Phosphonate monolayers functionalized by silver thiolate species as antibacterial nanocoatings on titanium and stainless steel†
Julien
Amalric
a,
P. Hubert
Mutin
*a,
Gilles
Guerrero
a,
Arnaud
Ponche
b,
Albert
Sotto
c and
Jean-Philippe
Lavigne
c
aInstitut Charles Gerhardt Montpellier UMR 5253 CNRS-UM2, Université Montpellier2, 34095 Montpellier cedex 5, France. E-mail: mutin@univ-montp2.fr
bInstitut de Chimie des Surfaces et Interfaces Mulhouse UPR-CNRS 9069, Mulhouse, France
cInstitut National de la Santé et de la Recherche Médicale, Espri 26, Université Montpellier 1, UFR de Médecine, Nîmes, France
First published on 14th November 2008
Abstract
Titanium and stainless steel substrates were modified by grafting with mercaptododecylphosphonic acid (MDPA) followed by reaction with silver nitrate (AgNO3), in order to investigate the potential of phosphonate self-assembled monolayers functionalized by silver thiolate species as antibacterial nanocoatings for inorganic biomaterials. The samples were characterized by Fourier transform infrared (FTIR) spectroscopy in grazing-incidence mode, water contact angle measurements, and X-ray photoelectron spectroscopy (XPS). The influence of the surface modification on bacterial adhesion and biofilm growth was investigated in vitro using Escherichia coli, Pseudomonas aeruginosa, Staphylococcus epidermidis, and Staphylococcus aureus strains. The stability of the monolayer in blood-mimicking medium was examined. Despite their very low silver content, MDPA + AgNO3 monolayers strongly decreased bacterial adhesion (>99.9% reduction in the number of viable adherent bacteria) and biofilm formation in comparison to the bare substrates.
1. Introduction
Orthopedic implants are increasingly used in modern medicine, and implant-related nosocomial infections affecting the health and capacity of the patient and multiplying the cost of treatment are a serious problem with important medical and socio-economic repercussions.1,2 These infections commonly result from the irreversible adhesion to the bare implant surface of bacteria entering the wound site, which may ultimately lead to the formation of tridimensional complex communities, known as biofilms, where the bacteria are encased in an exopolysaccharide gel. As bacteria living in biofilms are inherently protected from host defenses and antibiotics,3 it is essential to prevent bacterial adhesion and biofilm formation. Rather than developing new materials, a promising approach is to modify the surface of the implant by a coating immobilising or releasing a bactericide.1,4Self-assembled monolayers (SAMs) offer a powerful tool for the design of surface properties5 on inorganic substrates. In several studies, SAMs deposited on gold or silicon substrates have been used as model, passive surfaces to study the influence of the surface physicochemistry (notably hydrophilicity) on the adhesion of various bacteria.6–11 The covalent bonding of quaternary ammonium groups12 or antibiotics13 has also been investigated. On the other hand, the antibacterial effect of SAMs able to release bactericidal species is still largely unexplored.
The silver ion, Ag+, is a versatile bactericidal species with a broad-spectrum bactericidal activity14 and a very low toxicity toward mammalian cells.15 The antimicrobial efficiency in vitro and in vivo of silver-coated medical devices is well-established.4,16 It has been proposed that the reaction of Ag+ ions with thiol groups in the bacteria membrane proteins plays an essential role in bacterial inactivation.17,18 Several methods have been used for the deposition of silver-releasing coatings on orthopedic implant materials such as titanium or stainless steel, including ion implantation of silver,19,20physical vapor deposition of Ti/Ag,21 galvanic deposition of silver,16 and sol–gel deposition of Ag-doped silica22 or hydroxyapatite23 coatings.
In the present work, we propose to prevent bacterial adhesion on titanium and stainless steel by surface modification with a self-assembled phosphonate monolayer functionalized by silver thiolate species (Fig. 1). Indeed, phosphonic acids react with metal surfaces, leading to monolayers bound to the surface by iono-covalent M–O–P bonds24–29 ensuring good chemical and mechanical stability,30,31 while thiol groups react readily with silver cations to form silver thiolates with high formation constants.32 Accordingly, the silver thiolate groups should be stable toward hydrolysis, but silver ions can be selectively released by exchange between the silver thiolate groups at the surface of the monolayers and the free thiol groups exposed at the surface of the bacterial membrane proteins.18
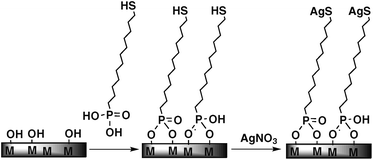 | ||
| Fig. 1 Schematic representation of the modification of a metallic surface (titanium or stainless steel) by a mercaptododecylphosphonic acid (MDPA) self-assembled monolayer and post-functionalization by reaction with silver nitrate to form silver thiolate end-groups. | ||
These monolayers are not expected to bring a permanent protection against bacteria: the amount of silver is extremely low compared to conventional, thick coatings. In addition, sooner or later the surface will be covered by a layer of proteins, cells, dead bacteria, etc., that will ‘mask’ the coating and lower its efficiency. Rather, the aim of these coatings is to prevent contamination during handling of the implant and surgery, then for the first few days after the implantation, which are considered critical.
