Photobleaching absorbed Rhodamine B to improve temperature measurements in PDMS microchannels
Tomasz
Glawdel
,
Zeyad
Almutairi
,
Shuwen
Wang
and
Carolyn
Ren
Department of Mechanical and Mechatronics Engineering, University of Waterloo, 200 University Ave W., Waterloo, Canada. E-mail: c3ren@mecheng1.uwaterloo.ca; Fax: +1 (519) 885-5862; Tel: +1 (519) 888-4567 x33030
First published on 20th October 2008
Abstract
Rhodamine B based fluorescence thermometry is commonly used in microfluidics to measure fluid temperatures in microchannels. Notable absorption of Rhodamine B into PDMS channel walls, however, causes difficulties in obtaining accurate temperature measurements due to a steady increase in the overall fluorescent signal. A simple and effective technique is reported that removes the fluorescent signal from absorbed Rhodamine B dye by means of photobleaching with a high intensity light source before taking images for thermometry analysis. The temperature field at the convergence of hot and cold streams in a Y-channel fabricated in PDMS/glass microfluidic chip is studied to demonstrate the execution of the photobleaching technique.
Introduction
Many lab-on-a-chip (LOC) applications require precise thermal control as part of chip operation. Several examples include continuous flow PCR,1isoelectric focusing with thermally generated pH gradients,2 temperature gradient focusing3 and joule heating studies.4 Characterizing the fluid temperature field is critical for optimizing chip design and performance in such devices. Measuring fluid temperatures in microchannels is difficult due to the constraints put on the size of the measurement probe. Most techniques rely on indicators added to the working fluid such as thermochromic liquid crystals,5 beads in µ-PIV thermometry,6 and temperature sensitive dye in fluorescence thermometry.7One of the widely used methods in microfluidics for measuring internal fluid temperature is a subset of fluorescence thermometry known as one-color ratiometric laser induced fluorescence (LIF). In this technique, a dilute concentration of Rhodamine B dye, which has a strong temperature dependent quantum efficiency, is added to the working fluid and the area of interest is imaged using a fluorescence microscope mounted with a CCD camera.7 To account for differences in setup during experiments, such as non-uniform light exposure, the test image is normalized with a base image at a known temperature. By measuring changes in the normalized intensity the temperature can be determined through a previously obtained calibration curve with high spatial and temporal resolution.
The majority of recently developed microfluidic chips are fabricated from poly(dimethylsiloxane) (PDMS) using soft lithography techniques. PDMS is an excellent material for a wide variety of microfluidics applications as it is optically transparent, chemically inert, biologically compatible, inexpensive and easy to work with. However, as outlined by Mukhopadhyay,8 a compatibility issue exists between small hydrophobic molecules and PDMS microchannels. For instance, Toepke and Beebe9 clearly demonstrated that PDMS absorbs the hydrophobic fluorophore Nile red and the hydrophobic drug quinine as indicated by the presence of residual fluorescent signals originating from the bulk PDMS after these molecules had been flushed through the microchannel. In fact a number of researchers have also reported the specific absorption of Rhodamine B in PDMS.10–12 For instance, the absorptive property of PDMS has been used to develop a Rhodamine B/PDMS thin film for the whole chip temperature measurement.10,11 In this technique, a thin PDMS film was saturated with Rhodamine B dye by soaking it in a Rhodamine B solution for several days. Pittman et al.12 also found that a fluorescence imaging technique developed to monitor in channel fluid flow based on periodic photobleaching of a dye added to the fluid could not be used in PDMS channels with Rhodamine B solutions because of the absorption of Rhodamine B molecules into the PDMS channel walls.
In terms of fluorescence thermometry, absorption causes an increase in the overall fluorescent intensity which will be interpreted as an erroneous change in temperature. The rate of absorption depends on a number of factors such as the pH and ions in the solution, temperature and material structure which makes it difficult to predict the amount of absorption beforehand.9 Without somehow accounting for the change in fluorescent intensity due to absorption it is difficult to obtain reliable and repeatable temperature measurements.
