Pd nanoparticles immobilized on sepiolite by ionic liquids: efficient catalysts for hydrogenation of alkenes and Heck reactions
Ranting
Tao
,
Shiding
Miao
,
Zhimin
Liu
*,
Yun
Xie
,
Buxing
Han
,
Guimin
An
and
Kunlun
Ding
Institute of Chemistry, The Chinese Academy of Sciences, Beijing, 100190, China. E-mail: liuzm@iccas.ac.cn
First published on 12th November 2008
Abstract
Palladium-sepiolite catalysts were prepared by immobilizing Pd2+ on sepiolite using an ionic liquid containing a guanidine cation, followed by reduction with hydrogen at 150 °C. The resulting composites were characterized by different techniques. X-Ray photoelectron spectroscopy analysis showed that the loaded Pd existed mainly in the form of Pd0, with a small amount of its oxides, and distributed uniformly on sepiolite with particle size about 5 nm, as confirmed by transmission electron microscopy examination. X-Ray diffraction analysis indicated that the sepiolite retained its original structure after deposition of Pd nanoparticles. The activities of the Pd-sepiolite catalysts for hydrogenations of some alkenes (e.g., cyclohexene and 1,3-cyclohexdiene) and Heck reactions were investigated. It was demonstrated that the as-prepared catalysts exhibited very high efficiency for these reactions.
Introduction
With the development of green chemistry, the design and preparation of green catalysts have attracted much attention recently, among which heterogeneous catalysts have been widely investigated. Natural clays are environmentally friendly materials, which are good catalyst supports because of their crystalline structures and large surface areas. Sepiolite, a kind of natural clay mineral with a formula of Si12Mg8O30(OH)6(OH2)4·8H2O, has a very special place among natural clay minerals due to its relatively high surface area and excellent ability to adsorb molecular, ionic, and polymeric species.1,2 Similar to other types of silicate minerals, it contains continuous two-dimensional tetrahedral sheets of T2O5 (T = Si, Al, Be,…), but has no continuous octahedral sheets.3 Owing to its special structure and properties, sepiolite has been widely used in a variety of industrial applications.4–6Ionic liquids (ILs), possessing negligible vapor pressure, reasonable thermal stability, strong ability to dissolve a wide range of organic, inorganic and organometallic compounds, have promising applications in many fields.7,8 In particular, ILs offer new opportunities for the development of catalytic materials. In industrial processes, heterogeneous catalysis is generally preferred to homogeneous catalysis, since the separation of the product and recovery of the catalyst are made easier.9 Therefore, the immobilization of ILs onto solid supports and the preparation of heterogeneous catalysts assisted by ILs have attracted significant attention.10–12 For example, Brønsted acid ILs were immobilized on solid supports, which displayed excellent recyclability and high activity for a range of esterification and nitration reactions.13Choline hydroxide was supported on MgO and shown to be an efficient catalyst for the aldol reaction for a range of aldehyde and ketone substrates.14 Metal nanoparticles (e.g.Pd, Rh, Ru, etc.) were supported on different solid supports with the aid of ILs, exhibiting high efficiency for hydrogenations of alkenes and arenes.15,16 The main advantage of using the supported IL system is that it requires significantly reduced amounts of the ionic media, which is favorable from an economic and toxicological point of view.
In our previous work, we developed an approach to support noble metal nanoparticles (e.g., Ru, Rh) on natural clays by ILs, and the resultant catalysts showed high efficiency for hydrogenations of benzene16 and cyclohexene.17 In this work, we extended this approach to immobilize Pd nanoparticles on sepiolite with the aid of an IL, aiming at developing new green catalysts. The as-prepared Pd catalysts were characterized in detail by different techniques, including X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TG), scanning electron microscopy (SEM), transmission electron microscopy (TEM), inductively coupled plasma-atomic emission spectrometer (ICP-AES), X-ray diffraction (XRD), N2 sorption, and their catalytic activities for hydrogenations of alkenes and Heck reactions were investigated. The combination of sepiolite as a support with an IL may widen the application of sepiolite and help develop new kinds of green catalysts as well.