Titanium and stainless steel samples were modified in two steps: (i) deposition of a thiol-functionalized monolayer by reaction with mercaptododecylphosphonic acid (MDPA); (ii) reaction of the terminating thiol groups with silver nitrate to form silver thiolate species. The surface was characterized by FTIR spectroscopy in grazing-incidence mode, water contact angle measurements, and X-ray photoelectron spectroscopy (XPS). The influence of the surface modification on bacterial adhesion and biofilm growth was investigated for different Gram-negative and Gram-positive bacterial strains:
Escherichia coli, genetically modified to express the green fluorescent protein (GFP), Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas aeruginosa, these four bacteria being responsible for more than 75% of orthopedic implant-related infections.1
2. Experimental
2.1 Surface modification
Absolute ethanol (VWR), pentane (VWR), HPLC-grade water (Acros), and chloroform (Carlo Erba) were used without further purification. Mercaptododecylphosphonic acid (MDPA) was prepared according to the literature.33 Stainless steel (AISI 316, Goodfellow, 0.15 mm thick) and titanium (99.7%, Aldrich, 0.127 mm thick) substrates were cut to dimensions of 18 × 18 mm for the biofilm assays, contact angle measurements and XPS characterization. Additionally, 20 × 50 mm samples were used for FTIR spectroscopy. The substrates were first washed by sonication in pentane for 4 min, then physisorbed organic species were removed by a 30 min treatment in a UV-O3 reactor. The freshly cleaned substrates were immersed in a 1 mM solution of MDPA in degassed ethanol for 48 h at 25 °C, then thoroughly rinsed with copious amounts of ethanol, water and chloroform, and dried under argon flow. The samples modified by MDPA were then treated by immersion in a 1 mM solution of AgNO3 in degassed water for 2 h in the dark at room temperature, to form the silver thiolate species. The samples were then thoroughly rinsed with copious amounts of water, ethanol, and chloroform and dried under argon flow. For comparison, bare titanium and stainless steel samples were also treated by AgNO3 in the same conditions.2.2 Characterization techniques
Water contact angle measurements were performed on a sessile drop video capture apparatus (Digidrop Fast 60 from GBX) using 2 µL drops of HPLC-grade water. All angles reported here are the averages of measurements on at least five drops. FTIR spectroscopy was performed using a Perkin-Elmer Spectrum 2000 spectrometer equipped with a MIR source and an MCT detector; the spectra were recorded at a resolution of 4 cm−1 in grazing-incidence mode, using a 80° angle of incidence and accumulating 128 scans. XPS analysis was carried out using a Gammadata Scienta SES 2002 X-ray photoelectron spectrometer under ultra-high vacuum (P < 10−9 mbar). The monochromated Al Kα source was operated at a current of 30 mA and 14 kV, with a 90° nominal take-off angle (angle between the sample surface and photoemission direction). The samples were outgassed in several ultra-high vacuum chambers with an isolated pumping system and pressure control until transfer to the analysis chamber. This ensured a complete removal of all degassing species. The samples were analyzed without further cleaning process and the adventitious carbon contamination is still on the surface. During acquisition, the pass energy was set to 500 eV for survey spectrum and 200 eV for high-resolution spectra. With this last pass energy setting, the resolution can be estimated to 0.05 eV for the position of the peak. Classical Scofield sensitivity factors were used for peak fitting procedures with CASAXPS software: C1s 1.00, O1s 2.83, Ti2p 7.81, S2p 1.68, Ag3d 18, and P2p 1.19. All lineshapes used in peak fitting procedures were a mix of 30% Gaussian and 70% Lorentzian shapes. To limit errors on background determination due to the low signal to noise ratio for the S2p high-resolution spectra, the two limit points of the Shirley-type background were averaged on 21 experimental points.2.3 Bacterial adhesion and biofilm assays
Bacterial adhesion and biofilm assays were performed using different clinical strains involved in biofilm formation: S. aureus NSA4201, S. epidermidis NSE4494, P. aeruginosa PA5903 and E. coli NECS19923 isolated from hospitalized patients. To allow the direct observation of adherent bacteria by fluorescence microscopy, the NECS19923 strain was genetically modified to express green fluorescent protein (GFP) using a pBBR-derived non-mobilisable plasmid carrying a GFP expression cassette as described in the ESI.†3. Results
3.1 Monolayer deposition and characterization
Thiol-terminated SAMs were deposited on the titanium and stainless steel substrates by reaction with 1 mM MDPA solutions in ethanol. Preliminary experiments indicated that the contact angle values reached a plateau after about 30 h; accordingly, the reaction time was fixed to 48 h. The water contact angle values measured for MDPA monolayers on titanium or stainless steel were close to 90°, indicating that these thiol-terminated SAMs were slightly less hydrophobic than methyl-terminated SAMs (100–110°).28 Further reaction of the thiol-terminated SAMs with AgNO3 did not significantly modify the contact angle values (Fig. 2).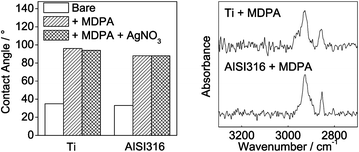 | ||
| Fig. 2 Water contact angle (left) and grazing-incidence FTIR spectra (right) of Ti and AISI316 substrates modified by MDPA and/or MDPA + AgNO3. | ||
The grazing-incidence FTIR spectra displayed in Fig. 2 confirmed the binding of MDPA to the surface, with peaks at 2852 cm−1 (νsCH2) and 2924 cm−1 (νasCH2) on both the titanium and the stainless steel samples. Such peak shifts suggest the formation of moderately ordered monolayers, compared to alkylphosphonic acid monolayers deposited on similar substrates.28
XPS analysis confirmed the effectiveness of the surface modification on both titanium and stainless steel substrates. The survey scans showed the presence of the expected elements, P, C, S, and, after treatment with AgNO3, Ag. The percentage atomic compositions of the samples were similar for both substrates (Table 1), except for the relatively high carbon content observed for the AISI316 + MDPA sample, likely due to surface contamination. The P/S ratio was consistent with the theoretical ratio of 1. After reaction with AgNO3, the Ag/S ratios were close to 1. Interestingly, the Ag content on the titanium and stainless steel substrates treated by AgNO3 only was not negligible, about 2 at%.