Several procedures have been developed in an attempt to alleviate the absorption of Rhodamine B into PDMS including surface modification using a sol–gel technique,13 coating the walls with a polybrene solution,10,14 and adding sodium dodecyl sulfate15 or a hybrid ionic liquid/nonionic surfactant to the working fluid.16 However, these methods may affect the intended operation of the microfluidic chip; for instance, in the case of electroosmotic flow the zeta potential may be altered. It may be possible to account for the absorption by subtracting a background image of the chip with absorbed dye, flushing out the dye when measurements are not being taken or calibrating the experiment to account for absorption. These methods, however, are highly unreliable and introduce significant errors as demonstrated previously.10
Herein a simple and versatile technique is proposed to improve Rhodamine B thermometry in PDMS channels without modifying the working fluid or channels walls. The approach is based on removing the fluorescent signal from absorbed dye particles by photobleaching the area of interest prior to taking images for temperature analysis. A similar technique is used in ultrasensitive single molecule detection to reduce background luminescence caused by impurities in the buffer.17 This principal of removing unwanted fluorescence by photobleaching is adapted to solve the problem of absorbed Rhodamine B dye particles in fluorescence thermometry. The technique is demonstrated by measuring the temperature field at the intersection of a Y-channel where hot and cold streams merge in PDMS/glass chip using only a conventional fluorescence microscope and mercury arc lamp.
Experimental
Images were recorded using an inverted microscope (GX-71, Olympus) mounted with a 1392 × 1040 pixel CoolSNAP ES Monochrome CCD camera (Photometrics). Laser grade Rhodamine B dye (Fisher Scientific) (100 µM in DI water) was excited through an appropriate filter set (U-MW6S3, exciter filter λ = 510–550, dichroic beam splitter λ = 570, barrier filter λ = 590,) with either a 100 W mercury arc lamp when photobleaching or a 100 W halogen lamp when capturing images. The mercury arc lamp was chosen for photobleaching due to its superior light intensity output near the excitation wavelength of Rhodamine B (λ = 546 nm). To achieve maximum light intensity (and photobleaching rate) the mercury arc lamp was properly centered prior to performing any experiment. The lower intensity halogen lamp was used to acquire images for temperature analysis so as to minimize photobleaching of the Rhodamine B solution and the associated error it produces in temperature measurements. Images taken during chip operation were normalized (ratio of 0 to 1) by a baseline image captured at 23 °C with Image Pro Plus®. The ratiometric intensity values were then converted to temperatures through the calibration curve provided by Ross et al.7 With Rhodamine B the normalized intensity decreases gradually with increasing temperature. Contour temperature plots were obtained from images taken during the experiment using an in-house Matlab program.A Y-channel design was selected to establish a non-uniform temperature field within the fluid that could be measured. The liquid temperature in one of the branch channels was increased by an embedded nichrome wire heater. The dimensions of the Y-channel are: 150 µm wide, 20 µm high, 1 cm long for the branch channels, and 4 cm long for the main vertical channel. The microfluidic chip was fabricated in a PDMS substrate and then bonded with a glass slide using standard soft lithography procedures. A glass reservoir was attached to the outlet of the main channel and connectors were attached to connect the two branch inlets of the Y-channel to a high precision dual syringe pump (11 Plus, Harvard Apparatus). The nichrome wire was fabricated into the PDMS replica during the PDMS curing process18 and heating was controlled using a DC power supply. A thermocouple was inserted beside the embedded heater for temperature monitoring. All tests were performed in the dark to limit unintentional photobleaching of the dyed fluid.