Experimental
Starting materials
The sepiolite clay, provided by the Mingyang company, was first treated as follows. 10 g of sepiolite was immersed in 20 mL of 1 M HNO3 solution at room temperature with stirring for 24 h. Then, the suspension was filtered and the resulting solid was washed with deionized water for ten times before drying at 105 °C for 24 h. The treated clay was pulverized and sieved down to 120 mesh, which was designated as SH.Other reagents including HNO3 (65%–68%), HCl (36%–38%), PdCl2, cyclohexene (AR), N,N-dimethylformamide (AR), methyl acrylate (AR) and triethylamine (AR) were purchased from the Beijing Chemical Reagent Company. 1,3-Cyclohexdiene was obtained from ACROS, and iodobenzene (98%) was provided by Alfa Aesar. The ILs (including 1,1,3,3-tetramethylguanidine trifluoroacetic acid, TMG+TFA−; 1,1,3,3-tetramethylguanidine lactic acid, TMG+LA− and 1,1,3,3-tetramethylguanidine acetic acid, TMG+AA−) were synthesized directly by neutralization of 1,1,3,3-tetramethylguanidine (TMG) with proportionable acid based on the procedures reported previously.18,19
Preparation of SH-IL-Pd catalysts
The catalyst SH-IL-Pd containing 1.0 wt% Pd (denoted as SH-IL-1.0%Pd) was prepared in the following way. 10.0 g of the activated sepiolite (i.e., SH) was dispersed in 50 mL of aqueous solution containing 0.7 g of TMG+TFA−, and stirred for about 6 h. Then the sepiolite was separated viacentrifugation, and treated with fresh IL aqueous solution again. These procedures were repeated three times. The sepiolite treated with IL aqueous solution was dried at 105 °C for 24 h and named SH-IL. 1.0 g of SH-IL was redispersed in 10.0 mL of H2PdCl4 (1M PdCl2 + 2M HCl) aqueous solution with a concentration of 1.0 mg/mL. Then the clay was dried at 60 °C under vacuum after the water was removed viaevaporation, and denoted as SH-IL-Pd2+. SH-IL-Pd2+ was hydrogenated with H2 at 150 °C for 4 h, resulting in a composite, designated as SH-IL-1.0 wt%Pd, since the mass content of Pd in the composite was 1.0 wt%, calculated on the basis of the amounts of the clay and the PdCl2 used. The catalyst containing 2.5 wt% Pd was synthesized following the same procedure, named SH-IL-2.5%Pd. Instead of TMG+TFA−, other ILs including TMG+LA−, TMG+AA− and TMG were also used to prepare the Pd catalysts in the similar way.Characterization
TG measurements were performed on a thermal analyzer (NETZSCH STA 409 PC/PG) with a heating rate of 3 °C/min. SEM examination was carried out on a scanning electron microscope (JEOL, JSM-4300) operated in a high-vacuum mode at 15 kV, which provided general textural information of the samples. The SEM samples were sputter-coated with gold before observation. TEM observation was performed on a transmission electron microscope (JEOL, JEM 1011) at an operating voltage of 100 kV, and the images were electronically captured using a CCD camera. XPS data of the as-prepared samples were obtained with an ESCALab220i-XL electron spectrometer from VG Scientific using 300 W MgKα radiation. The base pressure was about 3 × 10−9 mbar. The binding energies were referenced to the C 1s line at 284.8 eV from adventitious carbon. XRD was performed on a X'PERT SW X-ray diffractometer operated at 30 kV and 100 mA with CuKα radiation. The loading content of Pd in the catalysts was determined by ICP-AES (VISTA-MPX). The nitrogen sorption analysis was performed on an ASAP-2405N instrument at liquid nitrogen temperature.Catalytic activity test