| Sample | C (at%) | S (at%) | P (at%) | Ag (at%) | C/S | P/S | Ag/S |
|---|---|---|---|---|---|---|---|
| Ti + MDPA | 38.8 | 3.0 | 3.3 | — | 13 | 1.1 | — |
| Ti + MDPA + AgNO3 | 38.5 ± 2.2 | 2.4 ± 0.4 | 2.7 ± 0.6 | 2.5 ± 0.4 | 16 | 1.1 | 1.0 |
| Ti + AgNO3 | 20.5 | — | — | 2.4 | — | — | — |
| AISI316 + MDPA | 52.9 | 3.4 | 3.5 | — | 16 | 1.0 | — |
| AISI316 + MDPA + AgNO3 | 40.1 ± 3.2 | 5.1 ± 1.4 | 3.8 ± 0.7 | 3.5 ± 0.6 | 8 | 0.7 | 0.7 |
| AISI316 + AgNO3 | 27.8 | — | — | 1.6 | — | — | — |
The results of the high-resolution O1s scans of the samples modified by MDPA are displayed in Table 2. The spectra were resolved into three components at about 530.1, 531.6 and 532.9 eV. The major component at 530.1 eV was assigned to the surface metal oxide species. According to the literature, the component at about 531.6 eV was ascribed to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O–M sites, the component at about 532.9 eV to P–OH sites.25,29 In these studies, the ratio of the peak at 531.6 eV to that at 532.9 eV was 2.1–2.5, instead of 0.5 for unbound phosphonic acid, thus consistent with the covalent bonding of the phosphonic acids to the metal surface by conversion of P–OH sites to P–O–M sites. In our case, the ratios obtained were 3.2–3.7. These higher values probably resulted from the presence of oxidized sulfur species (as discussed below), which would account for about 25% of the component at 531.6 eV.36 Corrected ratios (2.4–2.9) were similar to those previously reported and confirmed the formation of P–O–M bonds in our monolayers. The high-resolution S2p scans of the modified samples confirmed the presence of free thiol groups after grafting with MDPA, and the formation of silver thiolate groups after reaction with AgNO3 (Fig. 3).
O and P–O–M sites, the component at about 532.9 eV to P–OH sites.25,29 In these studies, the ratio of the peak at 531.6 eV to that at 532.9 eV was 2.1–2.5, instead of 0.5 for unbound phosphonic acid, thus consistent with the covalent bonding of the phosphonic acids to the metal surface by conversion of P–OH sites to P–O–M sites. In our case, the ratios obtained were 3.2–3.7. These higher values probably resulted from the presence of oxidized sulfur species (as discussed below), which would account for about 25% of the component at 531.6 eV.36 Corrected ratios (2.4–2.9) were similar to those previously reported and confirmed the formation of P–O–M bonds in our monolayers. The high-resolution S2p scans of the modified samples confirmed the presence of free thiol groups after grafting with MDPA, and the formation of silver thiolate groups after reaction with AgNO3 (Fig. 3).
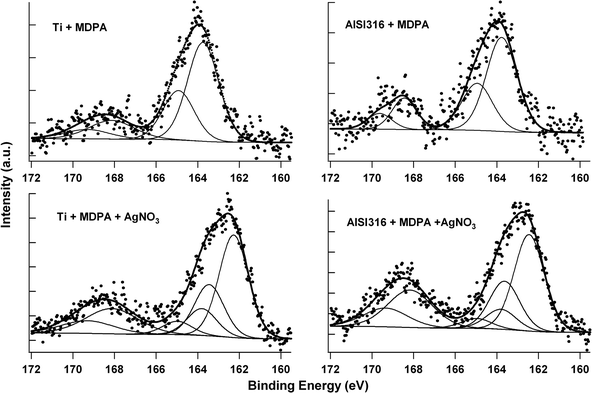 | ||
| Fig. 3 High-resolution S2p spectra of the Ti (left) and AISI316 (right) substrates, after modification by MDPA then AgNO3. | ||
The S2p peaks have a doublet structure due to the spin–orbit coupling with a split of 1.18 eV and a 2 : 1 peak area ratio. The spectra of the samples modified by MDPA only showed a doublet with a maximum at a binding energy of about 163.8 eV, characteristic of the S2p3/2 peak of unbound thiols or disulfide species.37 In addition, another doublet at a higher binding energy (ca. 168.1 eV) indicated the presence of oxidized sulfur species, most likely sulfonates.38 The absence of a doublet at about 162 eV, characteristic of thiolate species bound to stainless steel39 or a metal,40 indicated that the MDPA molecules were not anchored to the surface by the thiol end. Peak fitting indicated that the component arising from the free thiol groups represented about 80% of the total integrated S2p peak area (Table 3).
