bifurcation junction which has the disadvantage of incomplete sorting due to large droplets plugging the smaller daughter channel.11b Although the loop channel design can overcome the incomplete sorting issue, the larger droplet will split at the bifurcation junction.11b
To the best of our knowledge, the double T-junction geometry has not yet been proposed as the channel geometry for sorting. The goal of this study is to develop a method for producing chitosan microparticles of precisely controlled monodisperse size distribution by the microfluidic channels. A double T-junction is used to generate regular droplets and separate larger droplets away from smaller ones. The proposed microfluidic chip is easy to fabricate and set up, and is easily programmed to generate a large set of uniform chitosan microparticles.
2 Experimental
2.1 Principle
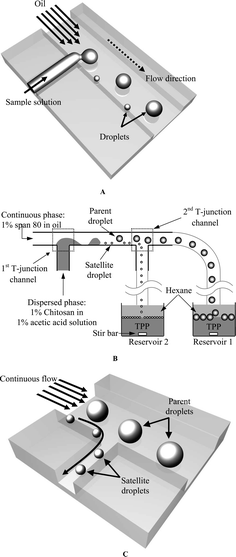 | ||
| Fig. 1 Schematic illustrations of the working principle of the multiple-functional microchannel (not to scale). (A) The top view of the 1st T-junction channel. Based on the ability of microfluidics to exert control over the cross-flowing streams a large set of self-assembling spheres can be obtained. (B) Illustration of microfluidic chip emulsification/separation (sorting) coupled with the ionic-crosslinking reaction process (the side view). (C) The 2nd T-junction channel design is employed to control the sorting of the droplets. | ||
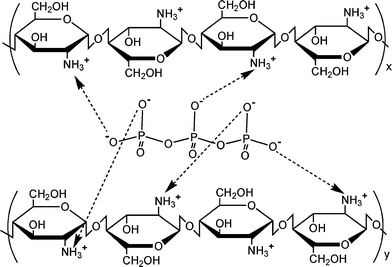 | ||
| Fig. 2 The mechanism of TPP-chitosan microspheres synthesis is based on the fact that in low pH, TPP is dissociated into P3O105− anions. Therefore, TPP-chitosan microparticles prepared in the acidic TPP solution are completely ionic-crosslinking dominated. | ||
2.2 Design and fabrication of a microfluidic platform
The proposed microfluidic chip was designed by AutoCAD®, and was constructed on a conventional poly methyl methacrylate (PMMA) substrate with a laser micromachining process by a CO2 laser machine (LaserPro Venus, GCC, Taiwan).9a,9c The microfluidic chip consists of three layers (for an expanded view see Fig. 3A) which are, from top to bottom: the cover layer (containing two inlets and 28 screw orifices for binding), the second layer (containing two T-junction channels and screw orifices) and the bottom layer (containing two outlets, screw orifices and a disk structure, 55 mm in radius, designed in a modular fashion for placing on an inverted fluorescent microscope). These three layers are then integrated by screws (0.5 mm pitch, 4.0 mm in diameter, tightened at 1.0∼1.2 Nm), see Fig. 3B.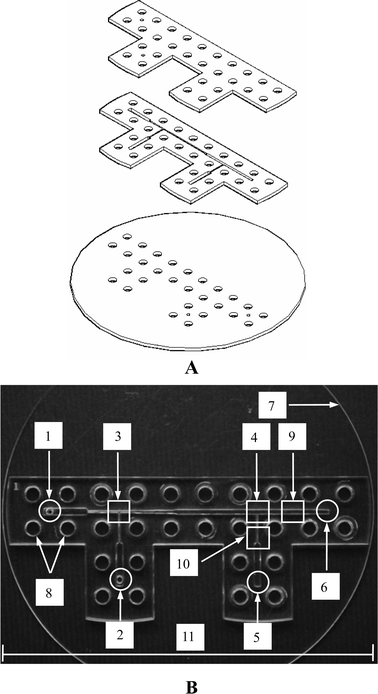 | ||
| Fig. 3 (A) Exploded view of the microfluidic chip. (B) Photograph of the chip after assembly: 1. and 2. inlets, 3. 1st T-junction, 4. 2nd T-junction, 5 and 6. outlets, 7. bottom layer disk, 8. screw orifices, 9. and 10. droplets observation areas, 11. scale bar is 11 cm. | ||
3 Results and discussion
Our strategy is based on the shear force at the 1st T-junction microchannel forming a narrow size distribution of self-assembling sphere structures, the so-called chitosan micro-emulsions. However, we find that satellite emulsions (see Fig. S1 in the ESI)† become a byproduct when the microfluidic T-junction is employed, even though a T-junction design is one of the most frequently used microfluidic geometries to produce immiscible fluid segments.6d,14 The 2nd T-junction design (down stream) can provide a separation (sorting) function in the microfluidic device. When satellite