To evaluate the catalytic characteristics of the as-prepared samples, hydrogenations of some alkenes (e.g., cyclohexene, 1,3-cyclohexdiene, styrene and 1-hexene) and Heck reactions were investigated. Hydrogenation reactions were carried out in a 50 mL stainless steel autoclave with a magnetic stirrer at 300 rpm. In a typical experiment, 12 mmol of alkene and the required amount of catalyst were placed in the reactor, and the air in the reactor was then replaced by H2 five times. After H2 was charged into the reactor up to 2.0 MPa, the reactor was moved to an oil bath at the desired temperature. The temperature of the oil bath was controlled with a temperature controller (Haake D3) with an accuracy of 0.1 °C. The pressure of the reaction system was monitored using a pressure transducer (Foxboro/ICT model 93). As the H2 pressure remained unchanged, the product was collected viacentrifugation (12000 rpm) and analyzed by a gas chromatograph (Agilent 4890 D) equipped with an Innovax capillary column and a Varian FID-GC flame ionization detector.The Heck reaction was performed as follows. Iodobenzene (2.0 mmol), methyl acrylate (2.2 mmol), triethylamine (Et3N, 2.4 mmol), and 2 mg of SH-IL-1.0wt%Pd composite were added into a 7 mL stainless steel autoclave with a magnetic stirrer. Then the reactor was sealed and placed in an air bath of 140 °C. After a desired reaction time, the autoclave was cooled by ice water. The product was dissolved by N,N-dimethylformamide (DMF, 10.0 mL) and analyzed by GC-MS. Conversion was determined from the amount of consumed iodobenzene. Bromobenzene instead of iodobenzene was also used to test the activity of the catalyst in the similar way.
Results and discussion
Characterization of the catalysts
Before being used as catalysts, SH, SH-IL, and SH-IL-Pd were characterized in detail by means of different techniques, including XPS, TG, SEM, TEM, ICP-AES, XRD, and N2 sorption analysis.The XPS analysis was used to detect the oxidation state of all species in the composites. Fig. 1a shows the survey spectrum of SH-IL-1.0wt%Pd, which indicates that no element Cl was detectable, suggesting that PdCl2 was completely converted during the H2reduction process. From Fig. 1a, it is obvious that element N was present in the composites. Fig. 1b illustrates the N 1s spectrum, in which the peak centered at 400.6 eV is attributed to N species bounding to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and –C, which originated from the cation of the IL, suggesting that IL existed in the composites. Fig. 1c displays the XPS spectrum of Pd 3d. Compared to the binding energy values of Pd 3d in pure palladium metal,20 both the predominant peak at 335.2 eV and the deconvolved peak at 340.8 eV are attributed to Pd0 species, and the peaks at 337.1 eV and 342.9 eV are assigned to the partially oxidized palladium species, which may result from the slight oxidation of Pd0. From XPS analysis, it can be deduced that the Pd0 species are predominant in the composites. XPS analysis shows that the Pd content on the surface of SH-IL-Pd was about 0.94%, which is almost identical with the initial quantities of Pd (1.0 wt%), implying that the Pd nanoparticals were mainly decorated on the surface of sepiolite. The loading content of Pd in SH-IL-1.0wt%Pd was also determined by ICP-AES. It was demonstrated that the Pd content was 0.95 wt%, nearly equal to the value obtained from XPS analysis, further confirming that the Pd nanoparticles were uniformly distributed on the sepiolite support.