| Sample | Binding energya/eV | FWHM/eV | % area | Attribution |
|---|---|---|---|---|
| a For the S2p3/2 component. | ||||
| Ti + MDPA | 163.7 | 1.77 | 78.6 | Unbound thiols |
| 168.1 | 2.75 | 21.4 | Oxidized sulfur species | |
| Ti + MDPA + AgNO3 | 162.3 | 1.80 | 58.9 | Silver thiolates |
| 164.5 | 1.80 | 15.4 | Unbound thiols | |
| 168.2 | 2.77 | 25.7 | Oxidized sulfur species | |
| AISI316 + MDPA | 163.7 | 1.94 | 79.1 | Unbound thiols |
| 168.5 | 2.44 | 20.9 | Oxidized sulfur species | |
| AISI316 + MDPA + AgNO3 | 162.5 | 1.75 | 56.2 | Silver thiolates |
| 164.5 | 1.75 | 11.6 | Unbound thiols | |
| 168.2 | 2.42 | 32.2 | Oxidized sulfur species | |
After reaction with AgNO3, the doublet corresponding to unbound thiols strongly decreased and a major doublet appeared at a lower binding energy (ca. 162.4 eV). This doublet at about 162.4 eV could be ascribed to silver thiolate species, as previously reported for alkanethiols assembled on silver surfaces40 or for alkanedithiols assembled on a gold surface and reacted with silver ions.41 Peak fitting indicated that the component arising from the silver thiolate species represented about 60% of the total integrated S2p peak area, free thiol groups accounting for 10–15% and oxidized species for 25–30%.
3.2 Bacterial adhesion
In order to assess the influence of the surface functionalization on bacterial adhesion, the different samples were first infected by incubating them for 1 h at 37 °C in a bacterial culture, then rinsing to remove non-adherent bacteria, and incubation in a sterile medium for 24, 48, or 72 h.In the case of the E. coli strain, fluorescence microscopy images demonstrated the high antibacterial efficiency of the surface modification by MDPA + AgNO3 (Fig. 4A). After incubation for 72 h, the surface of the unmodified samples was completely covered by a dense layer of bacteria, whereas the samples modified by MDPA then AgNO3 were practically bacteria-free. The bacterial counts (Fig. 4B) indicated that whatever the incubation time the population of viable adherent bacteria was comprised between 104 and 105 CFU on unmodified titanium and stainless steel samples. Surface modification by MDPA alone did not significantly change bacterial adhesion: surface modification by AgNO3 alone led to a significant decrease in the bacterial adhesion (p < 0.05), but the number of adherent bacteria remained relatively high, 102 to 103CFU per sample. On the other hand, very few viable bacterial cells (less than 10 CFU) were found on the samples modified by MDPA then AgNO3, which corresponds to a 3- to 4-log reduction of the number of viable adherent bacteria compared to the bare titanium or stainless steel surface.
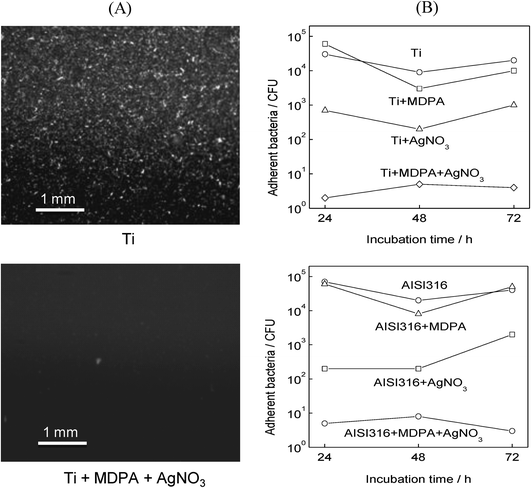 | ||
| Fig. 4 Effect of MDPA and AgNO3 treatment on the adhesion of E. coli: (A) fluorescence microscopy images of unmodified Ti (top) and Ti + MDPA + AgNO3 (bottom) after incubation for 1 h in a culture of E. coli. and for 3 days in a sterile medium; (B) total population of viable adherent bacteria (in CFU per sample) on titanium (top), and stainless steel (bottom) samples after incubation for 1 h in a E. coli culture and for 1, 2 and 3 days in a sterile medium. | ||
As shown in Fig. 5, the MDPA + AgNO3 coating also exhibited an excellent antimicrobial efficiency toward the bacteria that are responsible for the vast majority of orthopedic implant-related infections, S. aureus, S. epidermidis, and P. aeruginosa. In all cases, a 4- to 5-log reduction of the number of viable adherent bacteria was observed on the titanium or stainless steel substrates modified by MDPA then AgNO3, compared to the bare substrates.
