Temporal evolution of imposex in Nassarius reticulatus (L.) along the Portuguese coast: the efficacy of EC regulation 782/2003
Miléne
Rato
*a,
Nelson
Ferreira
b,
José
Santos
c and
Carlos
Barroso
a
aCESAM & Departamento de Biologia, Universidade de Aveiro, 3810-193, Aveiro, Portugal. E-mail: mileneg@bio.ua.pt; Fax: +351 234 426408; Tel: +351 234 370773
bDepartamento de Biologia, Universidade de Aveiro, 3810-193, Aveiro, Portugal
cISEGI, Universidade Nova de Lisboa, Campus de Campolide, 1070-312, Lisboa, Portugal
First published on 15th October 2008
Abstract
Imposex levels in Nassarius reticulatus (L.) were determined in 44 sites along the Portuguese coast in 2006 in order to describe spatial and temporal trends of TBT pollution in the area. The percentage of females with imposex across sites varied between 20 and 100, denoting the extent of this phenomenon throughout the Portuguese coast. The mean female penis length per site varied between 0.0–8.0 mm and the relative penis length index (mean female penis length × 100/mean male penis length) attained a maximum value of 92%, i.e., female penis never surpasses the size of the male penis but nevertheless it can almost approach the male dimensions. The vas deferens sequence index ranged from 0.2 to 4.5 and the oviduct convolution index varied between 0.0 and 1.3 across stations. The penis growth, the vas deferens development and the oviduct convolution were all correlated and constitute visible signs of a global virilisation progression in females in response to the proximity of harbours that constitute the main TBT pollution sources. The results indicate that about 95% of the surveyed sites were still exposed to TBT water concentrations above the OSPAR Environmental Assessment Criteria. Nevertheless, signs of recovery are shown by the significant reduction of VDSI levels in 2006 in comparison to 2003, which points to the efficacy of the EC Regulation 782/2003 in reducing TBT pollution levels in the Portuguese coast.
Introduction
Throughout history numerous methods have been used to counteract the settlement of organisms on the ship hulls. The most successful antifouling coatings known to date are those based on tributyltin (TBT), which were firstly introduced in the 1960s.1TBT paints have offered important economical benefits to shipowners, namely, fuel savings, extended dry-docking intervals and increased vessel availability due to less time spent in dry dock. Consequently, in 1996, for example, the TBT paints were used in 70% of the world fleet (approximately 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ships).2
000 ships).2
TBT was designed for toxic action at the ships surface but once released into the water it does not remain confined to the immediate vicinity of the ships since it is dispersed through the water where it is rapidly adsorbed to biota and to suspended particles that later deposit onto sediments.3,4 Organisms can accumulate TBT by ingestion of contaminated food or via contact with contaminated water or sediments. The deleterious effects of TBT released by antifouling paints became evident in the beginning of 1970s as females of different gastropod mollusc species started to appear along the Atlantic coasts with male characteristics, a phenomenon for which the term ‘imposex’ was coined by Smith.5 In 1974 the oyster (Crassostrea gigas) culture at Arcachon (France) showed failures in spat recruitment, whilst the shell calcification anomalies caused a decline in the marketable value of the remaining stock.6 After the mid-1980s, many other studies have described the TBT toxicity in organisms over a broad taxonomic spectrum, from bacteria to vertebrates, and its negative impacts at the individual, population and community levels.
Legislation to ban the use of organotin (OT) antifouling paints on small boats (<25 m) was introduced for the first time in France in 1982 and later in other European countries such as UK in 1987. These measures led to a reduction in imposex and clearance of oyster pathologies in many areas, accompanied by an amelioration of TBT contamination in the biota, water and sediments.7–11 For example, in the Bay of Arcachon the oyster farming returned to normal: as early as 1983 for spatfall and 1984–1985 for shell anomalies12 and in SW England the Nucella lapillus imposex and female sterility declined following the ban.13 The European Union (EU) applied the above restrictive measures to the member states in 1989 (Directive 89/677/EEC) by banning the retail sail or use of OT paints for pleasure boats (less than 25 m) and fish net cages. However, TBT pollution did not decrease at many EU areas, especially those subjected to large ship traffic. This was the case of Portugal: this country adopted Directive 89/677/EEC in 1993 but by 2000 there was no recovery of imposex in gastropods throughout the coast.14,15 In 2001, the International Maritime Organization (IMO) adopted the ‘International Convention on the Control of Harmful Antifouling Systems on Ships’ (AFS Convention), which stated the prohibition of the application of organotins as biocides after January 1st 2003 and the total ban of its usage after January 1st 2008. Nonetheless, this convention could only enter in force 12 months after 25 states, representing 25% of the world's merchant shipping tonnage, have ratified it. These numbers were only achieved on 17 September 2007 with the 25th state ratification, representing a total of 38% of the world's merchant shipping tonnage.16 However, anticipating a slow ratification process and being aware of the urgent need to implement restrictive measures, the EU put in place the statements of the AFS Convention in July 2003, through the EC Regulation 782/2003 (see also Directive 2002/62/EU) to prohibit the application of OT compounds on all kinds of vessels flying the flag of a member state.
The main objective of the present study is to evaluate the efficacy of EC Regulation 782/2003 in reducing the TBT pollution in Portugal, using the gastropod Nassarius reticulatus (L.) as a bioindicator. This is achieved by assessing the levels of imposex of this species in 2006 along the Portuguese coast and by comparing these levels with those reported previously in 2000 and 2003 for the same sites. Additionally, it is intended to characterize the spatial trend of imposex intensity in several nassariid species along the Portuguese coast.