C and –C, which originated from the cation of the IL, suggesting that IL existed in the composites. Fig. 1c displays the XPS spectrum of Pd 3d. Compared to the binding energy values of Pd 3d in pure palladium metal,20 both the predominant peak at 335.2 eV and the deconvolved peak at 340.8 eV are attributed to Pd0 species, and the peaks at 337.1 eV and 342.9 eV are assigned to the partially oxidized palladium species, which may result from the slight oxidation of Pd0. From XPS analysis, it can be deduced that the Pd0 species are predominant in the composites. XPS analysis shows that the Pd content on the surface of SH-IL-Pd was about 0.94%, which is almost identical with the initial quantities of Pd (1.0 wt%), implying that the Pd nanoparticals were mainly decorated on the surface of sepiolite. The loading content of Pd in SH-IL-1.0wt%Pd was also determined by ICP-AES. It was demonstrated that the Pd content was 0.95 wt%, nearly equal to the value obtained from XPS analysis, further confirming that the Pd nanoparticles were uniformly distributed on the sepiolite support.
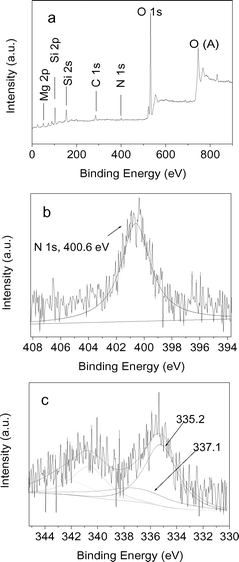 | ||
| Fig. 1 XPS spectra of SH-IL-1.0wt%Pd: (a) survey spectrum, (b) N 1s, (c) Pd 3d. | ||
To determine the loading content of IL on the sepiolite support, TG analysis for SH and SH-IL was carried out from room temperature to 700 °C at a heating rate of 3 °C min−1. At 700 °C, the residual contents of SH and SH-IL were 92.8 wt% and 91.2 wt%, respectively, which gives the information that the IL was indeed immobilized on the sepiolite support, and the loaded IL content was about 1.6 wt%.
The microstructure and morphology of the resultant composites were examined by SEM and TEM. The SEM images of SH-IL-1.0wt%Pd, as shown in Fig. 2, indicate that the sepiolite exhibited fibrous structure with an average diameter of about 100 nm and length in the range from 20 nm to 2 μm. Some fibers congregated into bundles, and a great deal of sepiolite fibers stacked up, leading to pores with different sizes. The TEM images provided more information about the morphology of SH and SH-IL-1.0wt%Pd. Fig. 2c shows a typical TEM image of SH, which indicates that sepiolite is composed of fibers with smooth surfaces. For the SH-IL-1.0wt%Pd composite, the surface of the fiber became rough, and there were numerous nanoparticles attached along the fibers. It is clear that the particle size was less than 5 nm, as shown in Fig. 2d; moreover, the TEM image also shows that the particle size distribution of the particles located on the rods was very narrow.
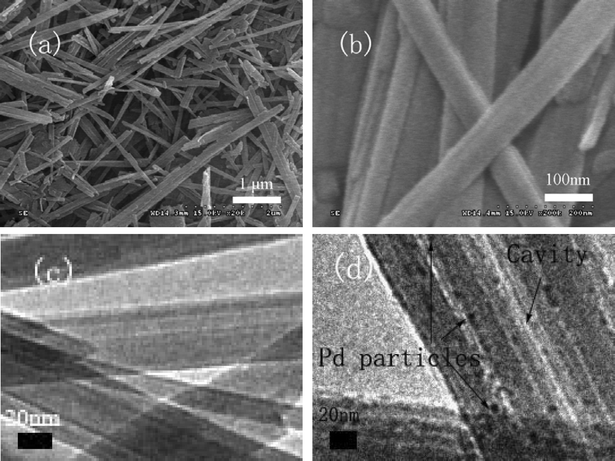 | ||
| Fig. 2 SEM images of SH (a), (b); and TEM images of SH (c) and SH-IL-Pd (d). | ||
The samples, SH, SH-IL, SH-IL-1.0wt%Pd, and SH-TMG, were also examined by XRD analysis. It was shown that these four samples exhibited almost identical XRD patterns, which suggests that the sepiolite in SH-IL, SH-IL-Pd, and SH-TMG retained its original structure without any damage. There is no expansion or collapse of the sepiolite framework, which suggests that no IL was impregnated into the channels of sepiolite. This is different from our previous work, in which the IL was intercalated into the interlayers of montmorillonite and expanded them to some extent.16 In the pattern of SH-IL-Pd, no detectable diffraction peak was assigned to Pd species in the wide angle range, which indicates that there were no large Pd particles in the SH-IL-Pd composites.