microspheres travel to the 2nd T-junction channel, they are drawn to the side channel, while parent droplets keep traveling the main stream/channel, thereby enhancing the uniformity of the chitosan micro-emulsions. When these emulsions are transported to a TPP solution through a teflon tube, they precipitate spontaneously to the bottom of the oil due to the fact that they have a higher density than oil. Therefore, chitosan micro-emulsions reacts with P3O105− ion at the interface between the oil phase and the water phase. After they have undergone ionic-crosslinking, the TPP-chitosan microparticles can be observed.The results of the numerical simulation are shown in Fig. 4. Fig. 4A indicates the streamlines of the flow field in the 2nd T-junction microchannel. The bottom streamlines alongside the main channel evolve into the side channel. The other streamlines go through the junction directly but a little shift to the side wall especially for those streamlines close to the bottom streamlines. Fig. 4B–D are the results of sorting the droplets by assuming the diameter of the parent droplets as 340 μm and those of the satellite droplets as 20 μm. As shown in Fig. 4B, initially the parent/satellite droplets have located near the middle and alongside the main channel, respectively. Fig. 4C and 4D are the evolution of the parent/satellite droplets motion in this channel at 0.4 sec and 0.8 sec. The satellite droplet fully immersed in the bottom streamlines travels toward the side channel and filters out at this junction. On the other hand the parent droplet goes through this junction but deviates from the original line towards the side wall of the channel due to the change in flow field after this junction. Fig. 4D clearly shows that the satellite droplet is drawn to the side channel and that the main channel is free of satellite droplets after this junction.
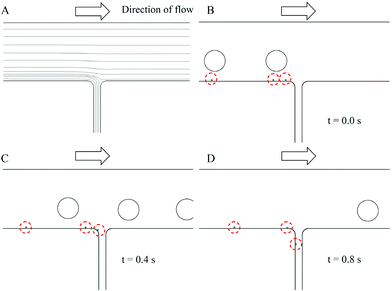 | ||
| Fig. 4 CFD-ACE+ simulation of the sorting behavior. (A) Streamlines of the 2nd T-junction channel. (B) Initial condition for droplets (C) Evolution (D) Separation of satellites droplets. The inlet oil flow rate is set as 0.3 mL/min and the outlet flow rate at the side channel is assumed as 0.012 mL/min. | ||
Fig. 5 shows the photo images of the generation and separation of droplets in the double T-junction microchannels (see the video in the ESI).† The sample fluid is sheared by an oil fluid, and then separated into parent/satellite droplets as shown in Fig. 5A. The hydrodynamic force makes the parent droplets flow toward the middle of the main channel while the satellite droplets flow along the side wall after the 1st T-junction. At the 2nd T-junction, the stream field forces the satellite droplets into the side channel while the parent droplets carry on along the main channel but shift slightly away from the centerline due to the effect of the side flow (Fig. 5B and 5C). Fig. 5D shows these satellite droplets are filtered from the parent droplets due to the 2nd T-junction geometry. Consequently, satellite droplets are absent downstream, as shown in Fig. 4C, and exit only before the junction in Fig. 5B. Fig. 6 shows the microscope images of the chitosan microparticles collected in the reservoirs. In reservoir 1 (Fig. 6A), microparticles are uniform in both morphology and size, and the average diameter is close to 340 μm (Fig. 6D). In reservoir 2 (Fig. 6B and 6C), the microparticles are less uniform and the size distribution is broad, ranging from 25 to 90 μm in diameter (Fig. 6E).