Study area
The study area corresponds to the Portuguese coast comprised between Vila Praia de Âncora (northern limit) and Faro (southern limit) (Fig. 1), in a coastal extension of about 880 km. The main Portuguese harbours lie along this study area and are located inside natural embayments, like estuarine systems. They may include commercial and fishing ports, marinas and dockyard facilities (Fig. 1), which in most cases are close to each other. Table 1 summarizes relevant information regarding boat traffic and dockyard activity in the Portuguese coast. Lisbon harbour corresponds to the main national commercial port, followed (in terms of gross tonnages (GTs)) by Sines, Leixões, Setúbal, Aveiro, Viana do Castelo, Figueira da Foz and Faro. Fishing boat GTs at each harbour (Table 1) provide an estimate of the relative distribution of the fleet in the Portuguese coast and show that Aveiro harbour encloses by far the highest ship tonnage (four times higher than the second on the rank, Viana do Castelo). Along the study area, there are 20 main marinas with an average berthing capacity (YBC) of approximately 300 yachts, although Lisbon and Portimão can berth, respectively, eight times and two times that number of vessels. There are also many small boat landings spread along the coast. The main dockyards in Portugal are located in the harbours of Viana do Castelo, Aveiro, Figueira da Foz, Peniche, Lisbon, Setúbal and Portimão (Table 1). Commercial ship traffic in the main harbours of Portugal for 2000, 2003 and 2006 is presented in Fig. 2.| Harbours | Commercial shipping | Fishing boats | Marinas | Dockyards | |
|---|---|---|---|---|---|
| N° | GT/103 T | GT/103 T | YBC | Presence | |
| Viana do Castelo | 211 | 926 | 8.2 | 307 | P |
| Póvoa de Varzim | — | — | 7.4 | 231 | |
| Leixões | 2725 | 20415 | 5.9 | 278 | |
| Aveiro | 1064 | 3141 | 34.5 | 270 | P |
| Figueira da Foz | 320 | 823 | 2.6 | 300 | P |
| Nazaré | — | — | 0.5 | 150 | |
| Peniche | — | — | 5.4 | 150 | P |
| Lisboa | 3335 | 35776 | 6.1 | 2335 | P |
| Sesimbra | — | — | 3.8 | 180 | |
| Setúbal | 1498 | 16202 | 1.9 | 150 | P |
| Sines | 1361 | 29893 | 2.4 | 230 | |
| Lagos | — | — | 1.9 | 462 | |
| Portimão | — | — | 4.1 | 620 | P |
| Faro | 23 | 67 | 4.4 | 375 | |
 | ||
| Fig. 1 Nassarius reticulatus. Map of the portuguese coast indicating the sites (1–44) where specimens were collected and location of the main harbours. Italic code numbers represent sampling stations located inside harbours. The graphic bars represent (A) percentage of female affected by imposex (%I), (B) vas deferens sequence index (VDSI5) and (C) relative penis length index (RPLI) for each sampling station. Error bars correspond to 1 standard deviation. The letter ‘b’s on VDSI chart represent stations where females exhibiting b-type VDS stages were found. The braces indicate the stations inside the harbours: VC, Viana de Castelo; Lx, Leixões; Av, Aveiro; FF, Figueira da Foz; N, Nazaré; P, Peniche; L, Lisbon; Sz, Sesimbra; St, Setúbal; Sn, Sines; Lg, Lagos; Pt, Portimão; F, Faro. | ||
 | ||
| Fig. 2 Data regarding the gross tonnage (GT) of ships entered in the main harbours of the Portuguese coast in 2000, 2003 and 2006. Data obtained from INE (Instituto Nacional de Estatística) Statistics Portugal (http://www.ine.pt). | ||
Materials and methods
Sampling and pre-treatment
Sampling of gastropods was performed between May and August 2006, from Vila Praia de Âncora to Faro. Sampling locations were selected in order to provide an extensive coverage of the mainland coast of Portugal, including the main national harbours (Fig. 1; Table 2).| Station code and name | Coordinates (EUR 50) | ♂(N) | H ♂ | ♀(N) | H ♀ | VDSI5 | VDSI4 | AOS | PLI | MPL | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Vila Praia de Âncora | 41° 41.93 N | 08° 51.94 W | 34 | 22.52(c) | 40 | 26.32(b) | 1.90 | 1.90 | 0.00 | 0.19 | 11.38 |
| 2. Praia Norte | 41° 41.85 N | 08° 51.13 W | 35 | 16.83(g) | 53 | 21.43(f) | 1.36 | 1.36 | 0.00 | 0.08 | 5.21 |
| 3. V. Castelo, Marina | 41° 41.70 N | 08° 49.20 W | 37 | 22.30(e) | 29 | 21.81(g) | 2.72 | 2.72 | 0.00 | 1.46 | 5.30 |
| 4. V. Castelo, Marégrafo | 41° 41.43 N | 08° 49.71 W | 35 | 20.52(f) | 42 | 25.16(d) | 3.24 | 3.10 | 0.15 | 1.74 | 8.27 |
| 5. V. Castelo, Estaleiro | 41° 41.38 N | 08° 50.01 W | 34 | 23.32(d) | 44 | 25.92(c) | 4.45 | 3.95 | 1.25 | 7.99 | 12.47 |
| 6. V. Castelo, Cais | 41° 41.34 N | 08° 50.26 W | 53 | 22.95(e) | 25 | 25.41(d) | 3.68 | 3.24 | 0.68 | 3.06 | 12.75 |