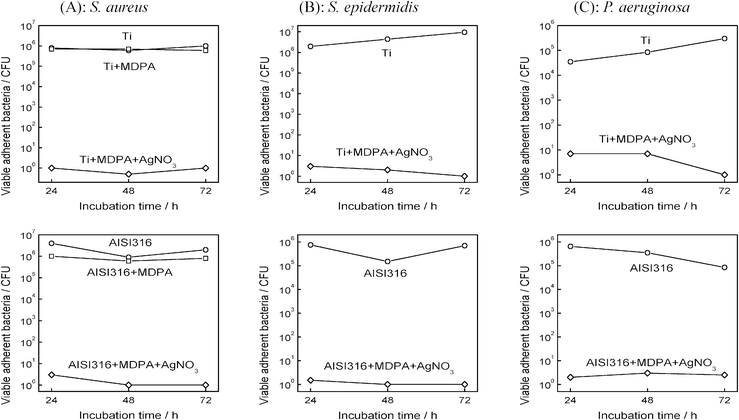 | ||
| Fig. 5 Effect of MDPA and AgNO3 treatment on the adhesion of (A) S. aureus, (B) S. epidermidis and (C) P. aeruginosa: total population of viable adherent bacteria (in CFU per sample) on titanium (top) and stainless steel (bottom) samples, after incubation for 1 h in a bacterial culture and for 1, 2 and 3 days in a sterile medium. | ||
The stability of the coating in a blood-mimicking medium was investigated by immersing Ti + MDPA + AgNO3 samples for 1–7 days at 37 °C in simulated body fluid (SBF) with ion concentrations close to those of human blood plasma. As shown in Fig. 6A, the water contact angle of Ti + MDPA + AgNO3 samples decreased with the immersion time in SBF, but remained significantly higher than that of bare Ti samples. XPS analysis of the Ti + MDPA + AgNO3 samples indicated that the surface concentration of Ag and S decreased continuously with the immersion time, whereas the surface concentration of P remained nearly constant (Fig. 6B). These results were consistent with a partial substitution of the surface phosphonate anchors by phosphate ions of the SBF, leading to a partial removal of the coating.
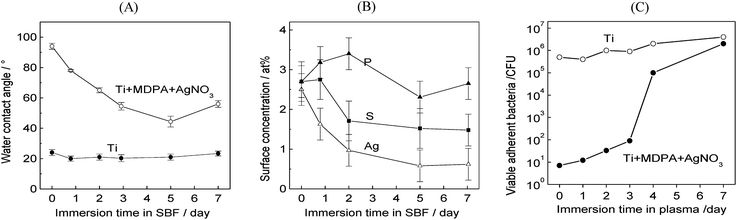 | ||
| Fig. 6 Stability of the MDPA + AgNO3 coating on Ti in blood-mimicking fluids: (A) influence of the immersion time in SBF on the water contact angle; (B) influence of the immersion time in SBF on the surface P, S, and Ag atomic concentration; (C) influence of the immersion time in fresh human blood plasma on the total population of viable adherent bacteria (in CFU per sample) on titanium (top) and titanium coated by MDPA + AgNO3 (bottom) samples (the samples were incubated for 1 h in a S. epidermidis culture and for 1 day in a sterile medium). | ||
The antibacterial efficiency of the MDPA + AgNO3 coating as a function of the immersion time in fresh human blood plasma at 37 °C was investigated for the S. epidermidis strain. After immersion in plasma for 1–7 days, the samples were incubated for 1 h in a S. epidermidis culture and for 1 day in a sterile medium. As shown in Fig. 6C, the antibacterial efficiency remained excellent for up to 3 days, with a 4-log reduction of the number of viable adherent bacteria on the coated substrate compared to the bare substrate. The number of viable adherent bacteria on the coated samples increased sharply after 4 days of immersion in plasma and after 7 days of immersion the number of viable adherent bacteria on the coated sample and on the bare Ti sample were not significantly different.
3.3 Inhibition of biofilm growth
The influence of the surface functionalization on the growth of a biofilm was investigated for both Gram-positive and Gram-negative bacteria by incubating the samples for 72 h at 37 °C in a culture of the bacteria in Mueller–Hinton broth. Under these conditions, fluorescence microscopy (Fig. 7A) showed that a very dense E. coli biofilm formed on the unmodified titanium or stainless steel surfaces; in comparison, the biofilms grown on the surfaces modified by MDPA then AgNO3 were much less dense.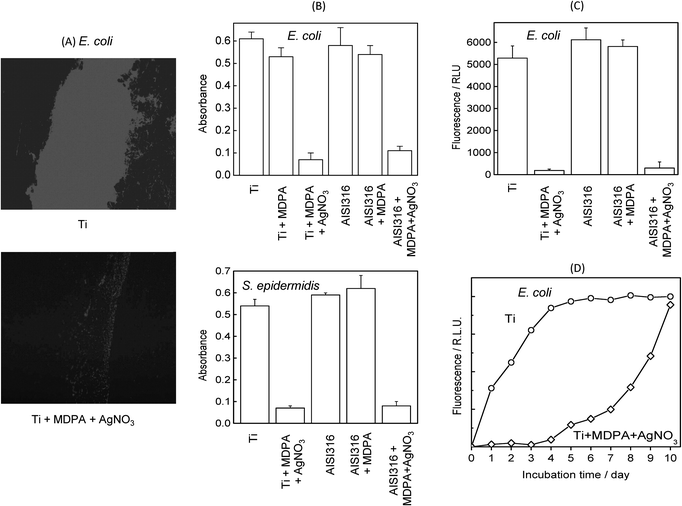 | ||
| Fig. 7 Effect of MDPA and AgNO3 treatment on biofilm growth after incubation for 3 days at 37 °C in E. coli and S. epidermidis cultures: (A) fluorescence microscopy images of E. coli biofilm on unmodified Ti (top) and Ti + MDPA + AgNO3 (bottom); (B) biofilm density on Ti and stainless steel substrates measured by colorimetry for E. coli (top) and S. epidermidis (bottom); (C) E. coli biofilm density on Ti and stainless steel substrates measured by fluorometry; (D) kinetics of E. colibiofilm formation monitored by fluorometry. | ||
The influence of surface modification on the density of the E. coli and S. epidermidis biofilm measured by colorimetry after crystal violet staining is displayed in Fig. 7B. Whatever the substrate (titanium or stainless steel), the density of biofilm on the samples modified by MDPA then AgNO3 was reduced by about 85% compared to the bare samples. On the other hand, the biofilm density on the unmodified samples and the samples modified by MDPA were not significantly different. Similar results were obtained for the P. aeruginosa strain (not shown).