Results and discussion
Photobleaching of absorbed Rhodamine B
Photobleaching is a destructive process where the fluorophore loses the ability to emit light under prolonged exposure to excitation. The rate of photobleaching depends on a number of factors including the illumination intensity and wavelength, exposure time, temperature, chemical environment and intrinsic properties of the dye.19 Generally photobleaching is not desirable for fluorescence thermometry because it creates unwanted intensity changes that cause a false temperature reading. However, in this work photobleaching is leveraged to remove the undesired fluorescent signal originating from absorbed Rhodamine B.Fig. 1 shows the fluorescence images and corresponding intensity profiles of a PDMS microchannel (20 µm W, 100 µm H) during a photobleaching experiment where a 100 µM solution of Rhodamine B was pumped continuosly at 20 µL min−1. First, the Rhodamine B solution was pumped for 30 min to allow the Rhodamine B to absorb. Afterwards, the area was exposed to excitation light from the 100 W mercury arc lamp (20× objective) and images were taken periodically over the next 30 min. The first image (0 min) clearly shows that absorption of Rhodamine B can be quite severe as indicated by the amount of fluorescence emitted from the microchannel surroundings. Subsequent images demonstrate that continuous exposure to the merucry lamp causes the absorbed Rhodamine B fluorescence signal to gradually decrease until eventually the only significant signal originates from Rhodamine B in the fluid.
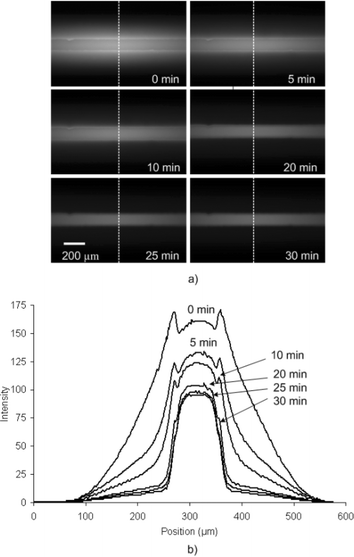 | ||
| Fig. 1 (a) Sequence of fluorescent images acquired during the course of photobleaching a PDMS microchannel with a 100 µM solution of Rhodamine B pumped at 20 µL min−1. (b) Corresponding light intensity profiles showing the decrease in signal from absorbed Rhodamine B. | ||
The factors which affect the photobleaching process may be determined by applying Beer's Law to a dilute optical sample:17
| [C]out = [C]inexp(−2303εQpdI0te) | (1) |
The quantum yield of photobleaching and the molar extinction coefficient are inherent properties of Rhodamine B, while the light intensity and exposure time can be altered to maximize photobleaching. Due to the exponential dependence, a small increase in time or light intensity results in a substantial increase in the amount of photobleaching. Note that the continuous absorption of fresh unbleached dye from the fluid stream is not considered in the equation. If this was considered an additional term accounting for the increase in emitting molecules from absorption would need to be included. Therefore, in practice the photobleaching technique only works if the rate of photobleaching exceeds the increase in concentration from absorption which may not occur for all system setups. For example, in this study the 20× objective was used because the lower 10× and 5× objectives produced insufficient light intensity to combat the continuous aborption.
Application of photobleaching technique
The procedure for applying this technique to fluorescence-based temperature measurements is as follows. First, the entire area of interest is exposed continuously to the high-intensity excitation light until the fluorescent signal from the absorbed Rhodamine B is removed. The processing time can be decreased by starting photobleaching at the beginning of the experiment when Rhodamine B is first introduced or photobleaching while the chip is operational (i.e. heated) since photobleaching rate increases significantly with temperature.10,11 Once the intensity has reached a stable condition the area is ready for performing temperature measurements. To take images for temperature analysis, the photobleaching beam is shut off for a few seconds so that fresh unbleached Rhodamine B solution fills the field of view. Then the low-intensity halogen lamp is turned on and an image is acquired. During this period of time absorption of dye is negligible. After one image is taken, the photobleaching lamp is turned back on and the area is continuously photobleached until the next image is to be taken. Photobleaching between images prevents the fluorescence intensity from increasing due to absorption.To demonstrate this technique thermal mixing between hot and cold streams at the intersection of the Y-channel PDMS/glass chip was studied as shown in Fig. 2. Rhodamine B solution was pumped at 50 µL min−1 for both streams. The thermocouple was used to assure that the system reached a thermal steady state while a constant current was applied to the heater. Bleaching of the intersection began as soon as the Rhodamine B solution was introduced in the channels. To verify that photobleaching was complete line intensity profiles were compared between subsequent images taken periodically until no difference was seen. The intersection was then imaged and photobleaching continued until another image for analysis was taken 5 min later. The heater was turned off and the chip was allowed to cool to room temperature, again while still photobleaching the area, and then a base image for normalization was obtained.