| 7. V. Castelo, Barra | 41° 41.06 N | 08° 50.24 W | 12 | 23.28(b) | 10 | 24.33(c) | 3.30 | 3.20 | 0.22 | 5.70 | 9.94 |
| 8. Praia da Amorosa | 41° 38.72 N | 08° 49.31 W | 36 | 19.73(e) | 62 | 25.26(c) | 1.42 | 1.42 | 0.00 | 0.12 | 9.04 |
| 9. Póvoa de Varzim | 41° 23.18 N | 08° 46.40 W | 43 | 21.39(d) | 61 | 21.68(f) | 0.56 | 0.56 | 0.00 | 0.02 | 9.18 |
| 10. Praia de Leça | 41° 12.21 N | 08° 42.82 W | 11 | 24.19(b) | 8 | 22.87(d) | 0.75 | 0.75 | 0.00 | 0.03 | 10.50 |
| 11. Porto de Leixões, Plat. 1 | 41° 11.42 N | 08° 41.43 W | 45 | 23.52(b) | 50 | 22.81(c) | 3.60 | 3.48 | 0.45 | 4.14 | 12.64 |
| 12. Porto de Leixões, Marina | 41° 11.30 N | 08° 42.24 W | 43 | 22.62(c) | 39 | 23.69(c) | 4.13 | 3.87 | 0.46 | 6.44 | 12.20 |
| 13. Porto de Leixões, Plat. 2 | 41° 11.26 N | 08° 41.89 W | 4 | 23.76(b) | 3 | 26.35(b) | 4.33 | 4.00 | 0.00 | 4.75 | 13.75 |
| 14. Praia da Foz | 41° 09.78 N | 08° 41.10 W | 27 | 23.73(b) | 78 | 25.70(b) | 0.81 | 0.81 | 0.12 | 0.07 | 11.55 |
| 15. Aveiro, Muranzel | 40° 42.49 N | 08° 42.25 W | 23 | 27.96(b) | 37 | 29.37(b) | 1.19 | 1.19 | 0.00 | 0.05 | 12.92 |
| 16. Aveiro, São Jacinto | 40° 39.48 N | 08° 43.56 W | 15 | 24.29(b) | 25 | 25.38(b) | 2.24 | 2.16 | 0.00 | 1.05 | 9.66 |
| 17. Aveiro, P. Com. Norte | 40° 39.06 N | 08° 43.76 W | 14 | 23.69(c) | 41 | 25.55(c) | 2.32 | 2.29 | 0.00 | 0.41 | 5.57 |
| 18. Aveiro, Terminal Químico | 40° 39.46 N | 08° 42.74 W | 26 | 25.23(b) | 31 | 25.80(b) | 3.32 | 3.16 | 0.00 | 1.45 | 9.45 |
| 19. Aveiro, Magalhães Mira | 40° 38.65 N | 08° 44.06 W | 18 | 27.02(b) | 36 | 27.38(b) | 2.94 | 2.83 | 0.00 | 1.16 | 7.88 |
| 20. Aveiro, Barra | 40° 38.71 N | 08° 44.82 W | 23 | 26.99(b) | 35 | 27.17(b) | 2.80 | 2.71 | 0.00 | 1.13 | 11.78 |
| 21. F. da Foz, Marina | 40° 08.91 N | 08° 51.67 W | 34 | 24.56(c) | 48 | 21.89(e) | 1.23 | 1.23 | 0.00 | 0.15 | 3.28 |
| 22. F. da Foz, Estaleiro | 40° 08.60 N | 08° 51.55 W | 19 | 25.73(b) | 14 | 26.95(b) | 1.29 | 1.29 | 0.00 | 0.21 | 7.04 |
| 23. Nazaré, Porto de Pesca | 39° 35.04 N | 09° 04.39 W | 10 | 26.70(b) | 5 | 26.79(b) | 3.40 | 3.20 | 0.40 | 3.88 | 7.80 |
| 24. Foz do Arelho | 39° 25.70 N | 09° 13.39 W | 27 | 20.41(c) | 80 | 25.28(c) | 0.20 | 0.20 | 0.00 | 0.00 | 10.56 |
| 25. Peniche, Porto de Recreio | 39° 21.15 N | 09° 22.52 W | 50 | 22.00(b) | 8 | 22.83(b) | 4.50 | 4.00 | 0.57 | 6.59 | 8.74 |
| 26. Praia do Guincho | 38° 43.74 N | 09° 28.46 W | 11 | 23.64(b) | 21 | 25.46(b) | 3.14 | 3.14 | 0.10 | 1.57 | 12.25 |
| 27. Praia das Avencas | 38° 41.21 N | 09° 21.27 W | 15 | 20.50(c) | 28 | 22.00(b) | 2.21 | 2.21 | 0.00 | 0.43 | 10.54 |
| 28. Lisboa, Marina de Belém | 38° 41.50 N | 09° 12.50 W | 48 | 22.45(c) | 22 | 23.57(c) | 3.36 | 3.36 | 0.00 | 2.01 | 10.67 |
| 29. Lisboa, Porto Brandão | 38° 40.77 N | 09° 12.29 W | 27 | 24.03(c) | 32 | 24.64(c) | 3.34 | 3.34 | 0.00 | 2.74 | 13.70 |
| 30. Lisboa, Trafaria | 38° 40.55 N | 09° 14.09 W | 26 | 22.58(c) | 19 | 24.61(b) | 3.47 | 3.42 | 0.11 | 3.53 | 10.69 |
| 31. Sesimbra, Porto de Pesca | 38° 26.25 N | 08° 06.76 W | 9 | 18.68(b) | 5 | 20.31(d) | 4.20 | 4.00 | 0.00 | 4.82 | 9.56 |
| 32. Portinho da Arrábida | 38° 28.58 N | 08° 58.97 W | 16 | 24.28(b) | 28 | 26.07(c) | 3.25 | 3.25 | 0.00 | 2.52 | 10.88 |
| 33. Setúbal, Lota | 38° 31.17 N | 08° 52.58 W | 51 | 21.32(b) | 25 | 20.72(b) | 4.20 | 3.88 | 0.00 | 5.57 | 8.96 |
| 34. Setúbal, Tróia | 38° 26.25 N | 09° 06.76 W | 30 | 21.45(b) | 26 | 21.05(b) | 4.15 | 3.88 | 0.12 | 4.38 | 8.18 |
| 35. Sines, Porto de Pesca | 37° 57.28 N | 08° 52.21 W | 54 | 20.51(b) | 17 | 20.66(b) | 4.41 | 4.00 | 0.00 | 6.94 | 7.55 |
| 36. Vila Nova de Mil Fontes | 37° 43.30 N | 08° 47.25 W | 24 | 21.47(b) | 49 | 21.23(g) | 0.20 | 0.20 | 0.00 | 0.00 | 7.36 |
| 37. Zambujeira do Mar | 37° 33.20 N | 08° 47.44 W | 26 | 21.67(b) | 34 | 22.95(b) | 0.76 | 0.76 | 0.00 | 0.02 | 10.22 |
| 38. Praia da Arrifana | 37° 17.82 N | 08° 52.11 W | 26 | 23.51(b) | 39 | 24.37(b) | 3.59 | 3.56 | 0.00 | 3.25 | 9.40 |
| 39. Lagos, Porto de Pesca | 37° 06.30 N | 08° 40.33 W | 13 | 18.29(b) | 24 | 19.96(c) | 3.83 | 3.67 | 0.00 | 1.88 | 8.00 |
| 40. Lagos, Barra | 37° 06.08 N | 08° 40.15 W | 21 | 20.09(b) | 36 | 21.46(d) | 3.42 | 3.33 | 0.00 | 2.08 | 10.00 |
| 41. Alvor, Aquacultura | 37° 07.97 N | 08° 37.48 W | 26 | 22.12(b) | 18 | 24.17(c) | 0.67 | 0.67 | 0.00 | 0.02 | 9.08 |
| 42. Alvor, Barra | 37° 07.22 N | 08° 37.14 W | 27 | 23.26(b) | 30 | 23.81(b) | 0.37 | 0.37 | 0.00 | 0.01 | 11.15 |
| 43. Portinho de Ferragudo | 37° 07.48 N | 08° 31.24 W | 18 | 20.61(c) | 11 | 29.91(c) | 4.45 | 3.91 | 0.00 | 2.89 | 7.04 |