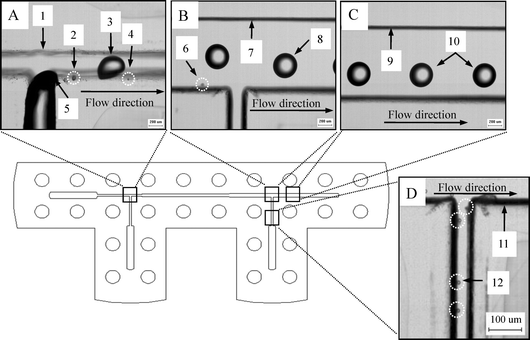 | ||
| Fig. 5 The top view photographs of droplets generation/sorting in the microchannels. (A) Parent/satellite droplets are generated under sheath fluid in the 1st T-junction channel. (B) Photo in the 2nd T-Junction region. (C) Photo in the downstream. (D) Photo in the side channel (1, 7, 9, 11 are channel wall; 2, 4, 6, 12 highlights are satellite droplets; 3, 8, 10 are parent droplets, 5 sample flow. Scale bars A to C are 200 μm, and scale bar D is 100 μm). | ||
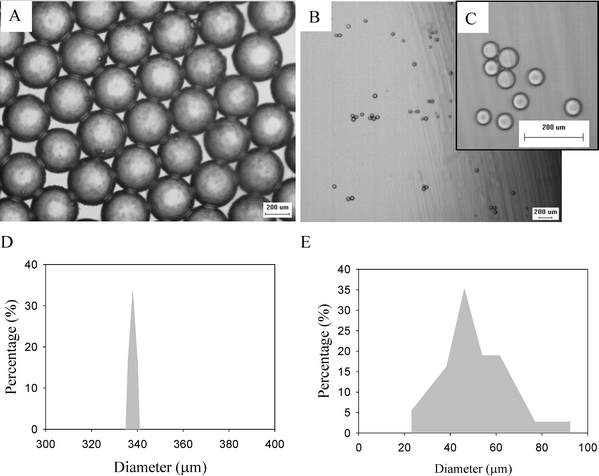 | ||
| Fig. 6 Microscope images of chitosan microparticles (A) in the Reservoir 1, and (B) and (C) in the Reservoir 2 (the scale bar is 200 μm). The size distribution of the microparticles regarding (D) is corresponding to (A), and (E) is corresponding to (B). | ||
Table 1 shows the relationships among the average droplet diameter, sorting efficiency, and various flow velocities. The results show that the droplet size was deeply affected by both water/oil flow rates. The relative standard deviation (RSD) data shows that the coefficient of variation in the satellite droplets is larger than in the parent droplets. The satellite droplets break away from the parent droplet. This is a highly unstable process, and the number and the volume of the satellite droplets per parent droplet that go through the necking and breaking process is random. In addition, a satellite droplet may fracture into two or more smaller droplets at higher flow velocity gradients (increasing shear rate) because interfacial tension can't afford the satellite droplet to remain intact. Due to these two reasons, satellite droplets have a larger RSD than the parent droplets. In each flow condition, the parent droplets have a very small RSD of less than 1.540%. This reflects the fact that the size of the droplets collected in reservoir 1 is monodisperse, and agrees with the result of Fig. 6D. Based on Table 1, the experimental results show no significant difference between sorting efficiency and flow velocities (P > 0.05). This experimental apparatus allows production of chitosan microparticles as small as 204 μm in diameter in which 95% of the droplets are within 5 μm of the average. By changing the water/oil flow rates, we can manufacture chitosan droplets of precisely controlled and monodisperse size distributions by using the proposed double T-junction microfluidic channels. Based on the above results, our proposed approach should be applicable to others types of materials (drug carriers).