Evaluation of the density of the E. coli biofilm by fluorometry (Fig. 7C) led to qualitatively similar results, confirming the antibacterial activity of the MDPA + AgNO3 coatings. The kinetics of formation of the E. coli biofilm was followed using fluorometry (Fig. 7D). On the unmodified titanium sample the density of the biofilm increased rapidly, reaching a plateau after about 4 days. On the other hand, on the sample modified by silver thiolate species the biofilm formation was completely inhibited for 4 days, and the growth rate was significantly reduced for about 1 week.
4. Discussion
The present study demonstrates the potential of phosphonate SAMs functionalized by silver thiolate species as antibacterial nanocoatings for inorganic biomaterials. The surface modification was performed simply by immersion in dilute MDPA and AgNO3 solutions. A combination of water contact angle measurements, FTIR and XPS spectroscopy was used to characterize the surface modification process. Atomic force microscopy or ellipsometry measurements were not possible due to the high roughness of the substrates. The FTIR spectra indicated that reaction of titanium or stainless steel substrates with MDPA led to the formation of moderately ordered monolayers.The formation of n-alkylthiol self-assembled monolayers on stainless steel has already been reported.39 Accordingly, MDPA molecules could be linked to the surface by the phosphonate or the thiol end, or both ends. However, no thiolate species bound to the metal surface were detected by XPS analysis, indicating that MDPA molecules were only linked to the surface by the phosphonate end. XPS analysis confirmed the covalent bonding of the monolayers by formation of M–O–P bonds at the expense of P–OH bonds. P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O–M species cannot be distinguished by XPS, but a solid state 17O NMR study indicated that the bonding of phosphonic acids to TiO2 also involved the formation of P–O–M bonds by coordination of phosphoryl oxygens to surface metal atoms.42XPS analysis indicated that the monolayers were predominantly thiol-terminated, although about 20% of the thiols groups were oxidized, despite the use of degassed solvents. This partial oxidation as well as the high roughness of the substrates probably accounted for the modest degree of ordering and the relatively low water contact angle values, close to 90°.
O and P–O–M species cannot be distinguished by XPS, but a solid state 17O NMR study indicated that the bonding of phosphonic acids to TiO2 also involved the formation of P–O–M bonds by coordination of phosphoryl oxygens to surface metal atoms.42XPS analysis indicated that the monolayers were predominantly thiol-terminated, although about 20% of the thiols groups were oxidized, despite the use of degassed solvents. This partial oxidation as well as the high roughness of the substrates probably accounted for the modest degree of ordering and the relatively low water contact angle values, close to 90°.
Post-modification with AgNO3 resulted in the conversion of most of the terminal thiol groups into silver thiolate species, which represented about 60% of all sulfur species in the final samples.
A grafting density of 4.3 molecules per nm2 has been reported for alkylphosphonic acid SAMs.43 From this value and the Ag/S ratio determined by XPS, the density of silver at the surface of the samples modified by MDPA then AgNO3 can be estimated to 3.5 ± 1 Ag nm−2, corresponding to about 0.6 nmol Ag cm−2 or 60 ng of silver per cm2. Thus, the amount of silver in our samples was very low compared to other antibacterial silver-coated materials reported in the literature. For instance, the silver content in stainless steel or titanium samples modified by ion implantation19,20 or by physical vapor deposition of Ti/Ag21 ranged from 80 to 1700 nmol Ag cm−2.
Despite their very low silver content, MDPA + AgNO3 monolayers strongly decreased the bacterial adhesion of the surface compared to the bare titanium or stainless steel substrates or to the samples modified by MDPA only: a 3- to 5-log reduction in the number of viable adherent bacteria was found for the four bacterial strains tested (E. coli, S. aureus, S. epidermidis and P. aeruginosa). In the adhesion assays, if all the silver on the MDPA + AgNO3 samples (ca. 6.5 cm2, 4 nmol Ag) was released in the culture medium (3 mL), the silver concentration in the well would be about 1.2 µM, thus about 50 times lower than the Ag+ minimum inhibitory concentration (MIC 0.06 ± 0.02 mM) or the minimum biofilm eradication concentration (MBEC 0.07 ± 0.02 mM) reported for E. coli.44 The efficiency of MDPA + AgNO3 monolayers confirmed the importance of the localization of the bactericidal species directly at the surface.1
The antibacterial efficiency of MDPA + AgNO3 monolayers remained excellent even after incubation for 3 days at 37 °C in fresh human blood plasma, with a 4-log reduction of the number of viable adherent bacteria on the coated substrate compared to the bare substrate.