| 44. Ilha da Armona | 37° 01.55 N | 07° 50.40 W | 9 | 21.23(c) | 8 | 21.21(b) | 0.75 | 0.75 | 0.00 | 0.27 | 7.71 |
Geographical coordinates were determined with a mobile global positioning system (GPS) at each sampling site. The specimens were collected by hand at the intertidal shore and with baited hoop nets at sublitoral sites. The animals were maintained in aquaria for about 1–3 days. Whenever possible, 30 or more animals of each gender were analysed per sampling station. Only adult animals were selected. They were narcotized using 7% MgCl2 in distilled water. The shell height (distance from shell apex to lip of siphonal canal) was measured with vernier callipers to the nearest 0.1 mm. The shells were cracked open with a bench vice and the individuals were sexed and dissected under a stereo microscope. Parasitized specimens were discarded from the analysis.
Imposex analysis
The imposex parameters determined for each sampling station were the mean female penis length index (PLI), the relative penis length index (RPLI = mean female penis length × 100/mean male penis length), the vas deferens sequence index (VDSI), the average oviduct convolution stage (AOS) and the percentage of females affected by imposex (%I). The penis length was measured using a stereomicroscope with a graduated eyepiece to the nearest 0.14 mm. The VDS stages in N. reticulatus were classified according to the scoring system proposed by Strobenet al.17 but the computation of the VDSI for each site was based on the methodology proposed by Barroso et al.15 (VDSI5), i.e., stages 4 and 4+ were computed with the numerical values of 4 and 5, respectively, instead of a common value of 4, which provides a better discrimination of imposex levels between different sites or different dates; nevertheless, the VDSI values computed according to Strobenet al.17 are also given in Table 2 (VDSI4) to allow comparisons with other studies. The progressive oviduct convolution in females was ranked according to the three-stage scale (0, 1, 2) of Barreiro et al.18 and the average value (AOS) was assessed per each station. Sterility of N. reticulatus females will be addressed elsewhere. Imposex determination in N. incrassatus was performed using the same methodology described for N. reticulatus except that VDS was classified according to Oehlmann et al.19Statistical analysis
The statistical analysis performed in the current work was based on nonparametric methods. The correlation analysis refers to the Spearman correlation coefficient. The comparison between groups was based on the Mann–Whitney test for independent samples and on the Friedman test for paired samples. The adopted significance level was 5%.Results
Nassarius reticulatus is very abundant along the Portuguese coast and represents, by far, the most common nassariid occurring in the surveyed area. We attempted to collect other nassariids but only a sufficient number of Nassarius incrassatus (Strőm, 1768) was collected by hand or baited hoop nets. Therefore, the available samples could only provide the assessment of imposex in N. reticulatus and N. incrassatus.Nassarius reticulatus imposex
The imposex levels of N. reticulatus at the different sampling stations are summarized in Table 2 and Fig. 1. Females with imposex occurred at all visited sites, denoting the extent of this phenomenon throughout the Portuguese coast. In fact, imposex (I%) affected between 20 and 100% of the females across the sampling sites. PLI varied between 0.0–8.0 mm and RPLI attained a maximum value of 92%, i.e., female penis never surpasses the size of the male penis but nevertheless it can almost approach the male dimensions. VDSI5 ranged from 0.2 to 4.5. Most females (88.6%) presented a-type VDS stages, i.e. with simultaneous penis development.17 Females exhibiting b-type VDS stages (’aphalic condition’) were less common (11.4%) but nevertheless they were spread across 22 sampling stations along the coast (see Fig. 1). There was a highly significant correlation between the VDSI5 and the Ln PLI (r = 0.94. P < 0.001) (Fig. 3) and also between VDSI5 and Ln RPLI (r = 0.93, P < 0.001) across stations, which means that the development of the vas deferens is accompanied by an increase of the penis length, both expressing the virilisation process in females. Another possible sign of female virilisation is the gonadal oviduct convolution, resembling the sinuous seminal vesicle of the males; the AOS index varied between 0.0 and 1.3 across stations. The average oviduct convolution stage estimated for all the females exhibiting a given VDS stage (0 to 5) is significantly correlated (r = 0.90, P < 0.05) with the VDS stage (Fig. 4). Hence, the intensity of oviduct convolution and vas deferens and penis development were all correlated and constitute visible signs of a global virilisation progression in females. | ||