| Flow rate of continuous phase (mL/min) | Flow rate of dispersed phase (mL/min) | Parent dropleta | Satellite dropletb | Sorting efficiency (%) | ||
|---|---|---|---|---|---|---|
| Average size (μm) | RSDc(%) | Average size (μm) | RSDc(%) | |||
| a Droplets in the reservoir 1. b Droplets in the reservoir 2. c RSD (relative standard deviation). | ||||||
| 0.002 | 0.15 | 268.02 | 0.473 | 7.34 | 33.082 | 100.0 |
| 0.20 | 229.67 | 1.018 | 8.67 | 51.944 | 99.4 | |
| 0.25 | 216.02 | 1.171 | 17.67 | 60.081 | 99.4 | |
| 0.30 | 204.33 | 0.964 | 13.68 | 52.911 | 99.6 | |
| 0.003 | 0.15 | 278.67 | 1.076 | 16.33 | 52.191 | 99.2 |
| 0.20 | 234.74 | 0.464 | 20.46 | 62.611 | 99.6 | |
| 0.25 | 227.82 | 1.541 | 14.52 | 84.756 | 99.4 | |
| 0.30 | 213.67 | 1.408 | 16.28 | 82.158 | 99.4 | |
| 0.004 | 0.15 | 284.82 | 0.997 | 11.63 | 37.702 | 99.6 |
| 0.20 | 268.08 | 1.251 | 16.33 | 14.314 | 99.6 | |
| 0.25 | 237.06 | 0.472 | 13.43 | 57.445 | 100.0 | |
| 0.30 | 233.67 | 1.138 | 16.35 | 14.314 | 99.4 | |
| 0.005 | 0.15 | 327.20 | 0.577 | 18.48 | 65.546 | 99.6 |
| 0.20 | 282.33 | 0.531 | 16.31 | 71.574 | 99.6 | |
| 0.25 | 244.18 | 0.548 | 21.34 | 69.061 | 99.4 | |
| 0.30 | 237.33 | 0.433 | 29.13 | 78.844 | 99.2 | |
| 0.006 | 0.15 | 329.01 | 0.838 | 10.33 | 59.235 | 99.4 |
| 0.20 | 287.05 | 0.383 | 16.28 | 11.181 | 99.2 | |
| 0.25 | 248.34 | 0.604 | 14.33 | 55.405 | 99.6 | |
| 0.30 | 242.33 | 0.618 | 17.67 | 34.647 | 99.4 | |
| 0.007 | 0.15 | 338.06 | 0.529 | 15.61 | 90.725 | 99.6 |
| 0.20 | 300.67 | 0.804 | 25.33 | 61.084 | 99.4 | |
| 0.25 | 267.05 | 0.408 | 19.38 | 76.206 | 99.2 | |
| 0.30 | 247.07 | 0.441 | 41.67 | 52.879 | 99.2 | |
Since field-flow fractionation (one of active sorting) has been reported by Gidding,10h,10i some researchers have used a similar approach for the microfluidic sorting.10 These methods, however, are still burdened by the need for an external power source to generate the driving force for sample separation. In addition, on-chip integration of interfacing components such as mechanical moving parts, electrodes, heaters, optical lattice, or piezoelectric materials often complicates fabrication procedures and increases the complexity of resulting devices.11a On the other hand, our T-junction is a relatively simple and useful strategy without the use of external power sources. Compared to the previous passive sorting,11 the T-junction design in this study is very attractive from a practical point of view, since its flow pattern substantially benefits the efficiency of sorting particles. The break-off positions of the satellite droplets are almost entirely located in the streamlines to the side channel rather than towards the main channel, thereby resulting in an extremely high sorting yield. The results show a separation ratio of the satellite microspheres of more than 99%. This sorting efficiency is similar to Huh's study (gravity-driven),11a and is a slight improvement over Gluckstad's study (optical).10b Most importantly, in addition to the rapid and high-purity sorting, our method has the advantage of monodisperse desired droplets that can be applied to chitosan microspheres.