The MDPA + AgNO3 coating deposited on titanium or stainless steel also strongly decreased (by about 85%) the density of bacterial biofilm formed after incubation for 3 days in a culture of E. coli, S. epidermidis or P. aeruginosa. In addition, the growth of E. coli biofilm on titanium modified by MDPA + AgNO3 was significantly inhibited for about 1 week.
Interestingly, XPS analysis of bare titanium or stainless steel substrates treated by AgNO3 showed the presence of about 2 at% Ag. The binding of Ag ions to the surface possibly resulted from the formation M–O–Ag bonds. However, the antibacterial efficiency was much lower than that observed for samples treated by MDPA and AgNO3 (2.5–3.5 at% Ag). Thus, the deposition of a thiol-terminated monolayer prior to silver nitrate treatment was necessary for a good antibacterial efficiency.
The use of silver thiolate functions for the controlled delivery of silver is quite original. Thiol groups react readily with silver cations to form silver thiolates with very high formation constants (about 1012).32 Accordingly, in our monolayers the release of silver ions by hydrolysis of the silver thiolate groups should be negligible. It is generally agreed that the reaction of Ag+ ions with thiol groups in the bacterial membrane proteins plays an essential role in bacterial inactivation.17,18 Thus, the antibacterial effect observed in this study could result from the exchange of silver between the thiolate groups at the surface of the MDPA/AgNO3 monolayers and the free thiol groups exposed at the surface of the bacterial membrane proteins.18
The approach presented here should apply to practically all the metallic or ceramic biomaterials currently employed in orthopedic applications. Indeed, phosphonic acids bind to metals (titanium, titanium alloys, stainless steel), metal oxides (alumina, zirconia), and calcium phosphates.24 Antibacterial phosphonate monolayers could also be easily applied to modify composite orthopedic implants with metallic and ceramic parts, for instance.
5. Conclusions
In this article, we report a new approach to confer antibacterial properties to inorganic (bio)materials, based on the grafting of phosphonate monolayers functionalized by silver thiolate species. This nanometer-thick coating deposited on titanium or stainless steel drastically decreased bacterial adhesion for the four bacterial strains tested (E. coli, S. aureus, S. epidermidis and P. aeruginosa). Moreover, the growth of a biofilm was significantly inhibited for about 1 week. The antibacterial effect was obtained for an extremely low Ag content compared to conventional coatings, which is important to avoid any toxicity issue and to minimize the release of silver in the environment, which could facilitate the selection of resistant strains. In addition, deposition of the monolayers and subsequent modification with Ag required only simple immersion procedures at room temperature in non-toxic solvents. These results suggest that these monolayers could be useful for the prevention of infections resulting from contamination during the surgical insertion of an orthopedic implant.Acknowledgements
CNRS, INSERM and the Languedoc-Roussillon Region are thanked for financial support.References
- D. Campoccia, L. Montanaro and C. R. Arciola, Biomaterials, 2006, 27, 2331–2339 CrossRef CAS.
- C. Defez, P. Fabbro-Peray, M. Cazaban, T. Boudemaghe, A. Sotto and J. P. Daurès, J. Hosp. Infect., 2008, 68, 130–136 CrossRef CAS.
- J. W. Costerton, L. Montanaro and C. R. Arciola, Int. J. Artif. Organs, 2005, 28, 1062–1068 Search PubMed.
- P. Wu and D. W. Grainger, Biomaterials, 2006, 27, 2450–2467 CrossRef CAS.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS.
- E. Ostuni, R. G. Chapman, M. N. Liang, G. Meluleni, G. Pier, D. E. Ingber and G. M. Whitesides, Langmuir, 2001, 17, 6336–6343 CrossRef CAS.
- V. A. Tegoulia and S. L. Cooper, Colloids Surf., B, 2002, 24, 217–228 CrossRef CAS.
- R. J. I. V. Emerson, T. S. Bergstrom, Y. Liu, E. R. Soto, C. A. Brown, W. G. McGimpsey and T. A. Camesano, Langmuir, 2006, 22, 11311–11321 CrossRef CAS.
- Y. Liu, J. Strauss and T. A. Camesano, Langmuir, 2007, 23, 7134 CrossRef CAS.
- L. Ploux, S. Beckendorff, M. Nardin and S. Neunlist, Colloids Surf., B, 2007, 57, 174–181 CrossRef CAS.
- S. Hou, E. A. Burton, K. A. Simon, D. Blodgett, Y.-Y. Luk and D. Ren, Appl. Environ. Microbiol., 2007, 73, 4300–4307 CrossRef CAS.
- B. Gottenbos, H. C. van der Mei, F. Klatter, P. Nieuwenhuis and H. J. Busscher, Biomaterials, 2002, 23, 1417–1423 CrossRef CAS.
- B. Jose, V. J. Antoci, A. R. Zeiger, E. Wickstrom and N. J. Hickok, Chem. Biol., 2005, 12, 1041–1048 CrossRef CAS.