| Fig. 3 Nassarius reticulatus. Relationship between the neparian logarithm of penis length index (PLI) and the vas deferens sequence index (VDSI5). | ||
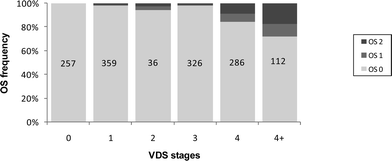 | ||
| Fig. 4 Nassarius reticulatus. Relative frequencies of oviduct stages (OS) observed for each female VDS stage. The numbers represent the total female number observed for each VDS stage. | ||
Nassarius incrassatus imposex
N . incrassatus samples were obtained in a relatively low number of sites, in some cases with a scarce number of animals, but still they could provide a coarse estimation of imposex levels in this species along the coast (Table 3). The percentage of affected females varied between 0 and 100%, VDSI between 0.0 and 3.5, PLI between 0.00 and 1.37 mm and RPLI between 0 and 31%. None of the observed females of N. incrassatus presented a convoluted gonadal oviduct. There was a significant correlation between imposex intensity in sympatric populations of N. incrassatus (only samples with 6 or more females were considered) and N. reticulatus for VDSI (r = 0.81, P < 0.015), PLI (r = 0.78, P < 0.025) and RPLI (r = 0.79, P < 0.025) but all imposex parameters presented lower values in N. incrassatus.| Station code | ♂(N) | H ♂ | ♀(N) | H ♀ | %I | VDSI | AOS | PLI | MPL | RPLI |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 12.15(b) | 7 | 11.85(b) | 0 | 0.00 | 0.00 | 0.00 | 10.67 | 0 |
| 9 | 6 | 11.65(b) | 4 | 10.54(c) | 0 | 0.00 | 0.00 | 0.00 | 11.00 | 0 |
| 10 | 20 | 12.76(b) | 20 | 12.72(b) | 0 | 0.00 | 0.00 | 0.00 | 7.43 | 0 |
| 14 | 22 | 11.45(c) | 29 | 10.98(c) | 3 | 0.03 | 0.00 | 0.00 | 8.44 | 0 |
| 17 | 3 | 11.49(c) | 4 | 11.02(c) | 25 | 0.25 | 0.00 | 0.20 | 6.67 | 3 |
| 23 | 14 | 12.34(b) | 12 | 11.86(c) | 100 | 3.50 | 0.00 | 1.37 | 4.45 | 31 |
| 27 | 2 | 12.79(c) | 2 | 12.58(a) | 0 | 0.00 | 0.00 | 0.00 | 12.00 | 0 |
| 29 | 19 | 13.92(b) | 14 | 13.59(b) | 71 | 0.71 | 0.00 | 0.44 | 7.31 | 6 |
| 33 | 23 | 12.07(b) | 22 | 12.09(b) | 73 | 0.73 | 0.00 | 0.52 | 4.74 | 11 |
| 34 | 23 | 12.37(b) | 33 | 12.46(a) | 15 | 0.15 | 0.00 | 0.05 | 1.71 | 3 |
Spatial evolution of imposex in Nassarius reticulatus
Due to the low abundance of other nassariid species, N. reticulatus is the only one used to describe spatial trends of imposex intensity along the Portuguese coast. There is an evident relationship between the imposex levels in N. reticulatus and the proximity of harbours enclosing potential TBT contamination sources such as commercial and fishing ports, dockyards and marinas. The highest values of VDSI5 (>4) were found at stations located inside the harbours of Viana do Castelo (stn 5), Leixões (stn 12 and 13), Peniche (stn 25), Sesimbra (stn 31), Setúbal (stn 33 and 34), Sines (stn 35) and Portimão (stn 43). The lowest values of VDSI5 (<1) occurred at sites distant from harbours, either on the open shore (stn 9, 10, 14, 24, 37) or inside estuarine systems (stn 36, 41, 42, 44). There were no pristine sites with unaffected females (see Fig. 1A), which probably due to the ubiquitous presence of small fishing and leisure boats and water current transport of TBT from contaminated areas. Considering all the surveyed sites, a highly significant difference (Mann–Whitney test, statistic = 125.0, P < 0.001) can be observed in the VDSI5 between two main groups of stations: distant from harbours (stn 1–2, 8–10, 14, 24, 26–27, 36–37, 41–42, 44) and close to harbours (remaining 30 stations). When the analysis is focused on a single region for which there is a reasonable number of sampling stations around a specific harbour, a clear increasing gradient of imposex is evident on approaching the harbour (Fig. 1). For instance, at Viana do Castelo the lowest imposex levels occurred at stations outside the estuary in the open coast (stn 2, 8) whereas the highest levels were registered in the harbour inside the estuary (stn 3–7). Similar trends were also observed around Leixões and Lisbon (Fig. 1B). The RPLI values presented the same general trend described above for VDSI5, which is expected since both parameters were significantly correlated to each other (Fig. 3). In fact, taking again Viana do Castelo as an example, the highest values of RPLI were found inside the estuary (stn 3–7) and the lowest values occurred outside the estuary (stn 2, 8). Once again the same pattern was observed for Leixões and Lisbon harbours (Fig. 1C).Temporal evolution of imposex in Nassarius reticulatus