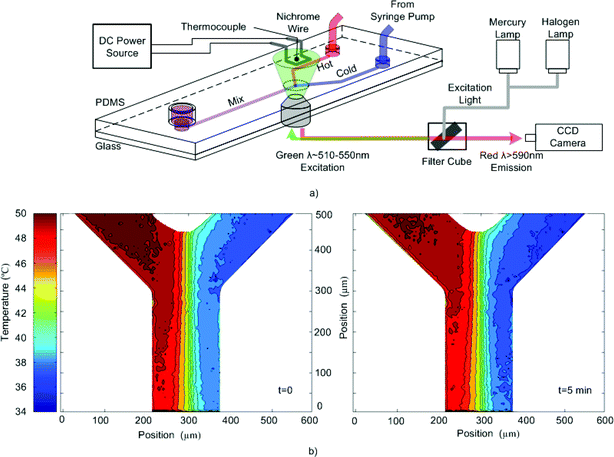 | ||
| Fig. 2 (a) Schematic of the experimental setup of the Y-channel PDMS chip. (b) Temperature contour plots of the intersection obtained from two images taken 5 min apart while applying the photobleaching technique to prepare the area for fluorescent based thermometry. | ||
Fig. 2b shows the temperature contour plots of the two images where the conduction of temperature cross-stream is clearly evident. In addition, the two plots are similar (within 2 °C of each other) which confirms that preparing the area using photobleaching can stabilize thermometry measurements. If the area was not photobleached, a significant decrease in the overall temperature (∼10 °C) would be expected for the second image due to absorption. A wider view of the intersection taken after the experiment (see Fig. 3) reveals the surprising extent of Rhodamine B absorption in the region surrounding the intersection as well as the complete removal of fluorescence signal in the photobleached area.
 | ||
| Fig. 3 Wide view (10×) of the intersection taken after the photobleaching experiment. The hot stream on the left hand side causes a larger amount of absorption than the cold stream on the right. | ||
Concluding remarks
Although the photobleaching technique has been successfully demonstrated, a number of precautions and considerations should be mentioned. First, photobleaching does not remove Rhodamine B from PDMS or solve the absorption problem; it only provides a means for eliminating the fluorescent intensity originating from absorbed dye particles in PDMS channel walls allowing for thermometry measurements to be performed. Absorption may still negatively impact the operational conditions of some devices. For example, absorbed dye particles are known to modify the zeta potential in electroosmotic flow.20Photobleaching is approximately characterized by an exponential decay of the overall intensity as described in eqn (1). Consequently, it is difficult to completely remove all of the fluorescent intensity from the absorbed dye; however, the method is still useful as long as the signal acquisition time is below a threshold limit for capturing the signal of the absorbed dye. Sufficient bleaching with a standard 100 W mercury arc lamp has been shown but the rate of photobleaching may be further increased by using a high-power lamp (500 W) or laser at the appropriate wavelength. Using a laser combined with a controlled shutter will also eliminate the need for the two lamp system used in this study. Experiments that require multiple locations to be studied will find the photobleaching process time consuming since at each new location photobleaching must first be performed to prepare the area before images can be taken.