To verify the changes of the surface area and porous characteristics of the sepiolite in SH-IL and SH-IL-Pd, the as-prepared samples were evaluated by N2 sorption analysis. Fig. 3 shows the corresponding isothermal curves of SH-IL-1.0wt%Pd, which belongs to type IV sorption isotherms with hysteresis loop of type H1 according to the IUPAC classification.21 The other samples (e.g.SH, SH-IL, SH-IL-2.5wt%Pd) exhibited similar sorption isotherms to the SH-IL-1.0wt%Pd composite, implying the immobilization of IL and Pd nanoparticles did not destroy the main structure of the sepiolite. The specific surface areas of the samples were achieved by the BET method (Brunauer-Emmett-Teller) in the relative pressure range of 0.05–0.20 from nitrogen desorption isotherms,22 and the pore size distribution was determined by the BJH method (Barrett-Joyner-Halenda). The specific surface area of the clay decreased from 72.3 m2/g for the sepiolite activated by nitric acid to 40.5 m2/g for SH-IL due to the immobilization of the IL, and further to 34.6 m2/g for SH-IL-Pd, which may result from the deposition of Pd nanoparticles on the surface of sepiolite. The small hysteresis of the composite indicates less mesoporosity present in the sepiolite. From the N2 sorption analysis, it is known that the total pore volume is reduced from 0.13 mL/g for SH to 0.09 mL/g for SH-IL composites, and to 0.07 mL/g for SH-IL-Pd, due to the absorption of IL and the deposition of Pd nanoparticles. Furthermore, the pore size distribution is much broader and has no significant change after the deposition of Pd nanoparticles in the composite.
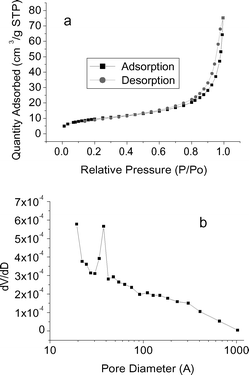 | ||
| Fig. 3 Nitrogen sorption isotherms (a) and pore size distribution (b) of SH-IL-1.0wt%Pd. | ||
Catalytic activities for hydrogenations and Heck Reactions of the catalysts
To evaluate their catalytic characteristics, the as-prepared SH-IL-Pd composites were used to catalyze the hydrogenations of some alkenes, including cyclohexene, 1-hexene, styrene and 1,3-cyclohexdiene, at different conditions, and the results are listed in Table 1. Both 1-hexene and styrene could almost completely convert into hexane and ethyl benzene within 0.5 h, respectively, at 60 °C and initial H2 pressure of 2 MPa with a substrate/Pd molar ratio of 5000 (Table 1, entries 1 and 2). The conversion of cyclohexene could approach 100% within 1 h with cyclohexane as the only product under the same conditions (Table 1, entry 3). The turnover frequencies (TOFs), defined as moles of substrate consumed per mole of palladium per hour, could reach 10000 or 5000 h−1, suggesting that the SH-IL-Pd catalyst was very active for these reactions. Taking the cyclohexene hydrogenation as an example, the stability of the SH-IL-1.0wt%Pd catalyst was investigated. It was demonstrated that there was almost no activity loss after the catalyst was reused for five times for this reaction (Table 1, entries 3–7), indicating that the as-prepared catalyst was rather stable. TEM observation for the five-time reused catalyst also support this, which shows that the Pd nanoparticles were still firmly adhered on the surface of sepiolite and no remarkable aggregation of Pd nanoparticles occurred after the catalyst was reused for five times, as shown in Fig. 4.| Entry | Catalyst | Substrate | Substrate/Pd (mol/mol) | T/°C | P(H2)/MPa | Time/h | Conversion (%) | TOF/h−1 |