Temporal comparisons of VDSI5 in N. reticulatus for common sites sampled in 2000, 2003 and 2006 are shown in Table 4. This comparison allows the assessment of the temporal evolution of TBT pollution in recent years, as the imposex analyses were performed on the three occasions using identical methods. The comparison included the samples collected in the three dates in 19 stations using VDSI5 as variable. The geographical locations of these stations were all the same for these three years, thus it was considered that the three samples were paired due to location reasons. The Friedman test20 indicated that there was a highly significant decrease in the VDSI5 levels (statistic = 16.9, P < 0.001). Multiple comparisons within the Friedman test were performed between these years concerning the levels of this variable and they showed a significant reduction of the VDSI5 (statistic = 20, P < 0.001) between 2003 and 2006, although there was no statistical difference between the VDSI5 levels (statistic = 5, P = 0.279) in the years 2000 and 2003.| Station code | VDSI5 | ||
|---|---|---|---|
| 2000 | 2003 | 2006 | |
| 2 | 2.50 | 2.72 | 1.36 |
| 5 | 4.50 | 4.88 | 4.45 |
| 8 | 1.70 | 2.20 | 1.42 |
| 12 | 4.40 | 4.36 | 4.13 |
| 13 | 4.60 | 4.45 | 4.33 |
| 14 | 2.50 | 1.30 | 0.81 |
| 16 | 2.60 | 2.45 | 2.24 |
| 17 | 4.30 | 4.21 | 2.32 |
| 19 | 3.30 | 3.87 | 2.94 |
| 20 | 2.70 | 3.00 | 2.80 |
| 25 | 4.90 | 5.00 | 4.50 |
| 26 | 3.40 | 3.27 | 3.14 |
| 27 | 4.40 | 4.08 | 2.21 |
| 30 | 4.90 | 4.85 | 3.47 |
| 33 | 4.90 | 4.32 | 4.20 |
| 34 | 4.60 | 4.56 | 4.15 |
| 36 | 0.50 | 0.73 | 0.20 |
| 37 | 0.60 | 0.39 | 0.76 |
| 38 | 0.70 | 3.09 | 3.59 |
Discussion
The current study shows that the netted whelk Nassarius reticulatus is a common gastropod in the Portuguese coast. Specimens were found in large quantities in sandy or muddy sediments of sheltered and rocky shores, particularly inside estuarine systems. Despite the abundance in most of the sites along the coast, in some visited stations the species was scarce or even absent, especially in the southern coast. Nevertheless, the stations where the species was present provided a good monitoring coverage of the Portuguese shore, comprising the main harbours of both western (Viana do Castelo, Leixões, Aveiro, Figueira da Foz, Lisbon, Setúbal and Sines) and southern (Portimão and Faro) coasts. Other nassariids were collected during this survey, namely N. pygmaeus (Lamarck, 1822), N. nitidus (Jeffreys, 1867) (see Rolán and Luque21) and N. incrassatus, but they were far less abundant than N. reticulatus. Although N. incrassatus is a suitable bioindicator,19 the current work shows that this species is less adequate than N. reticulatus for TBT pollution assessment along the Portuguese coast due to its poorer abundance and lower sensitivity. Other gastropods proposed in the literature as TBT pollution indicators, namely Nucella lapillus (L.), Ocenebra erinacea (L.), Ocinebrina aciculata (Lamark), Littorina littorea (L.) and Hydrobia ulvae (Pennant), do occur along the Portuguese coast and were also sampled in the current survey (to be published elsewhere). But among all these species N. reticulatus is by far the most abundant and ubiquitous in mainland Portuguese waters and should be regarded as the key bioindicator for national TBT pollution monitoring purposes.It is well established that imposex is a fairly specific and dose dependent response to TBT pollution.11 In N. reticulatus this relationship is supported by laboratory experiments22–24 and by field evidence of correlation between imposex and TBT female body burdens, whether in the same area of the current survey15,25 or in other coastal areas such as Spain,18 France17 and Britain.26 This fact allows the use of imposex per se as a reliable biomarker for the assessment of spatial and temporal trends of the level of TBT pollution, exempting the need to perform TBT determinations in the tissues or in the environment. The current work and the above studies have shown that imposex in N. reticulatus is expressed by three visible morphological changes: the development of a vas deferens, the growth of a penis and the convolution of the gonadal oviduct.