4 Conclusions
The manipulation of size-controlled chitosan microparticles with less variation than 2% in size has been successfully demonstrated in a very simple and useful approach. This is the first demonstration of using a double T-junction channel geometry on a microfluidic chip to generate droplets and separate satellite droplets from parent droplets. Our results show that the T-junction geometry filters out satellite droplets in a highly efficient manner, with a separation ratio of satellite microparticles of more than 99% being observed. This apparatus is very attractive from a practical point of view, since it easily emulsifies and yields extremely ordered and regular microparticles. This channel design on microfluidic chip will be suitable for many potential usages in the preparation of various monodisperse microparticles, especially for encapsulating biological entities for pharmaceutics.Acknowledgements
The authors gratefully acknowledge the financial support provided to this study by the National Science Council of Taiwan, R.O.C. under grant No. NSC 95–2320-B-214-002-MY2.References
- (a) V. Dodane and V. D. Vilivalam, Pharm. Sci. Technol. Today, 1998, 1, 246–253 CrossRef CAS; (b) W. Paul and C. P. Sharma, STP Pharma Sci., 2000, 10, 5–22 Search PubMed; (c) V. R. Sinha, A. K. Singla, S. Wadhawan, R. Kaushik, R. Kumria, K. Bansal and S. Dhawan, Int. J. Pharm., 2004, 274, 1–33 CrossRef CAS.
- (a) I. M. van der Lubben, J. C. Verhoef, A. C. van Aelst, G. Borchard and H. E. Junginger, Biomaterials, 2001, 22, 687–694 CrossRef CAS; (b) Y. Kawashima, H. Yamamoto, H. Takeuchi, T. Hino and T. Niwa, Euro. J. Pharm. Biopharm., 1998, 45, 41–48 Search PubMed; (c) T. J. Young, K. P. Johnston, K. Mishima and H. Tanaka, J. Pharm. Sci., 1999, 88, 640–650 CrossRef CAS.
- C. Berkland, K. Kim and D. W. Pack, J. Contr. Release, 2001, 73, 59–74 Search PubMed.
- (a) The typical monodispersity criterion has been defined as the coefficient of the variation of particle size distribution less than 10%. Refs. S. Inukai, T. Tanma, S. Orihara and M. Konno, Chem. Eng. Res. Des., 2001, 79, 901–905 Search PubMed; (b) S. Gu, T. Mogi and M. Konno, J. Colloid Interface Sci., 1998, 207, 113–118 CrossRef CAS; (c) Y. Hong and F. Wang, Microfluid Nanofluid, 2007, 3, 341–346 CrossRef CAS.
- (a) B. G. Amsden, Pharm. Res., 1999, 16, 1140–1143 CrossRef CAS; (b) T. Nakashima, M. Shimizu and M. Kukizaki, Adv. Drug Deliv. Rev., 2000, 45, 47–56 CrossRef CAS.
- (a) H. Becker and L. E. Locascio, Talanta, 2002, 56, 267–287 CrossRef CAS; (b) D. T. Eddington and D. J. Beebe, Adv. Drug Deliv. Rev., 2004, 56, 199–210 CrossRef CAS; (c) F. X. Gu, R. Karnik, A. Z. Wang, F. Alexis, E. Levy-Nissenbaum, S. Hong, R. S. Langer and O. C. Farokhzad, Nano Today, 2007, 2, 14–21 CrossRef; (d) G. F. Christopher and S. L. Anna, J. Phys. D: Appl. Phys., 2007, 40, R319–R336 CrossRef CAS; (e) S. Y. Teh, R. Lin, L. H. Hung and A. P. Lee, Lab Chip, 2008, 8, 198–220 RSC; (f) H. Song, D. L. Chen and R. F. Ismagilov, Angew. Chem. Int. Ed., 2006, 45, 7336–7356 CrossRef CAS.