Choosing the location to take measurements is also important. As an example, consider a continuous flow PCR chip which consists of a long serpentine channel (∼10 cm long) passing through three distinct temperature zones (∼95, 70, 55 °C).1 As the Rhodamine B solution is being pumped through the channel it is being absorbed into the PDMS at different rates along the channel. The concentration of Rhodamine B in the fluid will change with location and possibly with time leading to temperature errors. Due to the nature of the intensity-based measurements a number of other factors are of concern including contamination of the fluorescence signal from outside excitation light, reagant autofluorescence, material filtration, thermophoresis effects and cross-talk from sources outside the area of interest. These precautions outlined above should be judiciously considered when applying Rhodamine B with PDMS in florescence based imaging as well as with the photobleaching technique.
Acknowledgements
The authors gratefully acknowledge the support from the Natural Sciences and Engineering Research Council of Canada to Dr Carolyn Ren, the Canada Graduate Scholarship to Tomasz Glawdel, and the Department of Mechanical Engineering at King Saud University to Zeyad Almutairi.Discussions with Prof. Dayan Ban in the Department of Electrical and Computer Engineering at the University of Waterloo are also greatly acknowledged.
References
- J. A. Kim, J. Y. Lee, S. Seong, S. H. Cha, S. H. Lee, J. J. Kim and T. H. Park, Biochem. Eng. J., 2006, 29, 91–7 CrossRef CAS.
- T. Huang and J. Pawliszyn, Electrophoresis, 2002, 23, 3504–10 CrossRef CAS.
- G. J. Sommer, S. M. Kim, R. J. Littrell and E. F. Hasselbrink, Lab Chip, 2007, 7, 898–907 RSC.
- D. Erickson, D. Sinton and D. Q. Li, Lab Chip, 2003, 3, 141–9 RSC.
- V. N. Hoang, G. V. Kaigala and C. J. Backhouse, Lab Chip, 2008, 8, 484–7 RSC.
- V. Hohreiter, S. T. Wereley, M. G. Olsen and J. N. Chung, Meas. Sci. Technol., 2002, 13, 1072–8 CrossRef CAS.
- D. Ross, M. Gaitan and L. E. Locascio, Anal. Chem., 2001, 73, 4117–23 CrossRef CAS.
- R. Mukhopadhyay, Anal. Chem., 2007, 79, 3248–53 CrossRef CAS.
- M. W. Toepke and D. J. Beebe, Lab Chip, 2006, 6, 1484–6 RSC.
- R. Samy, T. Glawdel and C. L. Ren, Anal. Chem., 2008, 80, 369–75 CrossRef CAS.
- L. Gui and C. L. Ren, Appl. Phys. Lett., 2008, 92, 024102 CrossRef.
- J. L. Pittman, C. S. Henry and S. D. Gilman, Anal. Chem., 2003, 75, 361–70 CrossRef CAS.
- G. T. Roman, T. Hlaus, K. J. Bass, T. G. Seelhammer and C. T. Culbertson, Anal. Chem., 2005, 77, 1414–22 CrossRef CAS.
- D. Erickson, X. Z. Liu, R. Venditti, D. Q. Li and U. J. Krull, Anal. Chem., 2005, 77, 4000–7 CrossRef CAS.
- G. T. Roman, K. McDaniel and C. T. Culbertson, Analyst, 2006, 131, 194–201 RSC.
- Y. Xu, H. Jiang and E. Wang, Electrophoresis, 2007, 28, 4597–605 CrossRef CAS.
- R. L. Affleck, W. P. Ambrose, J. N. Demas, P. M. Goodwin, J. A. Schecker, J. M. Wu and R. A. Keller, Anal. Chem., 1996, 68, 2270–6 CrossRef CAS.
- R. Fu, B. Xu and D. Li, Int. J. Therm. Sci., 2006, 45, 841–7 CrossRef.
- L. L. Song, E. J. Hennink, I. T. Young and H. J. Tanke, Biophys. J., 1995, 68, 2588–600 CrossRef CAS.
- D. Ross and L. E. Locascio, Anal. Chem., 2003, 75, 1218–20 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