|---|---|---|---|---|---|---|---|---|
| a The amount of cyclohexene was 12 mmol. b SH-IL-1.0%Pd reused for the second time for cyclohexene hydrogenation. c SH-IL-1.0%Pd reused for the third time for cyclohexene hydrogenation. d SH-IL-1.0%Pd reused for the fourth time for cyclohexene hydrogenation e SH-IL-1.0%Pd reused for the fifth time for cyclohexene hydrogenation. f The product is cyclohexene. g The amount of cyclohexene was 100 mmol. | ||||||||
| 1 | SH-IL-1.0%Pd | 1-Hexene | 5000 | 60 | 2 | 0.5 | >99.0 | 10000 |
| 2 | SH-IL-1.0%Pd | Styrene | 5000 | 60 | 2 | 0.5 | >99.0 | 10000 |
| 3 | SH-IL-1.0%Pd | Cyclohexene | 5000 | 60 | 2 | 1 | >99.0 | 5000 |
| 4b | SH-IL-1.0%Pd | Cyclohexene | 5000 | 60 | 2 | 1 | >99.0 | 5000 |
| 5c | SH-IL-1.0%Pd | Cyclohexene | 5000 | 60 | 2 | 1 | >99.0 | 5000 |
| 6d | SH-IL-1.0%Pd | Cyclohexene | 5000 | 60 | 2 | 1 | >99.0 | 5000 |
| 7e | SH-IL-1.0%Pd | Cyclohexene | 5000 | 60 | 2 | 1 | >99.0 | 5000 |
| 8 | SH-IL-1.0%Pd | Cyclohexene | 5000 | 40 | 2 | 2 | 98.0 | 2450 |
| 9 | SH-IL-1.0%Pd | Cyclohexene | 5000 | 20 | 2 | 6 | 98.2 | 833 |
| 10 | SH-IL-1.0%Pd | Cyclohexene | 10000 | 60 | 2 | 2 | >99.0 | 5000 |
| 11 | SH-IL-2.5%Pd | Cyclohexene | 5000 | 60 | 2 | 1 | >99.0 | 5000 |
| 12 | SH-IL-2.5%Pd | Cyclohexene | 10000 | 60 | 2 | 2 | 88.0 | 4400 |
| 13f | SH-IL-1.0%Pd | 1,3-Cyclohexdiene | 5000 | 60 | 2 | 3.5 | >99.0 | 1428 |
| 14 | SH-IL-2.5%Pd | Cyclohexene | 5000 | 60 | 2 | 0.5 | 95.0 | — |
| 15 | Pd/C, 5%Pd | Cyclohexene | 5000 | 60 | 2 | 0.5 | 80.2 | — |
| 16g | SH-IL-1.0%Pd | Cyclohexene | 5000 | 60 | 2 | 1 | >99.0 | 5000 |
 | ||
| Fig. 4 TEM image of the SH-IL-1.0wt%Pd catalyst after it was reused for five times for hydrogenation of cyclohexene. | ||
The SH-IL-1.0wt%Pd catalyst also exhibited a good activity for cyclohexene hydrogenation at lower temperatures (e.g., Table 1, entries 8 and 9), but the TOFs decreased with decreasing temperature. The influence of Pd content on the hydrogenation was also investigated. For example, the SH-IL-2.5wt%Pd composite was used to catalyze the hydrogenation of cyclohexene, which displayed comparable activity with SH-IL-1.0wt%Pd at the C6H10/Pd molar ratio of 5000, as shown in Table 1 (entry 11). However, this catalyst was less active than SH-IL-1.0%Pd when the C6H10/Pd molar ratio was 10000, as listed in Table 1 (entries 10 and 12). This might result from the slight increment of Pd particle size in the SH-IL-2.5%Pd composite. For comparison, the commercially available catalyst Pd/C (5 wt%Pd) was used to catalyze the cyclohexene hydrogenation. As shown in Table 1 (entries 14 and 15), the catalyst prepared in this work was more active than the Pd/C catalyst.