RPLI is a useful index to describe spatial trends of imposex in the sense that it provides very interesting images of spatial gradients around hot spots of pollution along the coast (see Fig. 1C). However, indices based on ‘penis length’ must be regarded with some caution because the current survey denotes the occurrence of aphallic females (imposex-affected females lacking a penis) in half of the 44 stations sampled. As these females are computed by this index as ‘zero’ when in fact they exhibit imposex, the RPLI may incorporate some bias. Moreover, the male penis size depends on the testis maturation state, which varies along the year and forces RPLI to vary according to the sampling season.27 Hence, for ‘inter-site’ or ‘inter-date’ statistical comparisons we consider that VDSI is a more reliable index to be used. This index integrates the development of the vas deferens and the initial development of the penis and it does not change seasonally. However, the VDS scales that are applied by different authors for N. reticulatus differ in what regards to the maximum numerical value that VDS attains after VDS stage 4. Some authors seek for a coherent standardization of VDS classification among different prosobranch species (see Strobenet al.22 and Ohelman et al.19) so that VDS = 5 means that the vulva is blocked whereas VDS = 6 corresponds to a stage when aborted capsules accumulate inside the capsule gland as a consequence of the vulva closure (as originally defined by Gibbs et al.28 for N. lapillus). N. reticulatus females with aborted capsules have already been found in the European coast15,18,25,29 but there are still no experimental evidences that such condition is a direct consequence of vas deferens development in this species and so, according to these criteria, the maximum possible score attained in N. reticulatus cannot exceed the value of 4 (corresponding either to VDS stage 4 or 4+). Other authors attempt to increase the resolution power of the index in order to improve the discrimination between different polluted sites, independently of what happens in other species, and the score can attain a maximum value of 5 (corresponding to 4+)15 or 4.5 18 as the vas deferens grows beyond the vulva. Regardless which criteria is used there must be a special caution to provide VDSI values that can compare to results obtained by different authors. Hence, in the current work we apply VDSI4 (stages 4 and 4+ are computed as 4) according to Strobenet al.17 (Table 2) in order to compare our results with the OSPAR provisional assessment criteria (see below) and to make the data available to other authors but, on the other hand, we use VDSI5 (stage 4 is computed as 4 and stage 4+ is computed as 5) to increase the power of the spatial and temporal evolution analysis in the surveyed area.
The current study shows that the higher levels of imposex are found inside or in the vicinities of harbours. This trend was also registered in previous studies,15,25 strengthening the concept that areas in the proximity of harbours are ‘hotspots’ of TBT pollution. In fact, Portuguese harbours enclose commercial/fishing ports and marinas where many boats are gathered and are sources for TBT contamination to the environment through the leaching of this compound from antifouling coatings into the water. Besides, most of them contain ship/boat construction and repairing dockyards. At these facilities, boats are painted or repainted and the old layer of paint is removed from the hull, which results in slurry of wash-down water potentially contaminated with antifouling compounds and paint particles, which may represent a very important source of TBT input in the local area.9,30 Selecting the VDSI as a representative index of imposex, the current work reveals a highly significant difference in the values of VDSI5 between stations distant and stations close to harbours, providing evidence of imposex progression on approaching the harbours. Considering the specificity of imposex as a TBT pollution biomarker, which provides robust information of TBT exposure at a given location, the results of this survey indicate that most of the sites analysed are still highly contaminated by TBT. In fact, OSPAR has developed provisional assessment criteria to evaluate monitoring data on TBT-specific biological effects related to the existing Environmental Assessment Criteria (EAC) for TBT.31 According to these criteria, among the 44 stations surveyed in the Portuguese coast only two stations have been exposed to TBT concentrations below the EAC derived for TBT (VDSI4 < 0.3); thirteen stations fall into class C (0.3 < VDSI4 < 2.0), which means that they have been exposed to TBT concentrations higher than the EAC and there is a risk of adverse effects, such as reduced growth and recruitment in more sensitive species, caused by long-term exposure to TBT; eighteen stations are ranked in class D (2.0 < VDSI4 < 3.5) indicating that TBT exposure directly affects the reproductive capacity of more sensitive species; the remaining eleven stations are included in class E (VDSI4 > 3.5), i.e., the populations of more sensitive species are unable to reproduce, with the majority of the females sterilized.31 This analysis shows that there is currently a high ecological impact caused by TBT on the marine ecosystems along the Portuguese coast.
Barroso et al.15 and Santos et al.32 have found that the implementation of the European Directive 89/677/EEC (banning the use of organotins on ships under 25 m) was ineffective in reducing imposex in N. lapillus along the coast of Portugal, indicating that there was no decline of TBT concentrations in the environment. The current results demonstrate that imposex levels of N. reticulatus in 2006 are significantly lower when compared with those from 2003.25 It is meaningful to point out that no significant differences were found when comparing imposex levels from 2000 and 2003 surveys. It must be noted there was no decline in ship traffic along this period along the Portuguese coast (Fig. 2). It seems therefore that the total ban on the application of TBT antifouling paints on submerged structures imposed by EC Regulation 782/2003 did have a significant favourable impact on TBT pollution in Portugal. We expect that this decline will become more evident in the near future as ships will not be allowed to circulate with OT antifouling coatings after September 2008.