- (a) R. Clift, J. R. Grace, and M. E. Weber, Bubbles, drops, and particles, New York, Academic Press 1978, pp.321-351 Search PubMed; (b) X. Zhang, Chem. Eng. Sci., 1999, 54, 1759–1774 CrossRef CAS.
- (a) H. Andersson and A. van den Berg, Sens. Actuators B-Chem., 2003, 92, 315–325 CrossRef; (b) L. Kang, B. G. Chung, R. Langer and A. Khademhosseini, Drug Discov. Today, 2008, 13, 1–13 CrossRef CAS; (c) M. U. Kopp, A. J. deMello and A. Manz, Science, 1998, 280, 1046 CrossRef CAS; (d) B. H. Weigl, R. L. Bardell and C. R. Cabrera, Adv. Drug Deliv. Rev., 2003, 55, 349–377 CrossRef CAS; (e) G. M. Whitesides, Nature, 2006, 442, 368–373 CrossRef CAS.
- (a) C. H. Yang, K. S. Huang and J. Y. Chang, Biomed. Microdevices, 2007, 9, 253–259 CrossRef CAS; (b) J. Y. Chang, C. H. Yang and K. S. Huang, Nanotechnology, 2007, 18, 305305 CrossRef; (c) K. S. Huang, T. H. Lai and Y. C. Lin, Lab Chip, 2006, 6, 954–957 RSC.
- (a) S. K. Cho, Y. J. Zhao and C. J. Kim, Lab Chip, 2007, 7, 490–498 RSC; (b) J. Gluckstad, Nat. Mater., 2004, 3, 9–10 CrossRef; (c) Y. L. Li, C. Dalton, H. J. Crabtree, G. Nilsson and K. Kaler, Lab Chip, 2007, 7, 239–248 RSC; (d) G. T. Roman and R. T. Kennedy, J. Chromatogr. A, 2007, 1168, 170–188 CrossRef CAS; (e) D. Wu, J. Qin and B. C. Lin, J. Chromatogr. A, 2008, 1184, 542–559 CrossRef CAS; (f) C. Yi, C. W. Li, S. Ji and M. Yang, Anal. Chim. Acta, 2006, 560, 1–23 CrossRef CAS; (g) M. P. MacDonald, G. C. Spalding and K. Dholakia, Nature, 2003, 426, 421–424 CrossRef CAS; (h) J. C. Giddings, F. J. F. Yang and M. N. Myers, Science, 1976, 193, 1244–1245 CAS; (i) J. C. Giddings, Science, 2003, 260, 1244–1245.
- (a) D. Huh, J. H. Bahng, Y. B. Ling, H. H. Wei, O. D. Kripfgans, J. B. Fowlkes, J. B. Grotberg and S. Takayama, Anal. Chem., 2007, 79, 1369–1376 CrossRef CAS; (b) Y. C. Tan, J. S. Fisher, A. I. Lee, V. Cristini and A. P. Lee, Lab Chip, 2004, 4, 292–298 RSC; (c) Y. C. Tan, Y. Ho and A. P. Lee, Microfluidics Nanofluidics, 2007, 3, 495–499 CrossRef.
- (a) T. Nisisako, T. Torii and T. Higuchi, Proceedings of Micro Total Analysis Systems, 2004, 2, 312–314 Search PubMed; (b) M. Yamada, M. Nakashima and M. Seki, Anal. Chem., 2004, 76, 5465–5471 CrossRef CAS.
- X. Z. Shu, K. J. Zhu and W. Song, Int. J. Pharm., 2001, 212, 19–28 CrossRef CAS.
- Y. C. Tan and A. P. Lee, Lab Chip, 2005, 5, 1178–1183 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, experimental procedure, numerical simulations and supplementary video 1. See DOI: 10.1039/b807454b |
| This journal is © The Royal Society of Chemistry 2009 |