1,3-Cyclohexdiene hydrogenation was selected to investigate the activity of the catalyst SH-IL-1.0wt%Pd for selective hydrogenation. It was demonstrated that the catalyst showed high activity for the selective hydrogenation of 1,3-cyclohexdiene, and cyclohexene was the main product. For instance, at 60 °C and the initial H2 pressure of 2 MPa with a C6H8/Pd molar ratio of 5000, the conversion of cyclohexdiene could reach 99% within 3.5 hours, and the selectivity for cyclohexene could approach 97.6%. This phenomenon was similar to that reported previously,15 which originated from the properties of Pd metallic nanoparticles. Alkadienes are much more strongly adsorbed by the Pd nanoparticles than alkenes, which resulted in preferential hydrogenation of the alkadiene to the alkene. Moreover, the small size of the Pd nanopartilcles is also favorable in enhancing the selectivity.
The Pd catalysts mediated with other ILs including TMG+LA−, TMG+AA− and TMG, were also used to catalyze cyclohexene hydrogenation. It was demonstrated that these catalysts exhibited almost identical activity for this reaction, suggesting that 1,1,3,3-tetramethylguanidine cation played a key role in immobilizing the Pd nanoparticles on sepiolite.
Besides the catalyst for hydrogenation reactions, Pd is also an active catalyst for Heck reactions. For example, Clark et al. supported Pd nanoparticles on porous materials derived from starch in acetone solution, and the resultant Pd catalysts exhibited excellent activities for the Heck reaction of iodobenzene with methyl acetate under microwave irradiation.23 In this work, the catalytic activity of the SH-IL-1.0wt%Pd catalyst for Heck reaction was investigated using the model reaction of iodobenzene and methyl acrylate to give methyl cinnamate. The reaction of iodobenzene (1.0 equiv) and methyl acrylate (1.1 equiv) with the molar ratio of iodobenzene/Pd = 10000 was performed at 140 °C containing triethylamine (Et3N, 1.2 equiv) under solvent-free conditions. The conversion of iodobenzene could reach 100% within 65 min, and no production of biphenyl was detected. This indicates that the catalyst had high efficiency for this Heck reaction. However, as the reaction time was prolonged from 65 min to 20 h, a little cinnamic acid was obtained, which may result from ester hydrolysis of methyl cinnamate. The stability of SH-IL-1.0wt%Pd for the Heck reaction of iodobenzene with methyl acetate was examined by performing this reaction for 5 consecutive runs, and no noticeable activity loss was observed, suggesting that this catalyst was also stable for the Heck reaction. Bromobenzene instead of iodobenzene was investigated for Heck reaction under the same conditions. Unfortunately, the catalyst displayed little activity for this reaction.
To explore the scalability of the as-prepared SH-IL-Pd catalyst, SH-IL-1.0%Pd was used to catalyze hydrogenation of 100 mmol cyclohexene and Heck reaction of 16 mmol iodobenzene with methyl acrylate, respectively. It was demonstrated that the catalyst was still very effective for cyclohexene hydrogenation as the amount of cyclohexene increased (as listed in Table 1, entry 16), and it was also very active for Heck reaction of iodobenzene and methyl acrylate with the increment of iodobeneze.