In conclusion, N. reticulatus is a key indicator species for TBT pollution monitoring programs along the Portuguese coast. The coastal areas of Portugal are still heavily polluted by TBT, particularly around the harbours, as the imposex levels found in these sites are high. Nevertheless, the comparison of the current results with data collected in 2003 shows that imposex levels are decreasing. The EC Regulation 782/2003 seems to be effective but the rate at which TBT pollution and imposex will recover must be assessed through the continual monitoring of the Portuguese coast, for which the current work provides an important baseline.
Acknowledgements
This work was developed under the research project POCI/MAR/61893/2004 financed by the FCT and by the POCI 2010, co-financed by FEDER. This work was supported trough a PhD grant (SFRH/BD/12441/2003) attributed by the Portuguese Foundation for Science and Technology (FCT).References
- J. E. Hunter and C. D. Anderson, Proceedings of Control of TBT-based Antifouling Paints for Environmental Protection, World Maritime University, Malmö, Sweden, 2000 Search PubMed.
- CEFIC (European Chemical Industry Council), Document MEPC 36/14/4 for the 36th Meeting of the Marine Environmental Protection Committee of the International Maritime Organization, London, 1996 Search PubMed.
- C. Stewart and S. J. de Mora, Appl. Organomet. Chem., 1992, 6, 507 CrossRef CAS.
- C. C. ten Hallers-Tjabbes, Mar. Pollut. Bull., 2000, 40, 289 CAS.
- B. S. Smith, Proc. Malacol. Soc. London, 1971, 39, 377 Search PubMed.
- C. Alzieu, Sci. Total Environ., 2000, 258, 99 CrossRef CAS.
- S. K. Bailey and I. M. Davies, Mar. Environ. Res., 1991, 32, 187 CrossRef.
- S. M. Evans, T. Leksono and P. D. McKinnell, Mar. Pollut. Bull., 1995, 30, 14 CrossRef CAS.
- A. C. Birchenough, S. M. Evans, C. Moss and R. Welch, Mar. Pollut. Bull., 2002, 44, 652 CrossRef CAS.
- T. J. Reitsema, J. A. J. Thompson, P. Scholtens and J. T. Spickett, Mar. Pollut. Bull., 2002, 44, 257 CrossRef CAS.
- M. Huet, Y. M. Paulet and J. Clavier, Mar. Ecol. Progr. Ser., 2004, 270, 153 CrossRef.
- C. Alzieu, Mar. Environ. Res., 1991, 32, 7 CrossRef.
- P. E. Gibbs, G. W. Bryan, in Tributyltin: Case Study of an Environmental Contaminant, ed. S. J. de Mora, Cambridge Environmental Chemistry Series 8, Cambridge University Press, Cambridge, 1996, pp. 212–236 Search PubMed.
- C. M. Barroso and H. M. Moreira, Mar. Pollut. Bull., 2002, 44, 480 CrossRef CAS.
- C. M. Barroso, H. M. Moreira and M. J. Bebianno, Mar. Ecol.: Prog. Ser., 2002, 230, 127 CrossRef.
- IMO, Summary of Conventions as at 30 November 2007, International Maritime Organization, London, 2007, http://www.imo.org (accessed 10/12/07) Search PubMed.
- E. Stroben, J. Oehlmann and P. Fioroni, Mar. Biol., 1992, 113, 625 CrossRef.
- R. Barreiro, R. González, M. Quintela and J. M. Ruiz, Mar. Ecol.: Prog. Ser., 2001, 218, 203 CrossRef CAS.
- J. Oehlmann, E. Stroben, U. Schulte-Oehlmann and B. Barbara, Aquat. Toxicol., 1998, 43, 239 CrossRef.
- W. J. Conover, Practical Nonparametric Statistics, John Wiley & Sons, New York, 3rd edn, 1999, p. 597 Search PubMed.
- E. Rolán and A. A. Luque, Iberus, 1994, 12, 59 Search PubMed.
- E. Stroben, J. Oehlmann and P. Fioroni, Mar. Biol., 1992, 114, 289 CrossRef.
- C. Bettin, J. Oehlmann and E. Stroben, Helgol. Meeresunters., 1996, 50, 299 Search PubMed.
- C. M. Barroso, M. A. Reis-Henriques, M. S. Ferreira and M. H. Moreira, J. Mar. Biol. Assoc. U. K., 2002, 82, 249 Search PubMed.
- A. Sousa, S. Mendo and C. M. Barroso, Appl. Organomet. Chem., 2005, 19, 315 CrossRef CAS.
- G. W. Bryan, G. R. Burt, P. E. Gibbs and P. L. Pascoe, J. Mar. Biol. Assoc. U. K., 1993, 73, 913 Search PubMed.
- C. M. Barroso and M. H. Moreira, J. Mar. Biol. Assoc. U. K., 1998, 78, 1233 Search PubMed.
- P. E. Gibbs, G. W. Bryan, P. L. Pascoe and G. R. Burt, J. Mar. Biol. Assoc. U. K., 1987, 67, 507 Search PubMed.
- M. Huet, P. Fiorini, J. Oehlmann and E. Stroben, Hydrobiologia, 1995, 309, 29 CrossRef.
- S. J. de Mora, C. Stewart and D. Phillips, Mar. Pollut. Bull., 1995, 30, 50 CrossRef CAS.
- OSPAR, Provisional JAMP Assessment Criteria for TBT – Specific Biological Effects, Reference Number 2004-15-E, OSPAR Commission, London, UK, 2004 Search PubMed.
- M. M. Santos, C. C. ten Hallers-Tjabbes, A. M. Santos and N. Vieira, J. Sea Res., 2002, 48, 217 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2009 |