- S. Silver, T. Phung Le and G. Silver, J. Indust. Microbiol. Botechnol., 2006, 33, 627–634 Search PubMed.
- M. Bosetti, A. Masse, E. Tobin and M. Cannas, Biomaterials, 2002, 23, 887–892 CrossRef CAS.
- G. Gosheger, J. Hardes, H. Ahrens, A. Streitburger, H. Buerger, M. Erren, A. Gunsel, F. H. Kemper, W. Winkelmann and C. von Eiff, Biomaterials, 2004, 25, 5547–5556 CrossRef CAS.
- Q. L. Feng, J. Wu, G. Q. Chen, F. Z. Cui, T. N. Kim and J. O. Kim, J. Biomed. Mater. Res., 2000, 52, 662–668 CrossRef CAS.
- R. L. Davies and S. F. Etris, Catal. Today, 1997, 36, 107–114 CrossRef CAS.
- J. Zhao, H. J. Feng, H. Q. Tang and J. H. Zheng, Surf. Coatings Technol., 2007, 201, 5676–5679 Search PubMed.
- Y. Z. Wan, S. Raman, F. He and Y. Huang, Vacuum, 2007, 81, 1114–1118 CrossRef CAS.
- A. Ewald, K. Gluckermann Susanne, R. Thull and U. Gbureck, Biomed. Eng. Online, 2006, 5, 22 CrossRef.
- H.-J. Jeon, S.-C. Yi and S.-G. Oh, Biomaterials, 2003, 24, 4921–4928 CrossRef CAS.
- W. Chen, S. Oh, A. P. Ong, N. Oh, Y. Liu, H. S. Courtney, M. Appleford and J. L. Ong, J. Biomed. Mater. Res., Part A, 2007, 82A, 899–906 CrossRef CAS.
- P. H. Mutin, G. Guerrero and A. Vioux, J. Mater. Chem., 2005, 15, 3761–3768 RSC.
- N. Adden, L. J. Gamble, D. G. Castner, A. Hoffmann, G. Gross and H. Menzel, Langmuir, 2006, 22, 8197–8204 CrossRef CAS.
- C. Viornery, H. L. Guenther, B.-O. Aronsson, P. Pechy, P. Descouts and M. Gratzel, J. Biomed. Mater. Res., 2002, 62, 149–155 CrossRef CAS.
- J. G. Van Alsten, Langmuir, 1999, 15, 7605–7614 CrossRef.
- A. Raman, M. Dubey, I. Gouzman and S. Gawalt Ellen, Langmuir, 2006, 22, 6469–6472 CrossRef CAS.
- G. Zorn, I. Gotman, E. Y. Gutmanas, R. Adadi, G. Salitra and C. N. Sukenik, Chem. Mater., 2005, 17, 4218–4226 CrossRef CAS.
- A. Berman, S. Steinberg, S. Campbell, A. Ulman and J. Israelachvili, Tribol. Lett., 1997, 4, 43–48.
- J. Soullier, P. Tordjeman and P. H. Mutin, Mater. Res. Soc. Symp. Proc., 2005, 847, 299–304.
- N. W. H. Adams and J. R. Kramer, Aquat. Geochem., 1999, 5, 1–11 CrossRef CAS.
- M.-Y. Tsai and J.-C. Lin, J. Biomed. Mater. Res., 2001, 55, 554–565 CrossRef CAS.
- D. J. Balazs, K. Triandafillu, P. Wood, Y. Chevolot, C. van Delden, H. Harms, C. Hollenstein and H. J. Mathieu, Biomaterials, 2004, 25, 2139–2151 CrossRef CAS.
- G. A. O'Toole and R. Kolter, Mol. Microbiol., 1998, 28, 449–461 CrossRef CAS.
- D. Briggs and G. Beamson, Anal. Chem., 1993, 65, 1517–1523 CrossRef CAS.
- A.-S. Duwez, J. Electron Spectrosc. Relat. Phenom., 2004, 134, 97–138 CrossRef CAS.
- H. Rieley, G. K. Kendall, F. W. Zemicael, T. L. Smith and S. Yang, Langmuir, 1998, 14, 5147–5153 CrossRef CAS.
- C.-M. Ruan, T. Bayer, S. Meth and C. N. Sukenik, Thin Solid Films, 2002, 419, 95–104 CrossRef CAS.
- P. E. Laibinis, G. M. Whitesides, D. L. Allara, Y. T. Tao, A. N. Parikh and R. G. Nuzzo, J. Am. Chem. Soc., 1991, 113, 7152–7167 CrossRef CAS.
- M. J. Esplandiu and P. L. M. Noeske, Appl. Surf. Sci., 2002, 199, 166–182 CrossRef CAS.
- V. Lafond, C. Gervais, J. Maquet, D. Prochnow, F. Babonneau and P. H. Mutin, Chem. Mater., 2003, 15, 4098–4103 CrossRef CAS.
- R. Helmy and A. Y. Fadeev, Langmuir, 2002, 18, 8924–8928 CrossRef CAS.
- J. J. Harrison, H. Ceri, C. A. Stremick and R. J. Turner, Environ. Microbiol., 2004, 6, 1220–1227 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: genetic modification of E. coli gfp+ strain. See DOI: 10.1039/b813344a |
| This journal is © The Royal Society of Chemistry 2009 |