Conclusions
In summary, the sepiolite supported Pd catalysts were successfully prepared with the aid of ILs, and the as-prepared catalysts exhibited very high efficiency for hydrogenations of alkenes and Heck reaction of iodobenzene and methyl acrylate. Moreover, these catalysts were very stable and could be reused without activity loss. These catalysts combined the advantages of natural clay and ILs, resulting in environmentally friendly materials, which are expected to find promising applications in industrial catalysis.Acknowledgements
This work is financially supported by National Natural Science Foundation of China (No. 20773138), the Chinese Academy of Sciences (KJCX2.YW.H16) and the Opening Research Foundation of the State Key Laboratory of Heavy Oil Processing.References
- J. Santarén, J. Sanz and E. Ruiz-Hitzky, Clay Miner., 1990, 38, 63 CrossRef CAS.
- E. Ruiz-Hitzky, J. Mater. Chem., 2001, 11, 86 RSC.
- W. X. Kuang, G. A. Facey, C. Detellier, B. Casal, J. M. Serratosa and E. Ruiz-Hitzky, Chem. Mater., 2003, 15, 4956 CrossRef CAS.
- M. A. Aramendia, V. Borau, C. Jimenez, J. M. Marinas, A. Porras, F. J. Urbano and L. Villar, J. Mol. Catal., 1994, 94, 131 CAS.
- A. Corma and R. M. Martin-Aranda, Appl. Catal. A., 1993, 105, 271 CrossRef CAS.
- A. Corma, S. Iborra, S. Miquel and J. Primo, J. Catal., 1998, 173, 315 CrossRef CAS.
- R. Sheldon, Chem. Commun., 2001, 2399 RSC.
- Y. Chauvin, L. Mussman and H. Olivier, Angew. Chem. Int. Ed. Engl., 1995, 34, 2698 CrossRef.
- E. Lindner, T. Schneller, F. Auer and H. A. Mayer, Angew. Chem. Int. Ed., 1999, 38, 2154 CrossRef.
- C. P. Mehnert, E. J. Mozeleski and R. A. Cook, Chem. Commun., 2002, 3010–3011 RSC.
- A. Riisager, R. Fehrmann, S. Flicker, R. Hal, M. Haumann and P. Wasserscheid, Angew. Chem. Int. Ed., 2005, 44, 815–819 CrossRef CAS.
- V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615–2665 CrossRef CAS.
- K. Qiao, H. Hagiwara and C. Yokoyama, J. Mol. Catal. A, 2006, 246, 65 CrossRef CAS.
- S. Abelló, F. Medina, X. Rodríguez, Y. Cesteros, P. Salagre, J. E. Sueiras, D. Tichit and B. Coq, Chem. Commun., 2004, 1096 RSC.
- J. Huang, T. Jiang, H. Gao, B. X. Han, Z. M. Liu, W. Wu, Y. Chang and G. Zhao, Angew. Chem. Int. Ed., 2004, 43, 1397 CrossRef CAS.
- S. D. Miao, Z. M. Liu and B. X. Han, Angew. Chem. Int. Ed., 2006, 45, 266 CrossRef CAS.
- S. D. Miao, Z. M. Liu and B. X. Han, J. Phys. Chem. C, 2007, 111, 2185 CrossRef CAS.
- M. Hirao, H. Sugimoto and H. Ohno, J. Electrochem. Soc., 2000, 147, 4168 CrossRef CAS.
- N. M. M. Mateus, L. C. Branco, N. M. T. LourenKo and C. A. M. Afonso, Green Chem., 2003, 5, 347 RSC.
- J. F. Silvain and O. Fouassier, Sur. Interface Anal., 2004, 36, 769 Search PubMed.
- K. S. W. Sing, D. H. Everett and R. A. W. Haul, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- E. P. Barret, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373 CrossRef.
- V. L. Budarin, J. H. Clark, R. Luque, D. J. Macquarrie and R. J. White, Green Chem., 2008, 10, 382 RSC.
| This journal is © The Royal Society of Chemistry 2009 |
